The Ascidian-Derived Metabolites with Antimicrobial Properties
Abstract
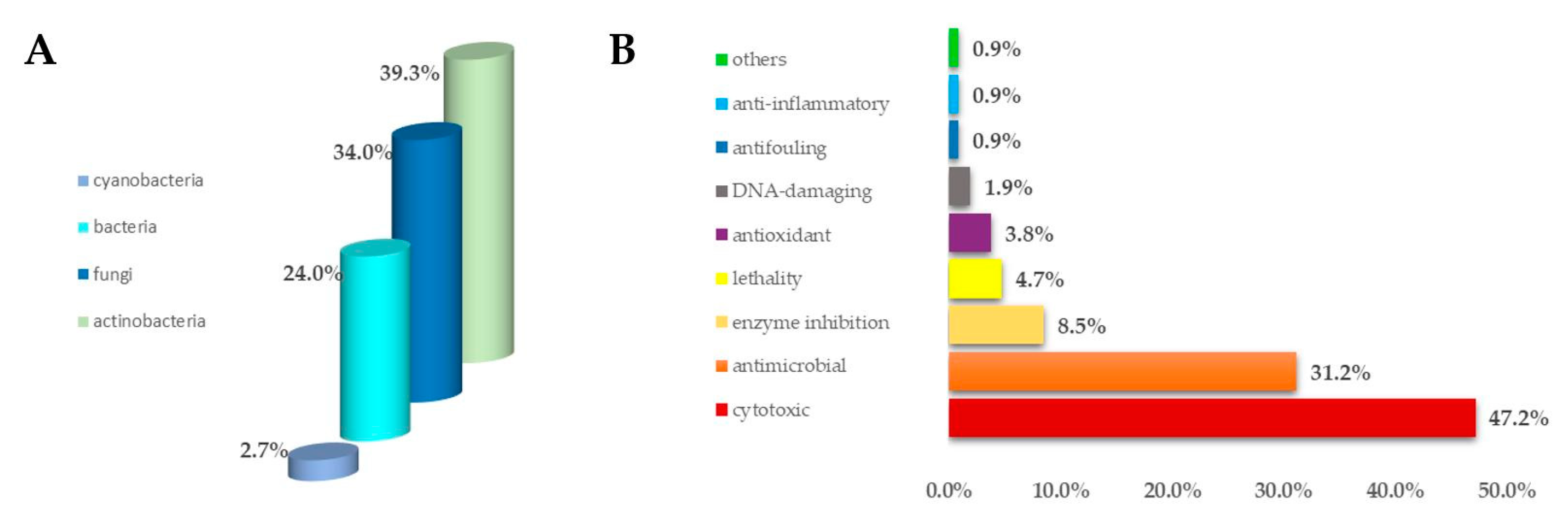
1. Introduction
2. Results and Discussion
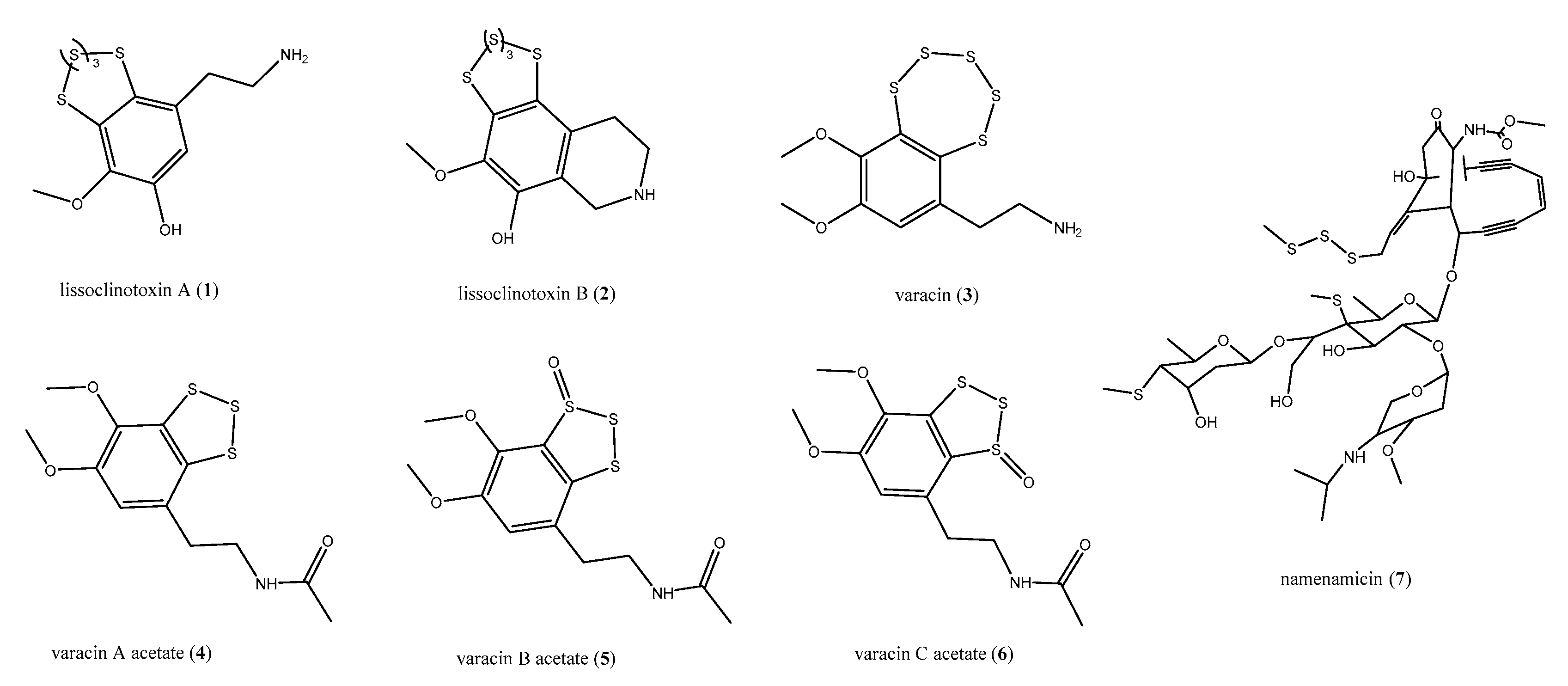
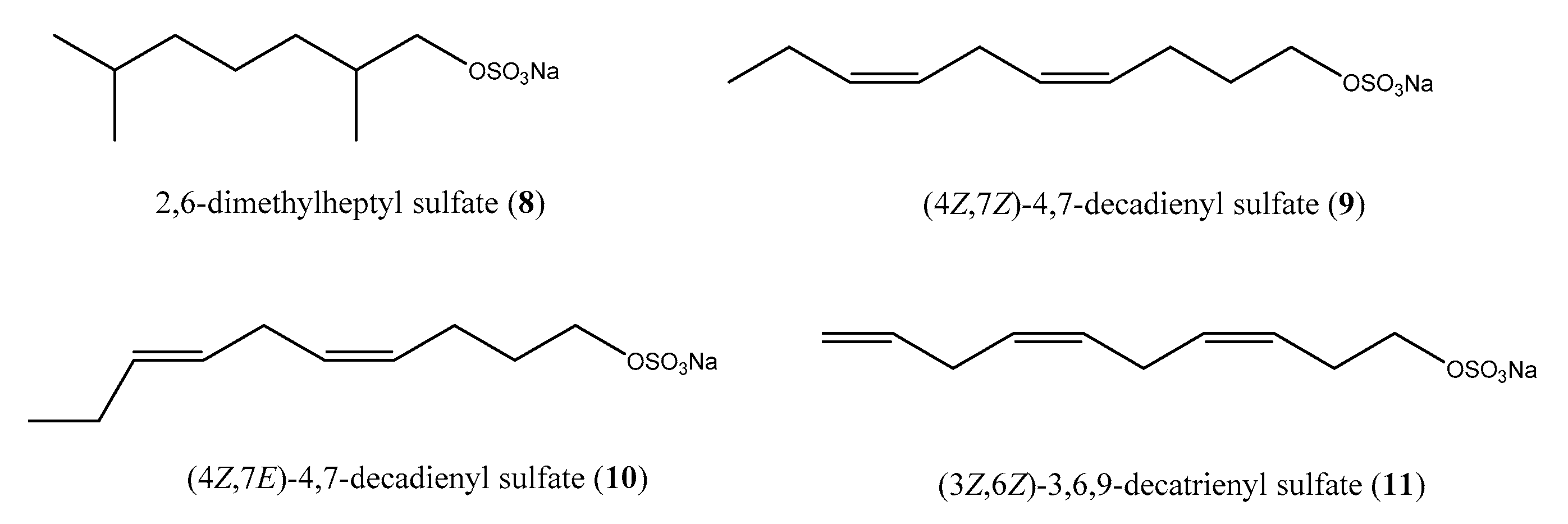
2.1. Sulfur-Containing Metabolites: Polysulfides and Alkyl Sulfates
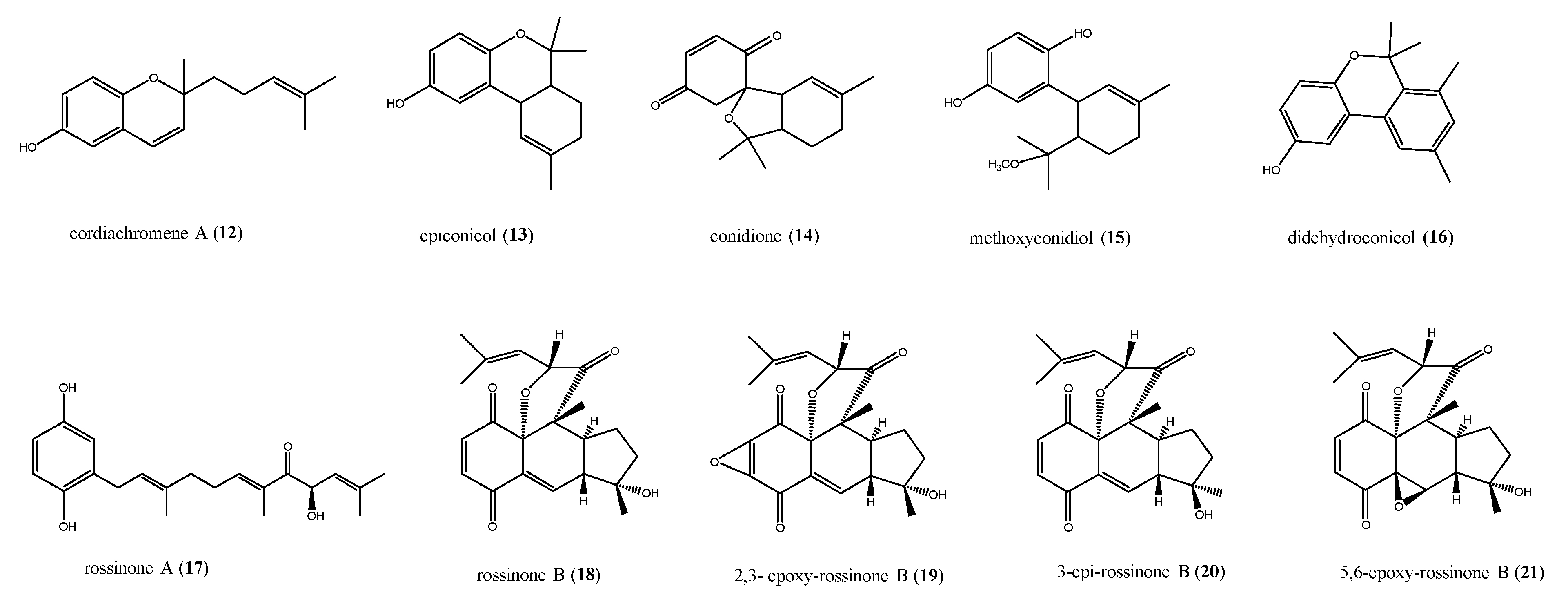
2.2. Meroterpenes
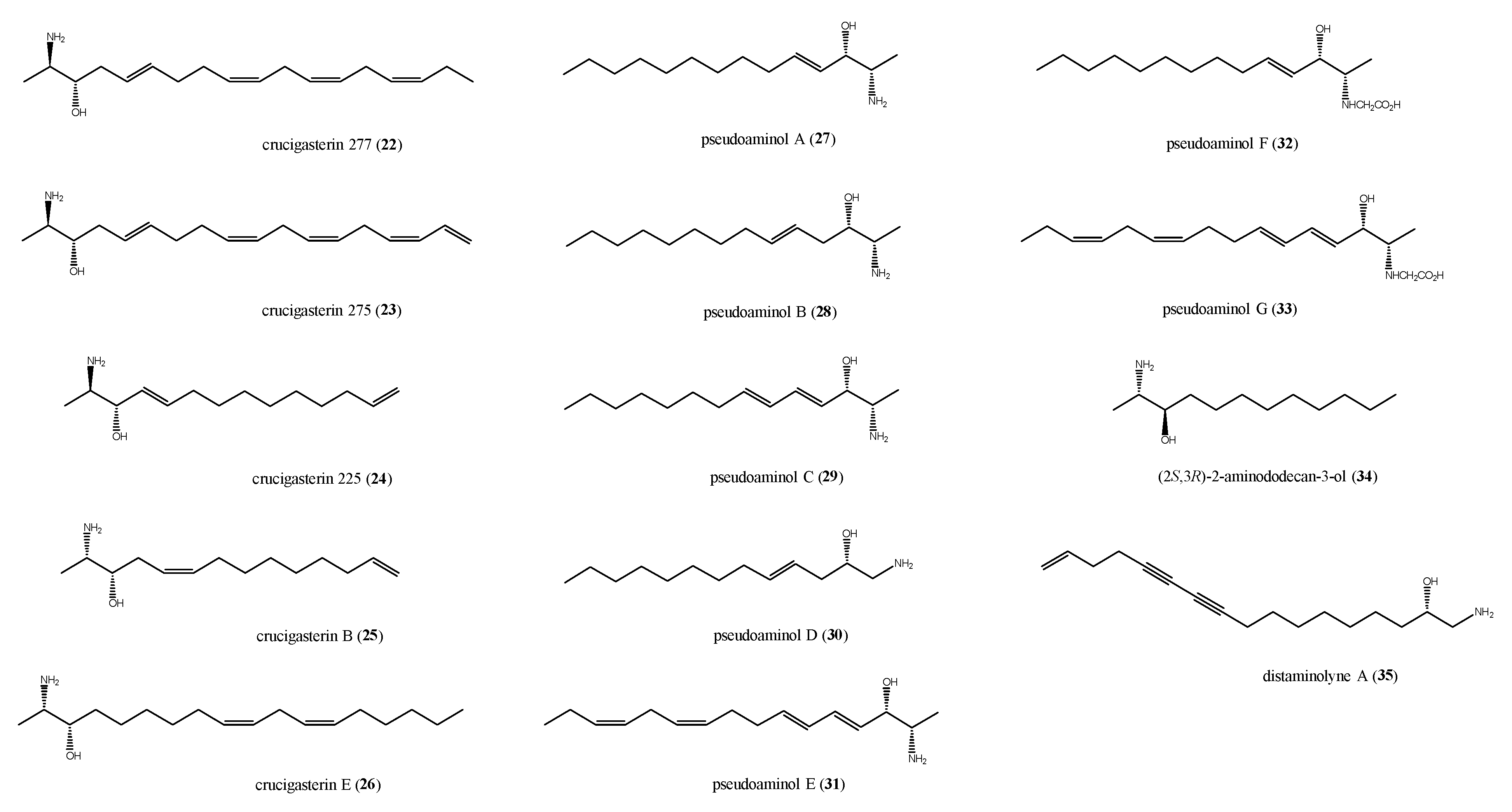
2.3. Saturated and Unsaturated Amino Alcohols
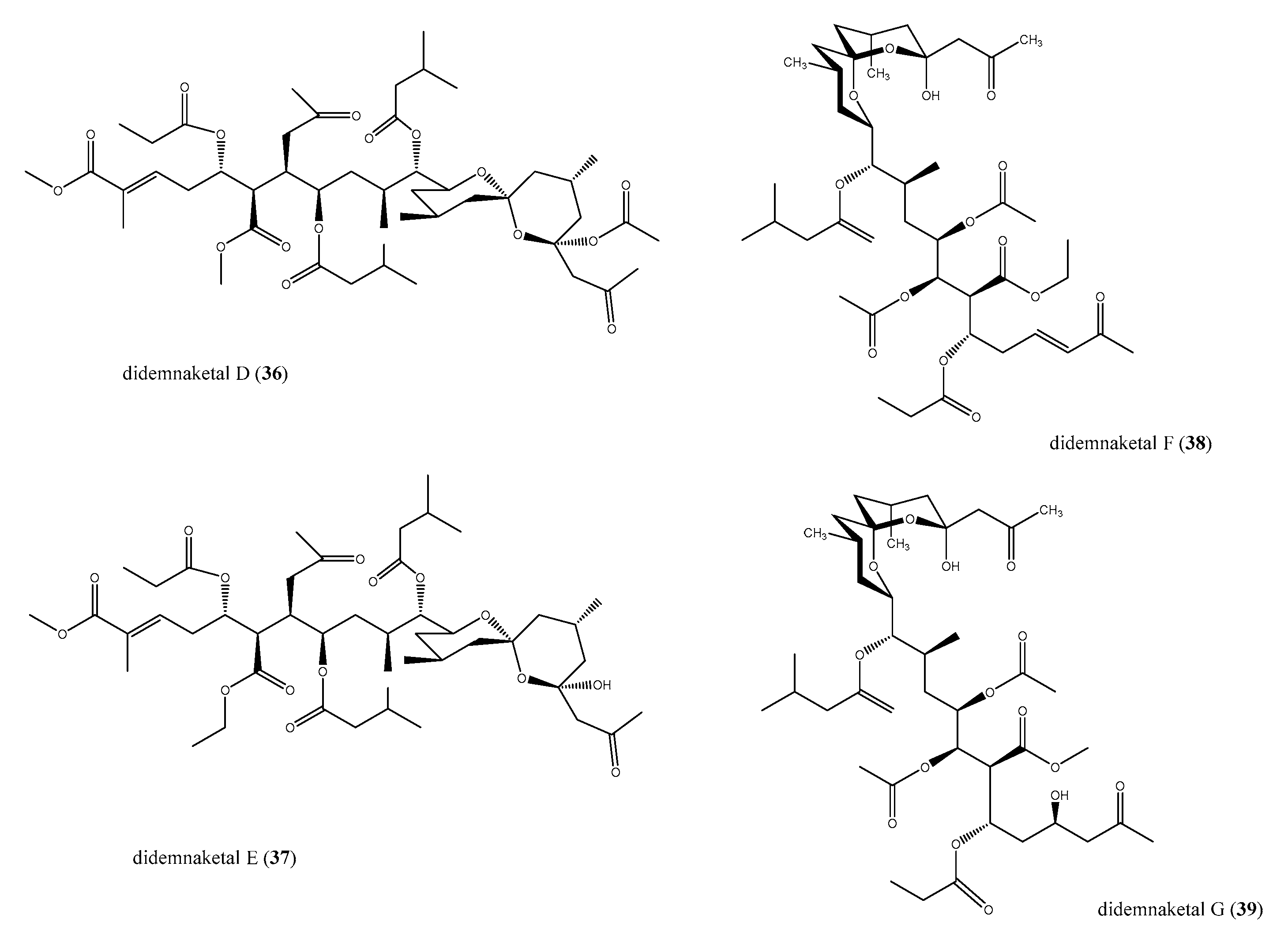
2.4. Spiroketals
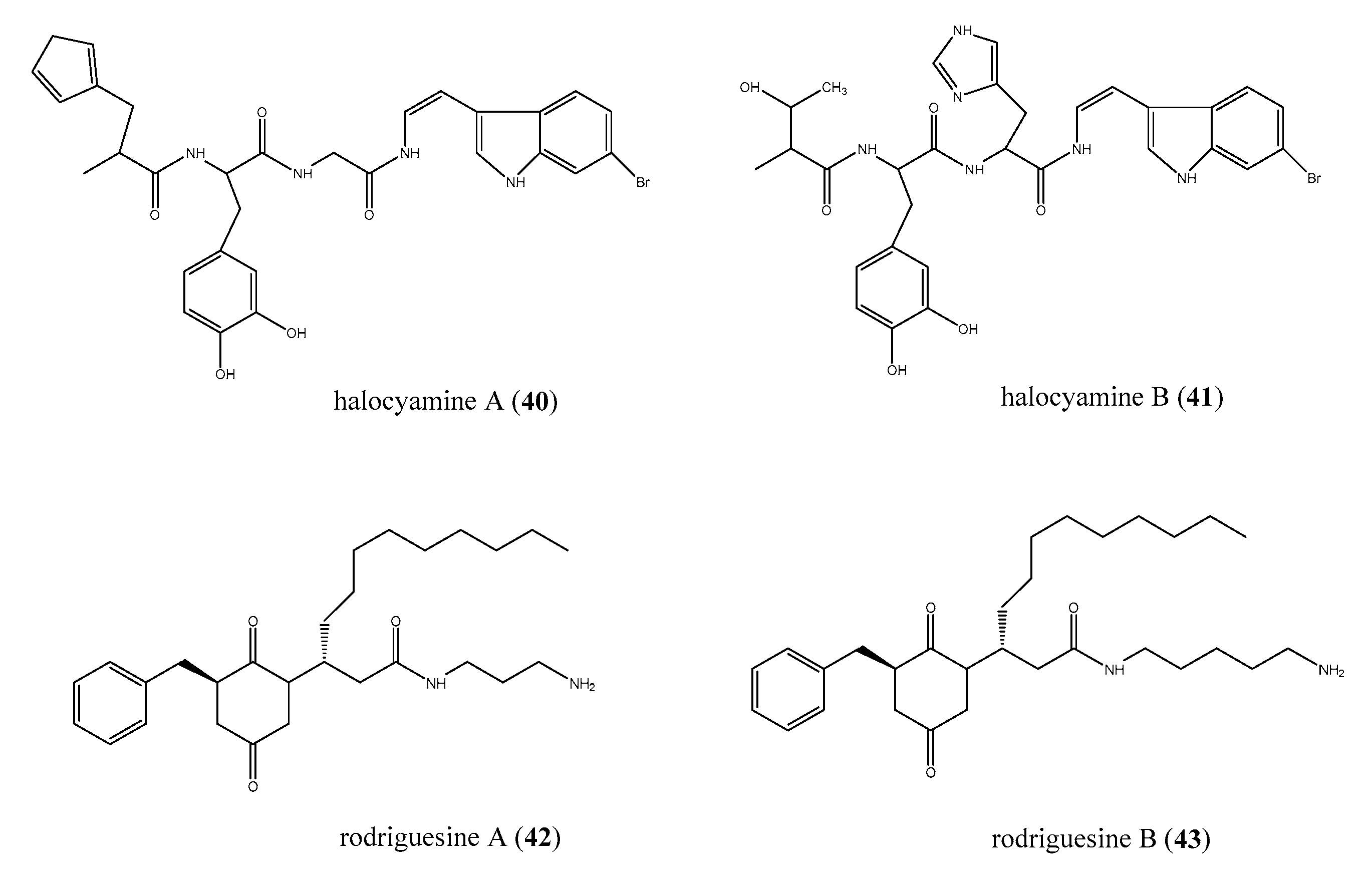
2.5. Peptides and Peptide-Like Structures
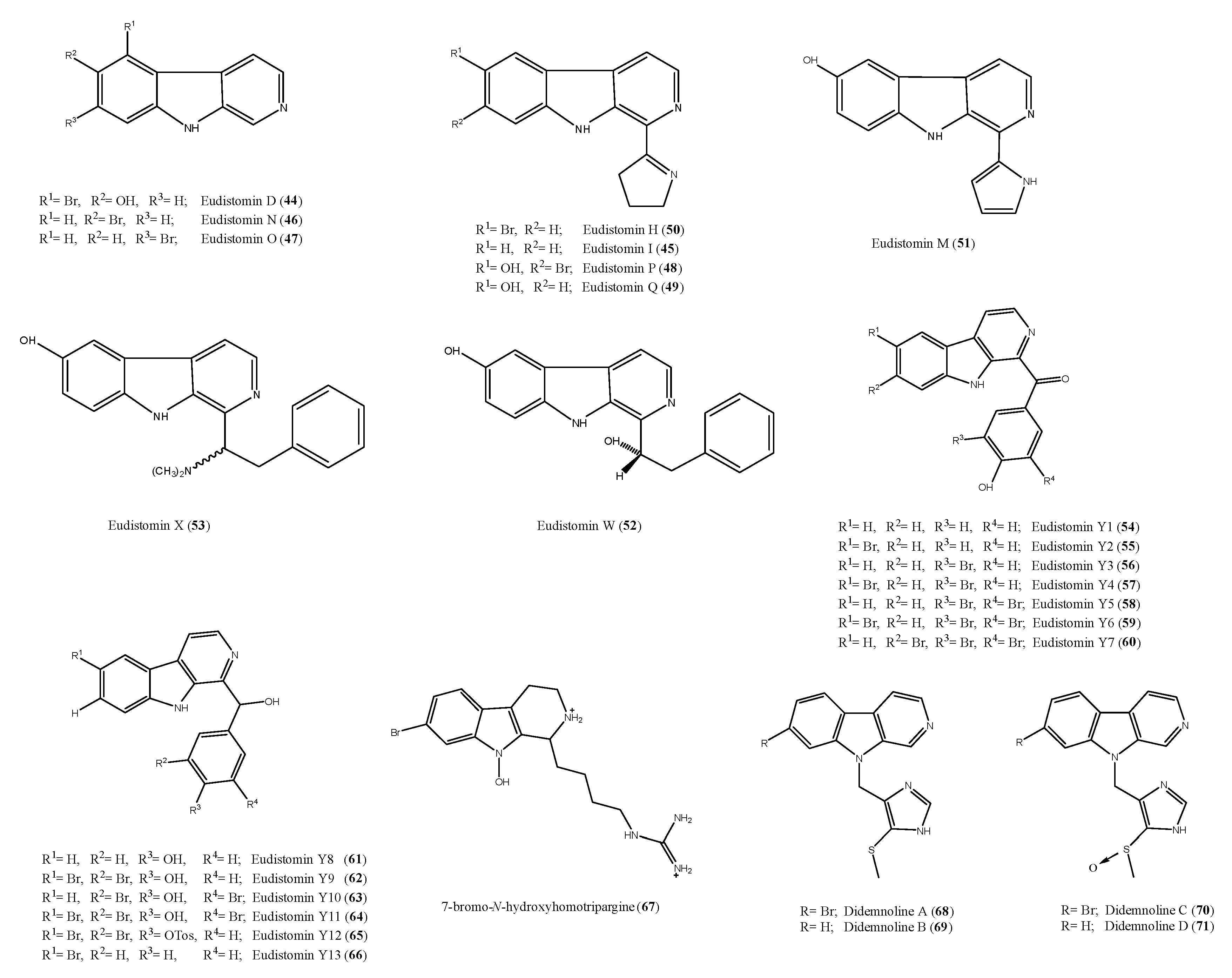
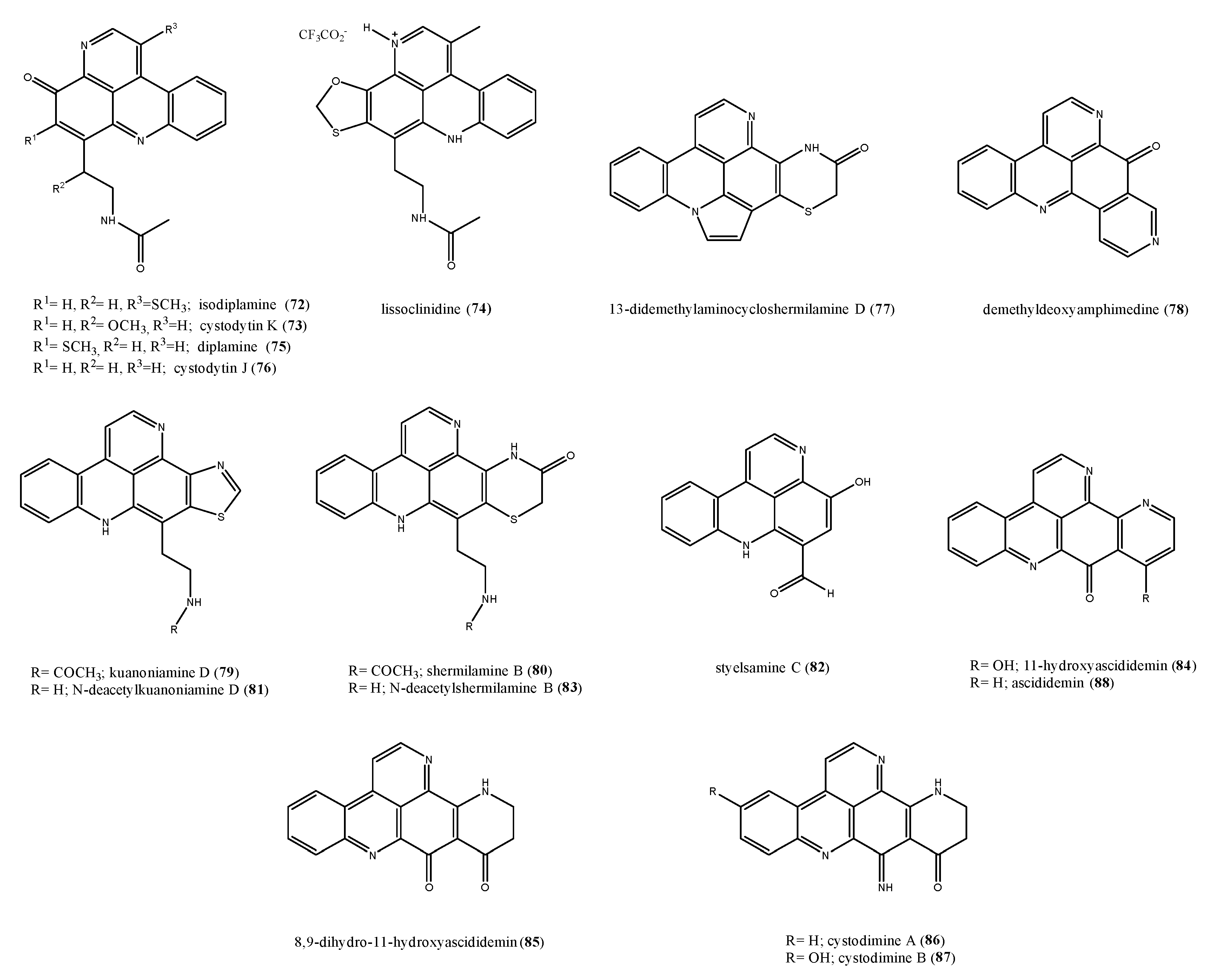
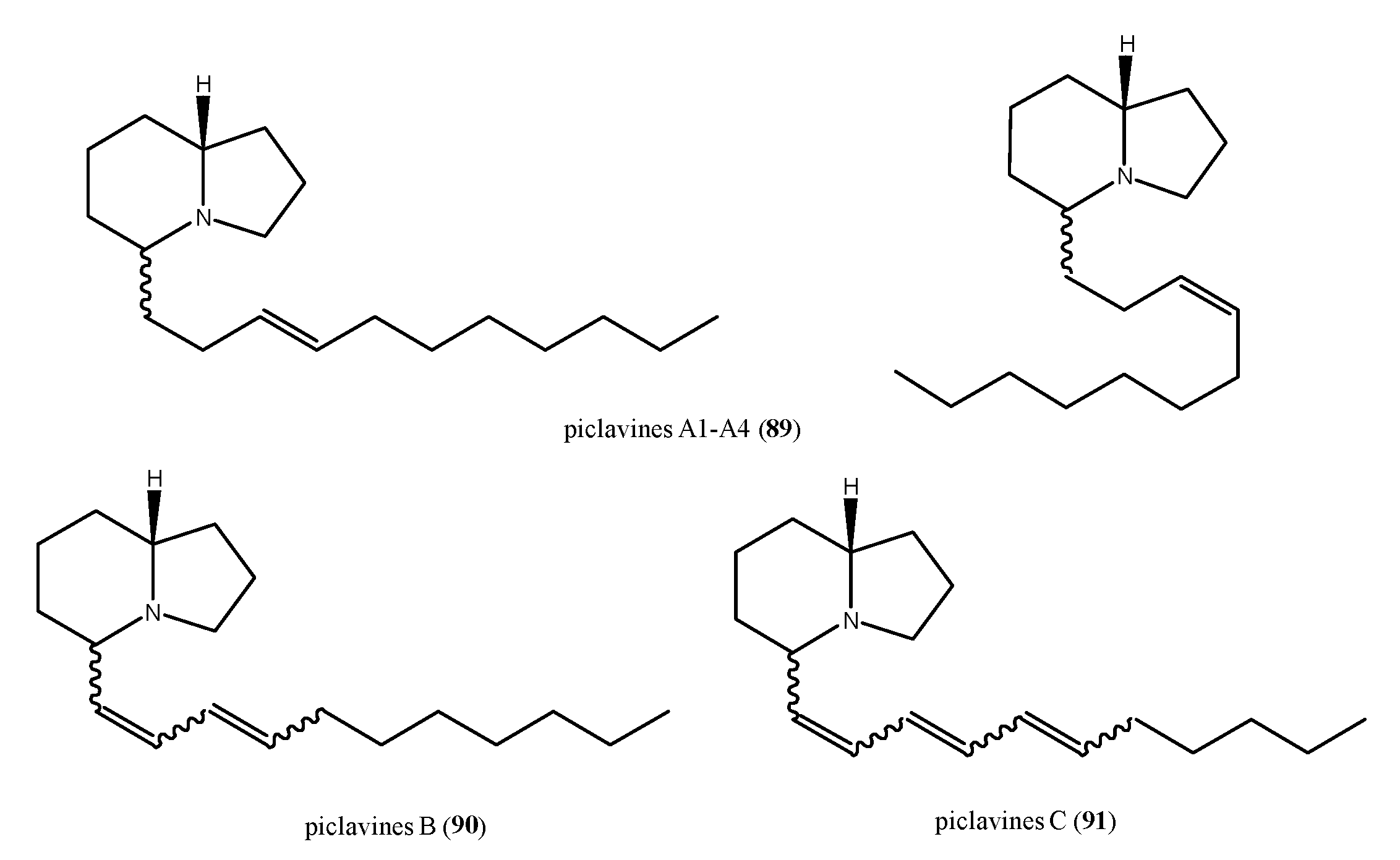
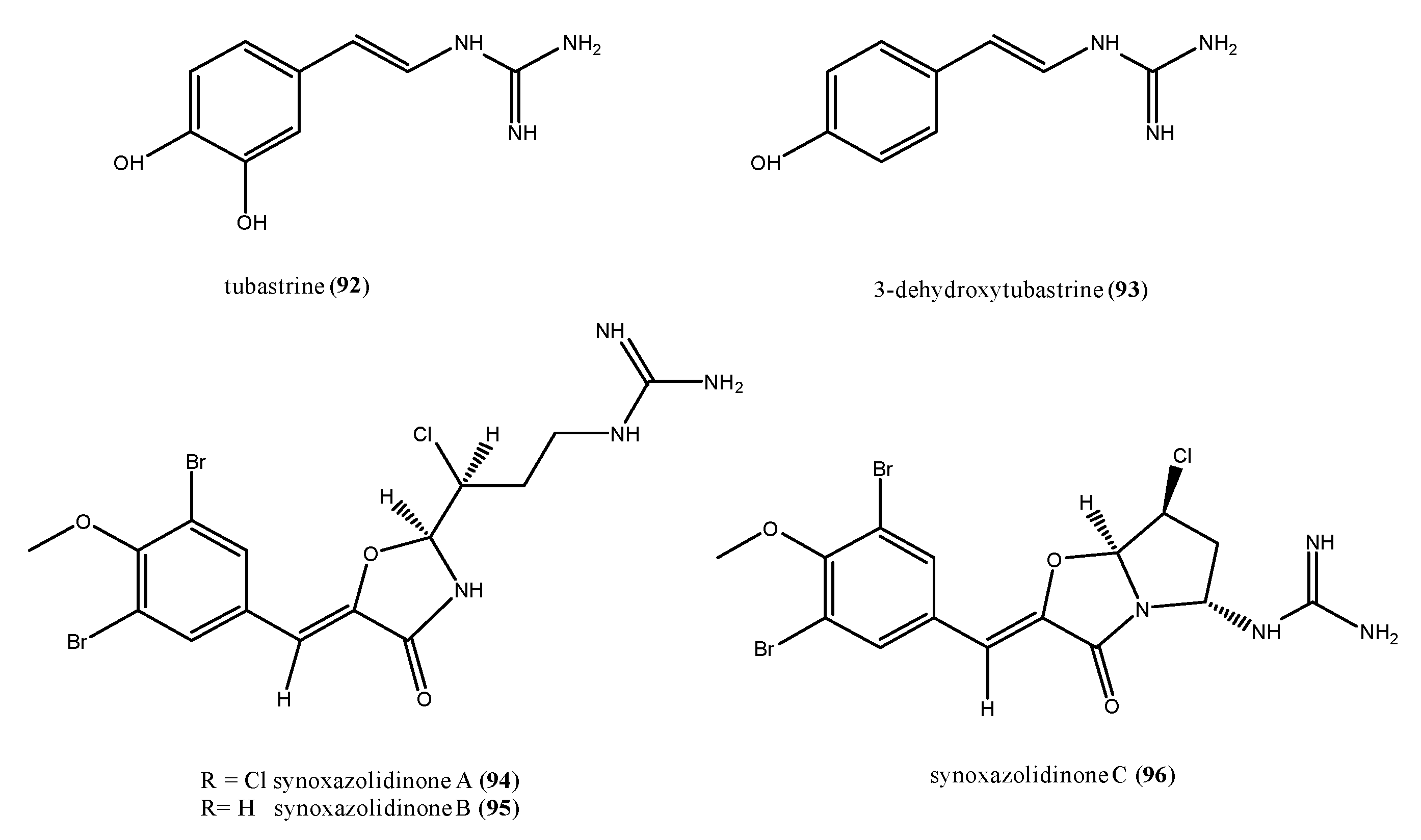
2.6. Alkaloid Structures
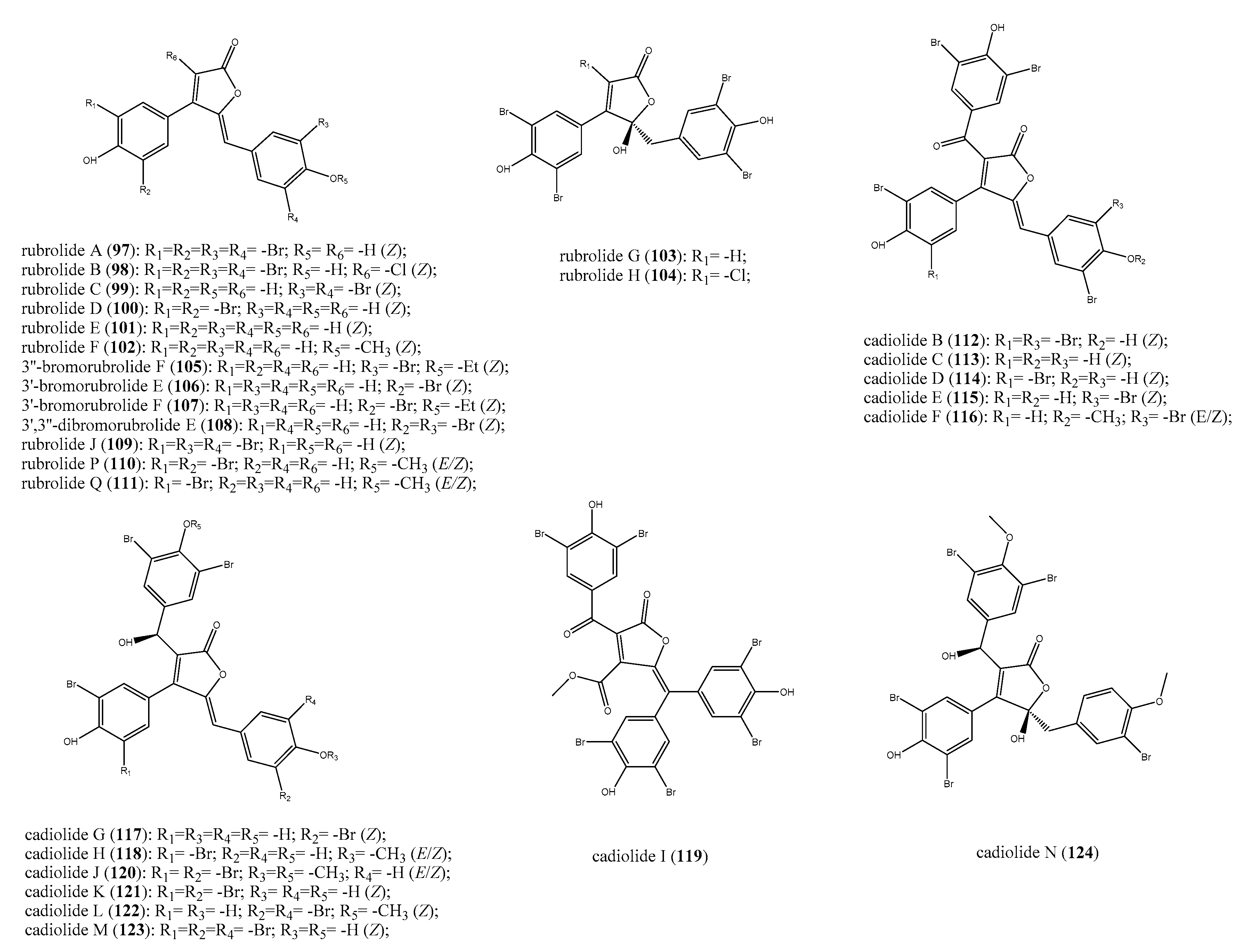
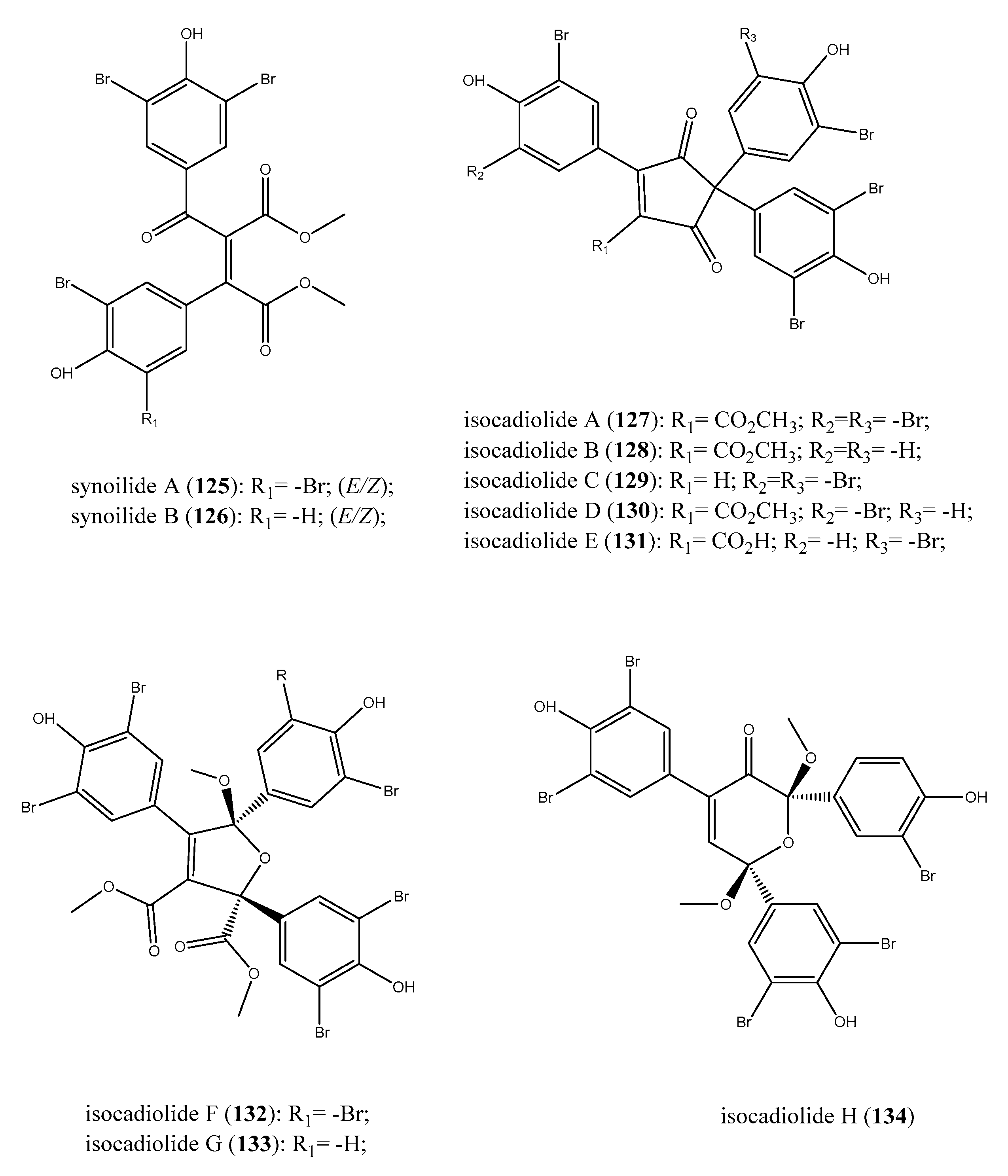
2.7. Furanones and Other Brominated Aromatic Derivatives
2.8. Exploring the Antimicrobial Properties of Metabolites from Ascidian-Associated Microorganisms
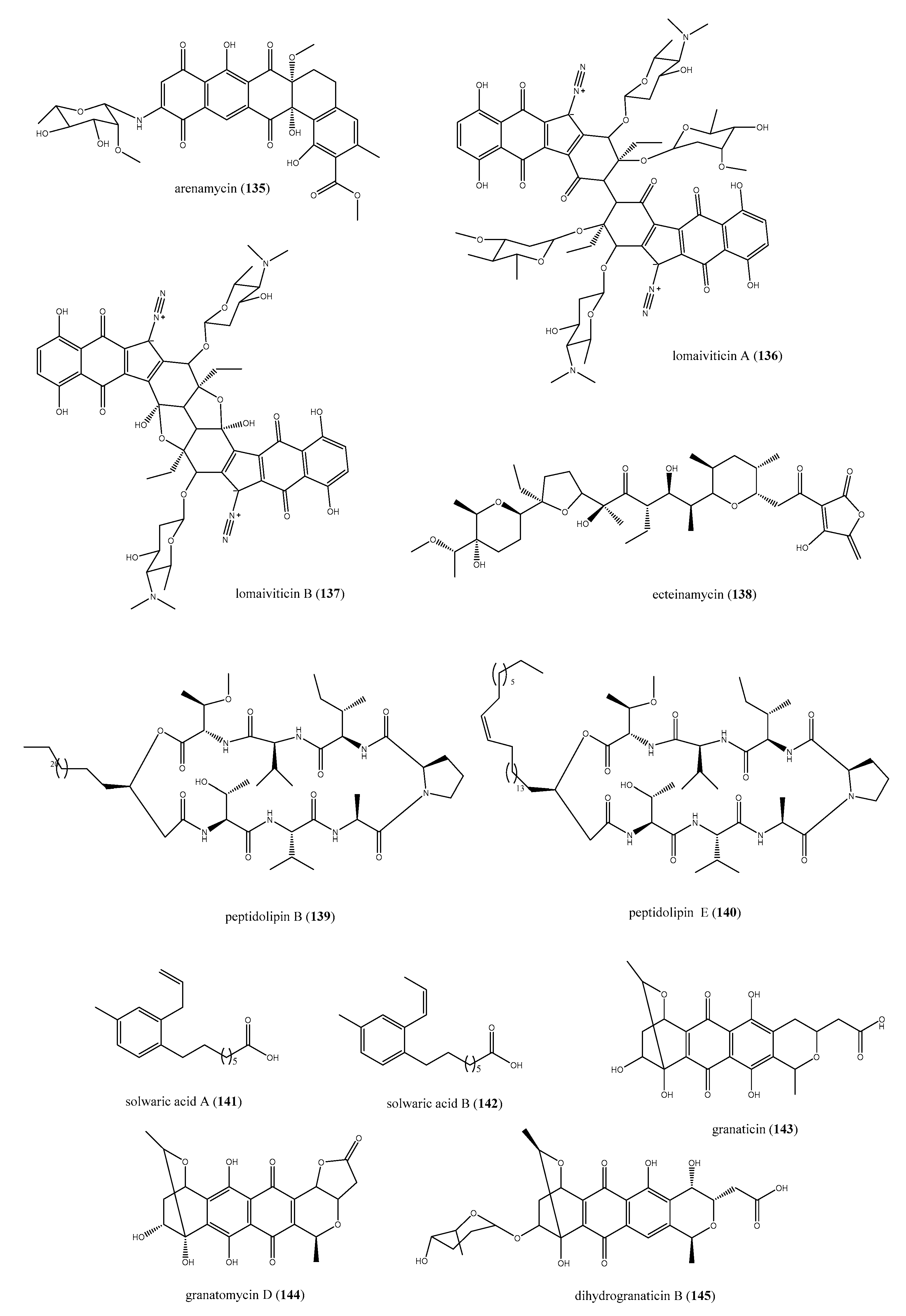
2.8.1. Ascidians-Associated Actinobacteria as Producers of Antimicrobial Compounds
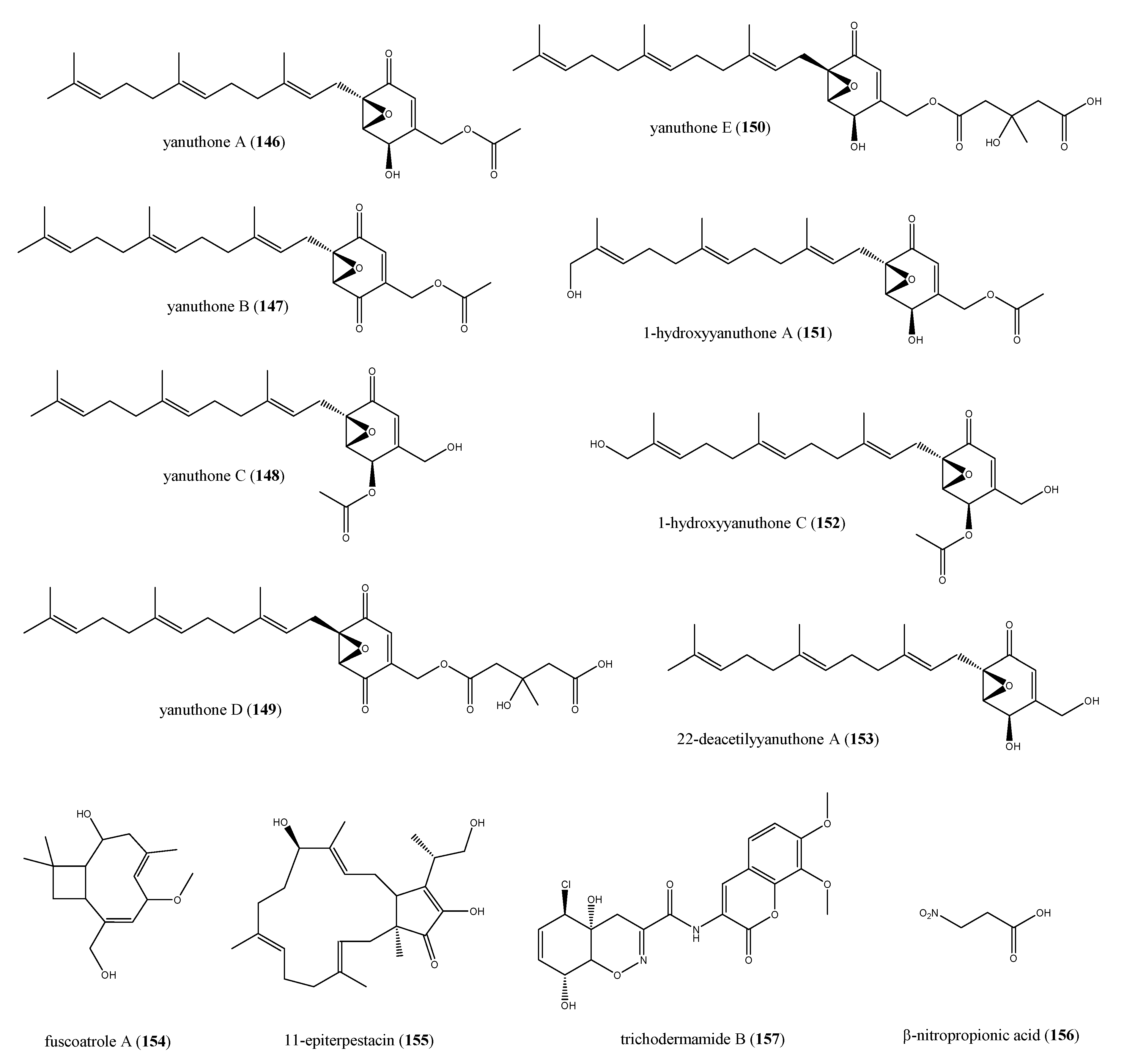
2.8.2. The Role of Fungi in the Biosynthesis of Antimicrobial Compounds
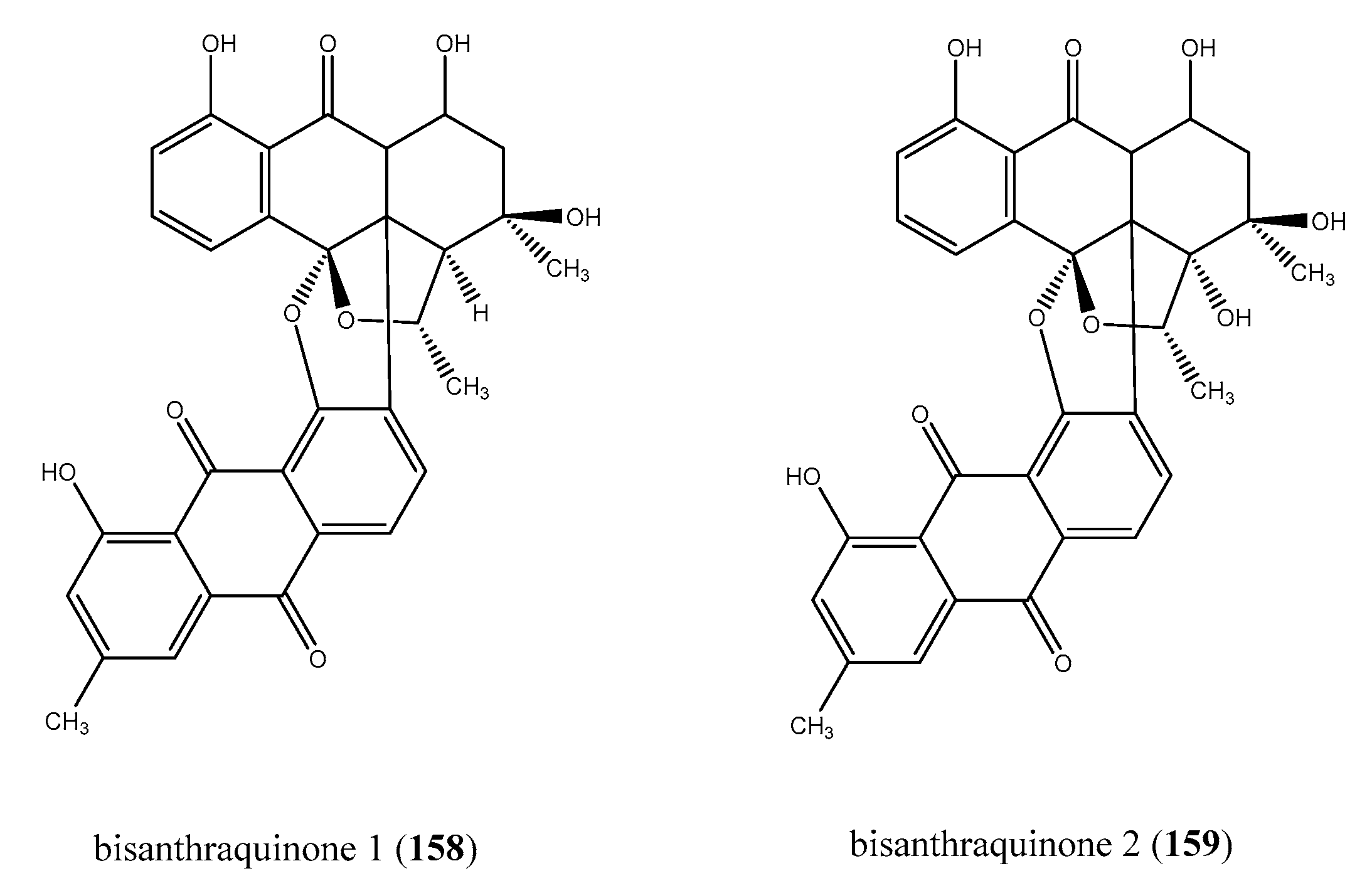
2.8.3. Bisanthraquinones from Cyanobacteria
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Furusawa, C.; Horinouchi, T.; Maeda, T. Toward Prediction and Control of Antibiotic-Resistance Evolution. Curr. Opin. Biotechnol. 2018, 54, 45–49. [Google Scholar] [CrossRef]
- WHO. No Time to Wait: Securing the Future from Drug-Resistant Infections. April 2019. Available online: https://www.who.int/antimicrobial-resistance/interagency-coordination-group/final-report/en/ (accessed on 30 April 2019).
- Silva, O.N.; de La Fuente-Núñez, C.; Haney, E.F.; Fensterseifer, I.C.; Ribeiro, S.M.; Porto, W.F.; Brown, P.; Faria-Junior, C.; Rezende, T.M.; Moreno, S.E.; et al. An Anti-Infective Synthetic Peptide with Dual Antimicrobial and Immunomodulatory Activities. Sci. Rep. 2016, 6, 35465. [Google Scholar] [CrossRef]
- Rosén, J.; Gottfries, J.; Muresan, S.; Backlund, A.; Oprea, T.I. Novel Chemical Space Exploration via Natural Products. J. Med. Chem. 2009, 52, 1953–1962. [Google Scholar]
- Millero, F.J. Descriptive Oceanography. In Chemical Oceanography, 4th ed.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2013. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Marine Natural Products and Related Compounds in Clinical and Advanced Preclinical Trials. J. Nat. Prod. 2004, 67, 1216–1238. [Google Scholar] [CrossRef]
- Panno, L.; Bruno, M.; Voyron, S.; Anastasi, A.; Gnavi, G.; Miserere, L.; Varese, G.C. Diversity, Ecological Role and Potential Biotechnological Applications of Marine Fungi Associated to the Seagrass Posidonia Oceanica. New Biotechnol. 2013, 30, 685–694. [Google Scholar] [CrossRef]
- Liang, X.; Luo, D.; Luesch, H. Advances in Exploring the Therapeutic Potential of Marine Natural Products. Pharmacol. Res. 2019, 147, 104373–104391. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, J.; Hu, G.; Yu, J.; Zhu, X.; Lin, Y.; Chen, S.; Yuan, J. Statistical Research on the Bioactivity of New Marine Natural Products Discovered during the 28 Years from 1985 to 2012. Mar. Drugs 2015, 13, 202–221. [Google Scholar] [CrossRef]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2019, 36, 122–173. [Google Scholar] [CrossRef]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2020, 37, 175–223. [Google Scholar] [CrossRef]
- Menna, M.; Aiello, A.; D’Aniello, F.; Fattorusso, E.; Imperatore, C.; Luciano, P.; Vitalone, R. Further Investigation of the Mediterranean Sponge Axinella polypoides: Isolation of a New Cyclonucleoside and a New Betaine. Mar. Drugs 2012, 10, 2509–2518. [Google Scholar] [CrossRef]
- Imperatore, C.; D’Aniello, F.; Aiello, A.; Fiorucci, S.; D’Amore, C.; Sepe, V.; Menna, M. Phallusiasterols A and B: Two New Sulfated Sterols from the Mediterranean Tunicate Phallusia fumigata and Their Effects as Modulators of the PXR Receptor. Mar. Drugs 2014, 12, 2066–2078. [Google Scholar] [CrossRef] [PubMed]
- Aiello, A.; Fattorusso, E.; Imperatore, C.; Luciano, P.; Menna, M.; Vitalone, R. Aplisulfamines, New Sulfoxide-Containing Metabolites from an Aplidium Tunicate: Absolute Stereochemistry at Chiral Sulfur and Carbon Atoms Assigned through an Original Combination of Spectroscopic and Computational Methods. Mar. Drugs 2012, 10, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Imperatore, C.; Luciano, P.; Aiello, A.; Vitalone, R.; Irace, C.; Santamaria, R.; Li, J.; Guo, Y.; Menna, M. Structure and Configuration of Phosphoeleganin, a Protein Tyrosine Phosphatase 1B Inhibitor from the Mediterranean Ascidian Sidnyum elegans. J. Nat. Prod. 2016, 79, 1144–1148. [Google Scholar] [CrossRef]
- Luciano, P.; Imperatore, C.; Senese, M.; Aiello, A.; Casertano, M.; Guo, Y.; Menna, M. Assignment of the Absolute Configuration of Phosphoeleganin via Synthesis of Model Compounds. J. Nat. Prod. 2017, 80, 2118–2123. [Google Scholar] [CrossRef]
- Lars, S.; Kjeldsen, K.U.; Funch, P.; Jensen, J.; Obst, M.; López-Legentil, S.; Schramm, A. Endozoicomonas Are Specific, Facultative Symbionts of Sea Squirts. Front. Microbiol. 2016, 7, 1042. [Google Scholar]
- Shenkar, N.; Swalla, B.J. Global Diversity of Ascidiacea. PLoS ONE 2011, 6, E20657. [Google Scholar] [CrossRef]
- Rangel, M.; Falkenberg, M. An Overview of the Marine Natural Products in Clinical Trials and on the Market. J. Coast. Life Med. 2015, 3, 421–428. [Google Scholar]
- Palanisamy, S.K.; Rajendran, N.M.; Marino, A. Natural Products Diversity of Marine Ascidians (Tunicates; Ascidiacea) and Successful Drugs in Clinical Development. Nat. Prod. Bioprospect. 2017, 7, 1–111. [Google Scholar] [CrossRef]
- Pereira, F. Have Marine Natural Product Drug Discovery Efforts Been Productive and How Can We Improve Their Efficiency? Expert Opin. Drug Dis. 2019, 14, 717–722. [Google Scholar] [CrossRef]
- Chen, L.; Hu, J.-S.; Xu, J.-L.; Shao, C.-L.; Wang, G.-Y. Biological and Chemical Diversity of Ascidian-Associated Microorganisms. Mar. Drugs 2018, 16, 362. [Google Scholar] [CrossRef]
- Schmidt, E.W. The Secret to a Successful Relationship: Lasting Chemistry between Ascidians and Their Symbiotic Bacteria. Invertebr. Biol. 2014, 134, 88–102. [Google Scholar] [CrossRef]
- Davison, E.K.; Sperry, J. Natural Products with Heteroatom-Rich Ring Systems. J. Nat. Prod. 2017, 80, 3060–3079. [Google Scholar] [CrossRef]
- Casertano, M.; Imperatore, C.; Luciano, P.; Aiello, A.; Putra, M.Y.; Gimmelli, R.; Ruberti, G.; Menna, M. Chemical Investigation of the Indonesian Tunicate Polycarpa aurata and Evaluation of the Effects Against Schistosoma mansoni of the Novel Alkaloids Polyaurines A and B. Mar. Drugs 2019, 17, 278. [Google Scholar] [CrossRef]
- Zabriskie, T.M.; Mayne, C.L.; Ireland, C.M. Patellazole C: A Novel Cytotoxic Macrolide from Lissoclinum patella. J. Am. Chem. Soc. 1988, 110, 7919–7920. [Google Scholar] [CrossRef]
- Davidson, B.S.; Ireland, C.M. Lissoclinolide, the First Non-Nitrogenous Metabolite from a Lissoclinum Tunicate. J. Nat. Prod. 1990, 53, 1036–1038. [Google Scholar] [CrossRef]
- Litaudon, M.; Guyot, M. Lissoclinotoxin A, an Antibiotic 1,2,3-Trithiane Derivative from the Tunicate Lissoclinum perforatum. Tetrahedron Lett. 1991, 32, 911–914. [Google Scholar] [CrossRef]
- Litaudon, M.; Trigalo, F.; Martin, M.T.; Frappier, F.; Guyot, M. Lissoclinotoxins: Antibiotic Polysulfur Derivatives From The Tunicate Lissodinum perforatum. Revised Structure of Lissoclinotoxin A. Tetrahedron 1994, 50, 5323–5334. [Google Scholar] [CrossRef]
- Davidson, B.S.; Molinski, T.F.; Barrows, L.R.; Ireland, C.M. Varacin: A Novel Benzopentathiepin from Lissoclinum vareau That Is Cytotoxic toward a Human Colon Tumor. J. Am. Chem. Soc. 1991, 113, 4709. [Google Scholar] [CrossRef]
- Makarieva, T.N.; Stonik, V.A.; Dmitrenok, A.S.; Grebnev, B.B.; Isakov, V.V.; Rebachyk, N.M. Varacin and Three Marine Antimicrobial Polysulfides from the Far-Eastern Ascidian Polycitor Sp. J. Nat. Prod. 1995, 58, 254–258. [Google Scholar] [CrossRef]
- McDonald, L.A.; Capson, T.L.; Krishnamurthy, G.; Ding, W.D.; Ellestad, G.A.; Bernan, V.S.; Maiese, W.M.; Lassota, P.; Discafani, C.; Kramer, R.A.; et al. Namenamicin, a New Enediyne Antitumor Antibiotic from the Marine Ascidian Polysyncraton lithostrotum. J. Am. Chem. Soc. 1996, 118, 10898–10899. [Google Scholar] [CrossRef]
- Aiello, A.; Fattorusso, E.; Menna, M.; Carnuccio, R.; Iuvone, T. A New Antiproliferative Sulfated Alkene from the Mediterranean Tunicate Microcosmus vulgaris. Tetrahedron 1997, 53, 11489–11492. [Google Scholar] [CrossRef]
- Aiello, A.; Carbonelli, S.; Fattorusso, E.; Iuvone, T.; Menna, M. New Bioactive Sulfated Metabolites from the Mediterranean Tunicate Sydnium turbinatum. J. Nat. Prod. 2001, 64, 219–221. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Kato, H.; Hirota, H.; Fusetani, N. Antibacterial and Antifungal Sulfated Alkane and Alkanes from the Hepatopancreas of the Ascidian Halocynthia Roretzi. J. Nat. Prod. 1994, 57, 1606–1609. [Google Scholar] [CrossRef]
- Menna, M.; Imperatore, C.; D’Aniello, F.; Aiello, A. Meroterpenes from Marine Invertebrates: Structures, Occurrence, and Ecological Implications. Mar. Drugs 2013, 11, 1602–1643. [Google Scholar] [CrossRef]
- Simon-Levert, A.; Menniti, C.; Soulère, L.; Genevière, A.; Barthomeuf, C.; Banaigs, B.; Witczak, A. Marine Natural Meroterpenes: Synthesis and Antiproliferative Activity. Mar. Drugs 2010, 8, 347–358. [Google Scholar] [CrossRef]
- Zubia, E.; Ortega, M.J.; Salva, J. Natural Products Chemistry in Marine Ascidians of the Genus Aplidium. Mini-Rev. Org. Chem. 2005, 2, 389–399. [Google Scholar]
- Imperatore, C.; Persico, M.; Aiello, A.; Luciano, P.; Guiso, M.; Sanasi, M.F.; Taramelli, D.; Parapini, S.; Cebrián-Torrejón, G.; Doménech-Carbó, A.; et al. Marine Inspired Antiplasmodial Thiazinoquinones: Synthesis, Computational Studies and Electrochemical Assays. RSC Adv. 2015, 5, 70689–70702. [Google Scholar] [CrossRef]
- Imperatore, C.; Gimmelli, R.; Persico, M.; Casertano, M.; Guidi, A.; Saccoccia, F.; Ruberti, G.; Luciano, P.; Aiello, A.; Parapini, S.; et al. Investigating the Antiparasitic Potential of the Marine Sesquiterpene Avarone, Its Reduced Form Avarol, and the Novel Semisynthetic Thiazinoquinone Analogue Thiazoavarone. Mar. Drugs 2020, 18, 112. [Google Scholar] [CrossRef]
- Imperatore, C.; Della Sala, G.; Casertano, M.; Luciano, P.; Aiello, A.; Laurenzana, I.; Piccoli, P.; Menna, M. In Vitro Antiproliferative Evaluation of Synthetic Meroterpenes Inspired by Marine Natural Products. Mar. Drugs 2019, 17, 684. [Google Scholar] [CrossRef]
- Fedorov, S.N.; Radchenko, O.S.; Shubina, L.K.; Balaneva, N.N.; Bode, A.M.; Stonik, V.A.; Dong, Z. Evaluation of Cancer-Preventive Activity and Structure-Activity Relationships of 3-Demethylubiquinone Q2, Isolated from the Ascidian Aplidium Glabrum, and Its Synthetic Analogs. Pharm. Res. 2006, 23, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Caroll, A.R.; Bowden, B.F.; Coll, J.C. Studies of Australian Ascidians. III. A New Tetrahydrocannabinol Derivative from the Ascidian Synoicum Castellatum. Aust. J. Chem. 1993, 46, 1079–1083. [Google Scholar] [CrossRef]
- Garrido, L.; Zubia, E.; Ortega, M.J.; Salva, J. New Meroterpenoids from the Ascidian Aplidium Conicum. J. Nat. Prod. 2002, 65, 1328–1331. [Google Scholar] [CrossRef] [PubMed]
- Simon-Levert, A.; Arrault, A.; Bontemps-Subielos, N.; Canal, C.; Banaigs, B. Meroterpenes from the Ascidian Aplidium Densum. J. Nat. Prod. 2005, 68, 1412–1415. [Google Scholar] [CrossRef]
- Benslimane, A.F.; Pouchus, Y.F.; Le Boterff, J.; Verbist, J.F.; Roussakis, C.; Monniot, F. Cytotoxic and Antibacterial Substances from the Ascidian Aplidium Antillense. J. Nat. Prod. 1988, 51, 582–583. [Google Scholar] [CrossRef]
- Appleton, D.R.; Chuen, C.S.; Berridge, M.V.; Webb, V.L.; Copp, B.R. Rossinones A and B, Biologically Active Meroterpenoids from the Antarctic Ascidian, Aplidium Species. J. Org. Chem. 2009, 74, 9195–9198. [Google Scholar] [CrossRef]
- Carbone, M.; Núñez-Pons, L.; Castelluccio, F.; Avila, C.; Gavagnin, M. New Meroterpenoids from the Antarctic Ascidian Aplidium Fuegiense. Tetrahedron 2012, 68, 3541–3544. [Google Scholar] [CrossRef]
- Núñez-Pons, L.; Carbone, M.; Vázquez, J.; Rodríguez, J.; Nieto, R.M.; Varela, M.M.; Gavagnin, M.; Avila, C. Natural Products from Antarctic Colonial Ascidians of the Genera Aplidium and Synoicum: Variability and Defensive Role. Mar. Drugs 2012, 10, 1741–1764. [Google Scholar] [CrossRef]
- Ciavatta, M.L.; Manzo, E.; Nuzzo, G.; Villani, G.; Varcamonti, M.; Gavagnin, M. Crucigasterins A-E, Antimicrobial Amino Alcohols from the Mediterranean Colonial Ascidian Pseudodistoma crucigaster. Tetrahedron 2010, 66, 7533–7538. [Google Scholar] [CrossRef]
- Jares-Erijman, E.A.; Bapat, C.P.; Lithgow-Bertelloni, A.; Rinehart, K.L.; Sakai, R. Crucigasterins, New Polyunsaturated Amino Alcohols from the Mediterranean Tunicate Pseudodistoma Crucigaster. J. Org. Chem. 1993, 58, 5732–5737. [Google Scholar] [CrossRef]
- Gulavita, N.K.; Scheuer, P.J. Two Epimeric Aliphatic Amino Alcohols from a Sponge, Xestospongia Sp. J. Org. Chem. 1989, 54, 366–369. [Google Scholar] [CrossRef]
- Kobayashi, J.; Naitoh, K.; Doi, Y.; Ishibashi, M. Pseudodistomin C, a New Piperidine Alkaloid with Unusual Absolute Configuration from the Okinawan Tunicate Pseudodistoma Kanoko. J. Org. Chem. 1995, 60, 6941–6945. [Google Scholar] [CrossRef]
- Won, T.H.; You, M.; Lee, S.H.; Rho, B.J.; Oh, D.C.; Oh, K.B.; Shin, J. Amino Alcohols from the Ascidian Pseudodistoma Sp. Mar. Drugs 2014, 12, 3754–3769. [Google Scholar] [CrossRef] [PubMed]
- Kossuga, M.H.; MacMillan, J.B.; Rogers, E.W.; Molinski, T.F.; Nascimento, G.G.F.; Rocha, R.M.; Berlinck, R.G.S. (2S,3R)-2-Aminododecan-3-Ol, a New Antifungal Agent from the Ascidian Clavelina Oblonga. J. Nat. Prod. 2004, 67, 1879–1881. [Google Scholar] [CrossRef]
- Wang, J.; Pearce, A.N.; Chan, S.T.S.; Taylor, R.B.; Page, M.J.; Valentin, A.; Bourguet-Kondracki, M.L.; Dalton, J.P.; Wiles, S.; Copp, B.R. Biologically Active Acetylenic Amino Alcohol and N-Hydroxylated 1,2,3,4-Tetrahydro-β-Carboline Constituents of the New Zealand Ascidian Pseudodistoma Opacum. J. Nat. Prod. 2016, 79, 607–610. [Google Scholar]
- Perron, F.; Albizati, K.F. Chemistry of Spiroketals. Chem. Rev. 1989, 89, 1617–1661. [Google Scholar] [CrossRef]
- Aho, J.E.; Pihko, P.M.; Rissa, T.K. Nonanomeric Spiroketals in Natural Products: Structures, Sources, and Synthetic Strategies. Chem. Rev. 2005, 105, 4406–4440. [Google Scholar] [CrossRef]
- Sperry, J.; Wilson, Z.E.; Rathwell, D.C.K.; Brimble, M.A. Isolation, Biological Activity and Synthesis of Benzannulated Spiroketalnatural Products. Nat. Prod. Rep. 2010, 27, 1117–1137. [Google Scholar] [CrossRef]
- Zang, F.M.; Zhang, S.Y.; Tu, Y.Q. Recent Progress in the Isolation, Bioactivity, Biosynthesis, and Total Synthesis of Natural Spiroketals. Nat. Prod. Rep. 2018, 35, 75–104. [Google Scholar] [CrossRef]
- Pika, J.; Faulkner, D.J. A Reinvestigation of the Didemnaketals from the Palauan Ascidian Didemnum Sp. Nat. Prod. Lett. 1995, 7, 291–296. [Google Scholar] [CrossRef]
- Potts, B.C.M.; Faulkner, D.J. Didemnaketals A and B, HIV-1 Protease Inhibitors from the Ascidian Didemnum Sp. J. Am. Chem. Soc. 1991, 113, 6321–6322. [Google Scholar] [CrossRef]
- Mohamed, G.A.; Ibrahim, S.R.M.; Badr, J.M.; Youssef, D.T.A. Didemnaketals D and E, Bioactive Terpenoids from a Red Sea Ascidian Didemnum Species. Tetrahedron 2014, 70, 35–40. [Google Scholar] [CrossRef]
- Shaala, L.A.; Youssef, D.T.A.; Ibrahim, S.R.M.; Mohamed, G.A.; Badr, J.M.; Risinger, A.L.; Mooberry, S.L. Didemnaketals F and G, New Bioactive Spiroketals from a Red Sea Ascidian Didemnum Species. Mar. Drugs 2014, 12, 5021–5034. [Google Scholar] [CrossRef] [PubMed]
- Cheung, R.C.; Ng, T.B.; Wong, J.H. Marine Peptides: Bioactivities and Applications. Mar. Drugs 2015, 13, 4006–4043. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.K.; Seo, C.H.; Park, Y. Marine Peptides and Their Anti-Infective Activities. Mar. Drugs 2015, 13, 618–654. [Google Scholar] [CrossRef]
- Falanga, A.; Lombardi, L.; Franci, G.; Vitiello, M.; Iovene, M.R.; Morelli, G.; Galdiero, M.; Galdiero, S. Marine Antimicrobial Peptides: Nature Provides Templates for the Design of Novel Compounds against Pathogenic Bacteria. Int. J. Mol. Sci. 2016, 17, 785. [Google Scholar] [CrossRef]
- Cruz, J.; Ortiz, C.; Guzman, F.; Fernandez-Lafuente, R.; Torres, R. Antimicrobial Peptides: Promising Compounds against Pathogenic Microorganisms. Curr. Med. Chem. 2014, 21, 2299–2321. [Google Scholar] [CrossRef]
- Hurdle, J.G.; O’Neill, A.J.; Chopra, I.; Lee, R.E. Targeting Bacterial Membrane Function: An Underexploited Mechanism for Treating Persistent Infections. Nat. Rev. Microbiol. 2011, 9, 62–75. [Google Scholar] [CrossRef]
- Lehrer, R.I.; Tincu, J.A.; Taylor, S.W.; Menzel, L.P.; Waring, A.J. Natural Peptide Antibiotics from Tunicates: Structures, Functions and Potential Uses. Integr. Comp. Biol. 2003, 43, 313–322. [Google Scholar] [CrossRef]
- Lee, I.H.; Cho, Y.; Lehrer, R.I. Styelins, Broad-Spectrum Antimicrobial Peptides from the Solitary Tunicate, Styela Clava. Comp. Biochem. Physiol. 1997, 118B, 515–521. [Google Scholar] [CrossRef]
- Taylor, S.W.; Craig, A.G.; Fischer, W.H.; Park, M.; Lehrer, R.I. Styelin D, an Extensively Modified Antimicrobial Peptide from Ascidian Hemocytes. J. Biol. Chem. 2000, 275, 38417–38426. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.H.; Zhao, C.; Cho, Y.; Harwig, S.S.L.; Cooper, E.L.; Lehrer, R.I. Clavanins, α-Helical Antimicrobial Peptides from Tunicate Hemocytes. FEBS Lett. 1997, 400, 158–162. [Google Scholar] [CrossRef]
- Saude, A.C.; Ombredane, A.S.; Silva, O.N.; Barbosa, J.A.; Moreno, S.E.; Guerra Araujo, A.C.; Falcão, R.; Silva, L.P.; Dias, S.C.; Franco, O.L. Clavanin Bacterial Sepsis Control Using a Novel Methacrylate Nanocarrier. Int. J. Nanomed. 2014, 9, 5055–5069. [Google Scholar]
- Galinier, R.; Roger, E.; Sautiere, P.E.; Aumelas, A.; Banaigs, B.; Mitta, G. Halocyntin and Papillosin, Two New Antimicrobial Peptides Isolated from Hemocytes of the Solitary Tunicate, Halocynthia Papillosa. J. Pept. Sci. 2009, 15, 48–55. [Google Scholar] [CrossRef]
- Lee, I.H.; Lee, Y.S.; Kim, C.H.; Kim, C.R.; Hong, T.; Menzel, L.; Boo, L.M.; Pohl, J.; Sherman, M.A.; Waring, A.; et al. Dicynthaurin: An Antimicrobial Peptide from Hemocytes of the Solitary Tunicate, Halocynthia Aurantium. Biochim. Biophys. Acta 2001, 1527, 141–148. [Google Scholar] [CrossRef]
- Jang, W.S.; Kim, K.N.; Lee, Y.S.; Nam, M.H.; Lee, I.H. Halocidin: A New Antimicrobial Peptide from Hemocytes of the Solitary Tunicate, Halocynthia Aurantium. FEBS Lett. 2002, 521, 81–86. [Google Scholar] [CrossRef]
- Jang, W.S.; Kim, H.K.; Lee, K.Y.; Kim, S.A.; Han, Y.S.; Lee, I.H. Antifungal Activity of Synthetic Peptide Derived from Halocidin, Antimicrobial Peptide from the Tunicate, Halocynthia Aurantium. FEBS Lett. 2006, 580, 1490–1496. [Google Scholar] [CrossRef]
- Jang, W.S.; Kim, C.H.; Kang, M.S.; Chae, H.J.; Son, S.M.; Seo, S.J.; Lee, I.H. CDNA Cloning of Halocidin and a New Antimicrobial Peptide Derived from the N-Terminus of Ci-META4. Peptides 2005, 26, 2360–2367. [Google Scholar] [CrossRef]
- Hansen, I.K.Ø.; Isaksson, J.; Poth, A.G.; Hansen, K.Ø.; Andersen, A.J.C.; Richard, C.S.M.; Blencke, H.-M.; Stensvåg, K.; Craik, D.J.; Haug, T. Isolation and Characterization of Antimicrobial Peptides with Unusual Disulfide Connectivity from the Colonial Ascidian Synoicum Turgens. Mar. Drugs 2020, 18, 51. [Google Scholar] [CrossRef]
- Oltz, E.M.; Bruening, R.C.; Smith, M.J.; Kustin, K.; Naganishi, K. The Tunichromes. A Class of Reducing Blood Pigments from Sea Squirts: Isolation, Structures, and Vanadium Chemistry. J. Am. Chem. Soc. 1988, 110, 6162–6172. [Google Scholar] [CrossRef]
- Azumi, K.; Yokosawa, H.; Ishii, S. Halocyamines: Novel Antimicrobial Tetrapeptide-like Substances Isolated from the Hemocytes of the Solitary Ascidian Halocynthia Roretzi. Biochemistry 1990, 29, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Azumi, K.; Yokosawa, H.; Ishii, S. Presence of 3,4-Dihydroxyphenylalanine-Containing Peptides in Hemocytes of the Ascidian, Halocynthia Roretzi. Experientia 1990, 46, 1020–1023. [Google Scholar] [CrossRef]
- Azumi, K.; Yoshimizu, M.; Suzuki, S.; Ezura, Y.; Yokosawa, H. Inhibitory Effect of Halocyamine, an Antimicrobial Substance from Ascidian Hemocytes, on the Growth of Fish Viruses and Marine Bacteria. Experientia 1990, 46, 1066–1068. [Google Scholar] [CrossRef] [PubMed]
- Kossuga, M.H.; Lira, P.A.; McHugh, S.; Torres, Y.R.; Lima, B.A.; Gonçalves, R.; Veloso, K.; Ferreira, A.G.; Rocha, R.M.; Berlinck, R.G.S. Antibacterial Modified Diketopiperazines from Two Ascidians of the Genus Didemnum. J. Braz. Chem. Soc. 2009, 20, 704–711. [Google Scholar] [CrossRef]
- Lima, B.D.A.; de Lira, S.P.; Kossuga, M.H.; Gonçalves, R.B.; Berlinck, R.G.S.; Kamiya, R.U. Halistanol Sulfate A and Rodriguesines A and B Are Antimicrobial and Antibiofilm Agents against the Cariogenic Bacterium Streptococcus Mutans. Rev. Bras. Farmacogn. 2014, 24, 651–659. [Google Scholar] [CrossRef]
- Menna, M.; Fattorusso, E.; Imperatore, C. Alkaloids from Marine Ascidians. Molecules 2011, 16, 8694–8732. [Google Scholar] [CrossRef]
- Choudhary, A.; Naughton, L.M.; Montánchez, I.; Dobson, A.D.W.; Rai, D.K. Current Status and Future Prospects of Marine Natural Products (MNPs) as Antimicrobials. Mar. Drugs 2017, 15, 272. [Google Scholar] [CrossRef]
- Imperatore, C.; Valadan, M.; Tartaglione, L.; Persico, M.; Ramunno, A.; Menna, M.; Casertano, M.; Dell’Aversano, C.; Singh, M.; d’Aulisio Garigliota, M.L.; et al. Exploring the Photodynamic Properties of Two Antiproliferative Benzodiazopyrrole Derivatives. Int. J. Mol. Sci. 2020, 21, 1246. [Google Scholar] [CrossRef]
- Wang, W.; Nam, S.-J.; Lee, B.-C.; Kang, H. b-Carboline Alkaloids from a Korean Tunicate Eudistoma Sp. J. Nat. Prod. 2008, 71, 163–166. [Google Scholar] [CrossRef]
- Schumacher, R.W.; Davidson, B.S. Didemnolines A-D, New N9-Substituted b-Carbolines from the Marine Ascidian Didemnum Sp. Tetrahedron 1995, 51, 10125–10130. [Google Scholar] [CrossRef]
- Wona, T.H.; Jeon, J.; Lee, S.H.; Rho, B.J.; Oh, K.B.; Shin, J. Beta-Carboline Alkaloids Derived from the Ascidian Synoicum Sp. Bioorg. Med. Chem. 2012, 20, 4082–4087. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, J.; Harbour, G.C.; Gilmore, J.; Rinehart, K.L., Jr. Eudistomins A, D, G, H, I, J, M, N, 0, P, and Q, Bromo-, Hydroxy-, Pyrrolyl-, and 1-Pyrrolinyl-P-Carbolines from the Antiviral Caribbean Tunicate Eudistoma Olivaceum. J. Am. Chem. Soc. 1984, 106, 1526–1528. [Google Scholar] [CrossRef]
- Rinehart, K.L., Jr.; Kobayashi, J.; Harbour, G.C.; Highes, R.G.; Mizsak, S.A.; Scahill, T.A. Eudistomins C, E, K, and L, Potent Antiviral Compounds Containing a Novel Oxathiazepine Ring from the Caribbean Tunicate Eudistoma Olivaceum. J. Am. Chem. Soc. 1984, 106, 1524–1526. [Google Scholar] [CrossRef]
- Hudson, J.B.; Saboune, H.; Abramowski, Z.; Towers, G.H.N.; Rinehart, K.L., Jr. The Photoactive Antimicrobial Properties of Eudistomins from the Caribbean Tunicate Eudistoma Olivaceum. Photochem. Photobiol. 1988, 47, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Schupp, P.; Poehner, T.; Edrada, R.; Ebel, R.; Berg, A.; Wray, V.; Proksch, P. Eudistomins W and X, Two New α-Carbolines from the Micronesian Tunicate Eudistoma Sp. J. Nat. Prod. 2003, 66, 272–275. [Google Scholar] [CrossRef]
- Imperatore, C.; Cimino, P.; Cebrián-Torrejón, G.; Persico, M.; Aiello, A.; Senese, M.; Fattorusso, C.; Menna, M.; Doménech-Carbó, A. Insight into the Mechanism of Action of Marine Cytotoxic Thiazinoquinones. Mar. Drugs 2017, 15, 335. [Google Scholar] [CrossRef]
- Casertano, M.; Menna, M.; Fattorusso, C.; Basilico, N.; Parapini, S.; Persico, M.; Imperatore, C. Antiplasmodial Activity of p-Substituted Benzyl Thiazinoquinone Derivatives and Their Potential against Parasitic Infections. Molecules 2020, 25, 1530. [Google Scholar] [CrossRef]
- Gimmelli, R.; Persico, M.; Imperatore, C.; Saccoccia, F.; Guidi, A.; Casertano, M.; Luciano, P.; Pietrantoni, A.; Bertuccini, L.; Paladino, A.; et al. Thiazinoquinones as New Promising Multistage Schistosomicidal Compounds Impacting Schistosoma Mansoni and Egg Viability. ACS Infect. Dis. 2020, 6, 124–137. [Google Scholar] [CrossRef]
- Imperatore, C.; Persico, M.; Senese, M.; Aiello, A.; Casertano, M.; Luciano, P.; Basilico, N.; Parapini, S.; Paladino, A.; Fattorusso, C.; et al. Exploring the Antimalarial Potential of the Methoxy-Thiazinoquinone Scaffold: Identification of a New Lead Candidate. Bioorganic Chem. 2019, 85, 240–252. [Google Scholar] [CrossRef]
- Molinski, T.F. Marine Pyridoacridine Alkaloids: Structure, Synthesis, and Biological Chemistry. Chem. Rev. 1993, 93, 1825–1838. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Mohamed, G.A. Marine Pyridoacridine Alkaloids: Biosynthesis and Biological Activities. Chem. Biodiversity 2016, 13, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Appleton, R.D.; Pearce, A.N.; Lambert, G.; Babcock, R.C.; Copp, B.R. Isodiplamine, Cystodytin K and Lissoclinidine: Novel Bioactive Alkaloids from the New Zealand Ascidian Lissoclinum Notti. Tetrahedron 2002, 58, 9779–9783. [Google Scholar] [CrossRef]
- Kobayashi, J.; Cheng, J.; Walchli, M.R.; Nakamura, H.; Hirata, Y.; Sasaki, T.; Ohizumi, Y. Cystodytins A, B, and C, Novel Tetracyclic Aromatic Alkaloids with Potent Antineoplastic Activity from the Okinawan Tunicate Cystodytes Dellechiajei. J. Org. Chem. 1988, 53, 1800–1804. [Google Scholar] [CrossRef]
- Kobayashi, J.; Cheng, J.; Nakamura, H.; Ohizumi, Y.; Hirata, Y.; Sasaki, T.; Ohta, T.; Nozoe, S. Ascididemin, a novel pentacyclic aromatic alkaloid with potent antileukemic activity from the Okinawan tunicate Didemnum sp. Tetrahedron Lett. 1988, 29, 1177–1180. [Google Scholar] [CrossRef]
- Bonnard, I.; Bontemps, N.; Lahmy, S.; Banaigs, B.; Combaut, G.; Francisco, C.; Colson, P.; Houssier, C.; Waring, M.J.; Bailly, C. Binding to DNA and Cytotoxic Evaluation of Ascididemin, the Major Alkaloid from the Mediterranean Ascidian Cystodytes Dellechiajei. AntiCancer Drug Des. 1995, 10, 333–346. [Google Scholar]
- Schmitz, F.J.; De Guzman, F.S.; Hossain, M.B.; Van Der Helm, D. Cytotoxic Aromatic Alkaloids from the Ascidian Amphicarpa Meridiana and Leptoclinides Sp.: Meridine and 11-Hydroxyascididemin. J. Org. Chem. 1991, 56, 804–808. [Google Scholar] [CrossRef]
- Delfourne, E.; Bontemps-Subielos, N.; Bastide, J. Structure Revision of the Marine Pentacyclic Aromatic Alkaloid: Cystodamine. Tetrahedron Lett. 2000, 41, 3863–3864. [Google Scholar] [CrossRef]
- Kobayashi, J.; Tsuda, M.; Tanabe, A.; Ishibashi, M.; Cheng, J.F.; Yamamura, S.; Sasaki, T. Cystodytins D-I, New Cytotoxic Tetracyclic Aromatic Alkaloids from the Okinawan Marine Tunicate Cystodytes Dellechiajei. J. Nat. Prod. 1991, 54, 1634–1638. [Google Scholar] [CrossRef]
- Torres, Y.R.; Bugni, T.S.; Berlinck, R.G.S.; Ireland, C.M.; Magalhães, A.; Ferreira, A.G.; Moreira, D.A.; Rocha, R. Sebastianines A and B, Novel Biologically Active Pyridoacridine Alkaloids from the Brazilian Ascidian Cystodytes Dellechiajei. J. Org. Chem. 2002, 67, 5429–5432. [Google Scholar] [CrossRef]
- Bry, D.; Banaigs, B.; Long, C.; Bontemps, N. New Pyridoacridine Alkaloids from the Purple Morph of the Ascidian Cystodytes Dellechiajei. Tetrahedron Lett. 2011, 52, 3041–3044. [Google Scholar] [CrossRef]
- Carroll, A.R.; Scheuer, P.J. Kuanoniamines A, B, C, and D: Pentacyclic Alkaloids from a Tunicate and Its Prosobranch Mollusk Predator Chelynotus Semperi. J. Org. Chem. 1990, 55, 4426–4431. [Google Scholar] [CrossRef]
- Carroll, A.R.; Cooray, N.M.; Poiner, A.; Scheuer, P.J. A Second Shermilamine Alkaloid from a Tunicate Trididemnum Sp. J. Org. Chem. 1989, 54, 4231–4232. [Google Scholar] [CrossRef]
- Eder, C.; Schupp, P.; Proksch, P.; Wray, V.; Steube, K.; Muller, C.E.; Frobenius, W.; Herderich, M.; van Soest, R.W.M. Bioactive Pyridoacridine Alkaloids from the Micronesian Sponge Oceanapia Sp. J. Nat. Prod. 1998, 61, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Copp, B.R.; Jompa, J.; Tahir, A.; Ireland, C.M. Styelsamines A-D: New Tetracyclic Pyridoacridine Alkaloids from the Indonesian Ascidian Eusynstyela Latericius. J. Org. Chem. 1998, 63, 8024–8026. [Google Scholar]
- Zeng, C.; Ishibashi, M.; Matsumoto, K.; Nakaike, S.; Kobayashi, J. Two New Polycyclic Aromatic Alkaloids from the Okinawan Marine Sponge Biemna Sp. Tetrahedron 1993, 49, 8337–8342. [Google Scholar] [CrossRef]
- Lopez-Legentil, S.; Turon, X.; Schupp, P. Chemical and Physical Defenses against Predators in Cystodytes (Ascidiacea). J. Exp. Mar. Biol. Ecol. 2006, 332, 27–36. [Google Scholar] [CrossRef]
- Raub, M.F.; Cardellina, J.H., II. The Piclavines, Antimicrobial Indolizidines from the Tunicate Clavelina Picta. Tetrahedron Lett. 1992, 33, 2257–2260. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.-W.; Guo, Y.-W. Recent Advances in the Isolation, Synthesis and Biological Activity of Marine Guanidine Alkaloids. Mar. Drugs 2017, 15, 324. [Google Scholar] [CrossRef]
- Sakai, R.; Higa, T. Tubastrine, a New Guanidinostyrene from the Coral Tubastrea Aurea. Chem. Lett. 1987, 127–128. [Google Scholar] [CrossRef]
- Barenbrock, J.S.; Köck, M. Screening Enzyme—Inhibitory Activity in Several Ascidian Species from Orkney Islands Using Protein Tyrosine Kinase (PTK) Bioassay—Guided Fractionation. J. Biotechnol. 2005, 117, 225–232. [Google Scholar] [CrossRef]
- Pearce, A.N.; Chia, E.W.; Berridge, M.W.; Maas, E.; Page, M.J.; Harper, J.L.; Webb, V.L.; Copp, B.R. Orthidines A-E, Tubastrine, 3,4-Dimethoxyphenethyl-β-Guanidine, and 1,14-Sperminedihomovanillamide: Potential Anti-Inflammatory Alkaloids Isolated from the New Zealand Ascidian Aplidium Orthium That Act as Inhibitors of Neutrophil Respiratory Burst. Tetrahedron 2008, 64, 5748–5755. [Google Scholar] [CrossRef]
- Tadesse, M.; Tørfoss, V.; Strøm, M.B.; Hansen, E.; Andersen, J.H.; Stensvåg, K.; Haug, T. Isolation and Biological Activity of (E)-1-(4-Hydroxystyryl)Guanidine from the Sub-Arctic Ascidian, Dendrodoa Aggregata. Biochem. Syst. Ecol. 2010, 38, 827–829. [Google Scholar] [CrossRef]
- Urban, S.; Capon, R.J.; Hooper, J.N.A. A New Alkaloid from an Australian Marine Sponge, Spongosorites Sp. Aust. J. Chem. 1994, 47, 2279–2282. [Google Scholar] [CrossRef]
- Tadesse, M.; Strøm, M.B.; Svenson, J.; Jaspars, M.; Milne, B.F.; Tørfoss, V.; Andersen, J.H.; Hansen, E.; Stensvåg, K.; Haug, T. Synoxazolidinones A and B: Novel Bioactive Alkaloids from the Ascidian Synoicum Pulmonaria. Org. Lett. 2010, 12, 4752–4755. [Google Scholar]
- Tadesse, M.; Svenson, J.; Jaspars, M.; Strøm, M.B.; Abdelrahman, M.H.; Andersen, J.H.; Hansen, E.; Kristiansen, P.E.; Stensvåg, K.; Haug, T. Synoxazolidinone C; a Bicyclic Member of the Synoxazolidinone Family with Antibacterial and Anticancer Activities. Tetrahedron Lett. 2011, 52, 1804–1806. [Google Scholar] [CrossRef]
- Miao, S.; Andersen, R.J. Rubrolides A-H, Metabolites of the Colonial Tunicate Ritterella Rubra. J. Org. Chem. 1991, 56, 6275–6280. [Google Scholar] [CrossRef]
- Smith, C.J.; Hettich, R.L.; Jompa, J.; Tahir, A.; Buchanan, M.V.; Ireland, C.M. Cadiolides A and B, New Metabolites from an Ascidian of the Genus Botryllus. J. Org. Chem. 1998, 63, 4147–4150. [Google Scholar] [CrossRef]
- Wang, W.; Kim, H.; Nam, S.J.; Rho, B.J.; Kang, H. Antibacterial Butenolides from the Korean Tunicate Pseudodistoma Antinboja. J. Nat. Prod. 2012, 75, 2049–2054. [Google Scholar] [CrossRef]
- Won, T.H.; Jeon, J.; Kim, S.H.; Lee, S.H.; Rho, B.J.; Oh, D.C.; Oh, K.B.; Shin, J. Brominated Aromatic Furanones and Related Esters from the Ascidian Synoicum Sp. J. Nat. Prod. 2012, 75, 2055–2061. [Google Scholar] [CrossRef]
- Sikorska, J.; Parker-Nance, S.; Davies-Coleman, M.T.; Vining, O.B.; Sikora, A.E.; McPhail, K.L. Antimicrobial Rubrolides from a South African Species of Synoicum Tunicate. J. Nat. Prod. 2012, 75, 1824–1827. [Google Scholar] [CrossRef]
- Wang, W.; Kim, H.; Patil, R.S.; Giri, A.G.; Hwan Won, D.; Hahn, D.; Sung, Y.; Lee, J.; Choi, H.; Nam, S.-J.; et al. Cadiolides J-M, Antibacterial Polyphenyl Butenolides from the Korean Tunicate Pseudodistoma Antinboja. Bioorganic Med. Chem. Lett. 2016, 27, 574–577. [Google Scholar] [CrossRef]
- Zhu, T.; Chen, Z.; Liu, P.; Wang, Y.; Xin, Z.; Zhu, W. New Rubrolides from the Marine-Derived Fungus Aspergillus Terreus OUCMDZ-1925. J. Antibiot. 2014, 67, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Cho, E.; Park, J.S.; Won, T.H.; Seo, S.Y.; Oh, D.C.; Oh, K.B.; Shin, J. Isocadiolides A−H: Polybrominated Aromatics from a Synoicum Sp. Ascidian. J. Nat. Prod. 2020, 83, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Cai, S.; Gu, G.; Guo, Z.; Long, Z. Recent Progress in the Development of Sortase A Inhibitors as Novel Anti-Bacterial Virulence Agents. RSC Adv. 2015, 5, 49880–49889. [Google Scholar] [CrossRef]
- Bhusal, R.P.; Bashiri, G.; Kwai, B.X.C.; Sperry, J.; Leung, I.K.H. Targeting Isocitrate Lyase for the Treatment of Latent Tuberculosis. Drug Discov. Today 2017, 22, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Lyla, P.S.; Khan, S.A. Marine Microbial Diversity and Ecology: Importance and Future Perspectives. Curr. Sci. 2006, 90, 1325–1335. [Google Scholar]
- Dou, X.; Dong, B. Origins and Bioactivities of Natural Compounds Derived from Marine Ascidians and Their Symbionts. Mar. Drugs 2019, 17, 670. [Google Scholar] [CrossRef] [PubMed]
- Donia, M.S.; Fricke, W.F.; Partensky, F.; Cox, J.; Elshahawi, S.I.; White, J.R.; Phillippy, A.M.; Schatz, M.C.; Piel, J.; Haygood, M.G.; et al. Complex Microbiome Underlying Secondary and Primary Metabolism in the Tunicate-Prochloron Symbiosis. Proc. Natl. Acad. Sci. USA 2011, 108, E1423–E1432. [Google Scholar] [CrossRef]
- Rath, C.M.; Janto, B.; Earl, J.; Ahmed, A.; Hu, F.Z.; Hiller, L.; Dahlgren, M.; Kreft, R.; Yu, F.; Wolff, J.J.; et al. Metaomic Characterization of the Marine Invertebrate Microbial Consortium That Produces the Chemotherapeutic Natural Product ET-743. ACS Chem. Biol. 2011, 6, 1244–1256. [Google Scholar] [CrossRef]
- Xu, Y.; Kersten, R.D.; Nam, S.G.; Lu, L.; Al-Suwailem, A.M.; Zheng, H.; Fenical, W.; Dorrestein, P.C.; Moore, B.S.; Qian, P.Y. Bacterial Biosynthesis and Maturation of the Didemnin Anti-Cancer Agents. J. Am. Chem. Soc. 2012, 134, 8625–8632. [Google Scholar] [CrossRef]
- Tianero, M.D.; Kwan, J.C.; Wyche, T.P.; Presson, A.P.; Koch, M.; Barrows, L.R.; Bugni, T.S.; Schmidt, E.W. Species Specificity of Symbiosis and Secondary Metabolism in Ascidians. ISME J. 2015, 9, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Asolkar, R.N.; Kirkland, T.N.; Jensen, P.R.; Fenical, W. Arenimycin, an Antibiotic Effective against Rifampin- and Methicillin-Resistant Staphylococcus Aureus from the Marine Actinomycete Salinispora Arenicola. J. Antibiot. 2010, 63, 37–39. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Ding, W.D.; Bernan, V.S.; Richardson, A.D.; Ireland, C.M.; Greenstein, M.; Ellestad, G.A.; Carter, G.T. Lomaiviticins A and B, Potent Antitumor Antibiotics from Micromonospora Lomaivitiensis. J. Am. Chem. Soc. 2001, 123, 5362–5363. [Google Scholar] [CrossRef] [PubMed]
- Janso, J.E.; Haltli, B.A.; Eustáquio, A.S.; Kulowski, K.; Waldman, A.J.; Zha, L.; Nakamura, H.; Bernan, V.S.; He, H.; Carter, G.T.; et al. Discovery of the Lomaiviticin Biosynthetic Gene Cluster in Salinispora Pacifica. Tetrahedron 2014, 70, 4156–4164. [Google Scholar] [CrossRef]
- Wyche, T.P.; Alvarenga, R.F.R.; Piotrowski, J.S.; Duster, M.N.; Warrack, S.R.; Cornilescu, G.; DeWolfe, T.J.; Hou, Y.; Braun, D.R.; Ellis, G.A.; et al. Chemical Genomics, Structure Elucidation, and in Vivo Studies of the Marine-Derived Anticlostridial Ecteinamycin. ACS Chem. Biol. 2017, 12, 2287–2295. [Google Scholar] [CrossRef]
- Fenical, W.; Jensen, P.R. Developing a New Resource for Drug Discovery: Marine Actinomycete Bacteria. Nat. Chem. Biol. 2006, 2, 666–673. [Google Scholar] [CrossRef]
- Wyche, T.P.; Hou, Y.; Vazquez-Rivera, E.; Braun, D.; Bugni, T.S. Peptidolipins B–F, Antibacterial Lipopeptides from an Ascidian-Derived Nocardia Sp. J. Nat. Prod. 2012, 75, 735–740. [Google Scholar] [CrossRef]
- Stefani, S.; Chung, D.R.; Lindsay, J.A.; Friedrich, A.W.; Kearns, A.M.; Westh, H.; MacKenzie, F.M. Meticillin-Resistant Staphylococcus Aureus (MRSA): Global Epidemiology and Harmonisation of Typing Methods. Int. J. Antimicrob. Agents 2012, 39, 273–282. [Google Scholar] [CrossRef]
- De Kraker, M.E.A.; Wolkewitz, M.; Davey, P.G.; Koller, W.; Berger, J.; Nagler, J.; Icket, C.; Kalenic, S.; Horvatic, J.; Seifert, H.; et al. Clinical Impact of Antimicrobial Resistance in European Hospitals: Excess Mortality and Length of Hospital Stay Related to Methicillin-Resistant Staphylococcus Aureus Bloodstream Infections. Antimicrob. Agents Chemother. 2011, 55, 1598–1605. [Google Scholar] [CrossRef]
- Ellis, G.A.; Wyche, T.P.; Fry, C.G.; Braun, D.R.; Bugni, T.S. Solwaric Acids A and B, Antibacterial Aromatic Acids from a Marine Solwaraspora Sp. Mar. Drugs 2014, 12, 1013–1022. [Google Scholar] [CrossRef]
- Valliappan, K.; Sun, W.; Li, Z. Marine Actinobacteria Associated with Marine Organisms and Their Potentials in Producing Pharmaceutical Natural Products. Appl. Microbiol. Biotechnol. 2014, 98, 7365–7377. [Google Scholar] [CrossRef] [PubMed]
- Sung, A.A.; Gromek, S.M.; Balunas, M.J. Upregulation and Identification of Antibiotic Activity of a Marine-Derived Streptomyces Sp. via Co-Cultures with Human Pathogens. Mar. Drugs 2017, 15, 250. [Google Scholar] [CrossRef] [PubMed]
- Cuomo, V.; Palomba, I.; Perretti, A.; Guerriero, A.; D’Ambrosio, M.; Pietra, F. Antimicrobial Activities from Marine Fungi. J. Mar. Biotechnol. 1995, 2, 199–204. [Google Scholar]
- Rahbaek, L.; Breinholt, J. Circumdatins D, E, and F: Further Fungal Benzodiazepine Analogues from Aspergillus Ochraceus J. Nat. Prod. 1999, 62, 904–905. [Google Scholar] [CrossRef]
- Yurchenko, A.N.; Ivanets, E.V.; Smetanina, O.F.; Pivkin, M.V.; Dyshlovoi, S.A.; Amsberg, G.V.; Afiyatullov, S.S. Metabolites of the Marine Fungus Aspergillus Candidus KMM 4676 Associated with a Kuril Colonial Ascidian. Chem. Nat. Compd. 2017, 53, 747–749. [Google Scholar] [CrossRef]
- Bugni, T.S.; Abbanat, D.; Bernan, V.S.; Maiese, W.M.; Greenstein, M.; Van Wagoner, R.M.; Ireland, C.M. Yanuthones: Novel Metabolites from a Marine Isolate of Aspergillus Niger. J. Org. Chem. 2000, 65, 7195–7200. [Google Scholar] [CrossRef]
- Smetanina, O.F.; Kuznetsova, T.A.; Gerasimenko, A.V.; Kalinovsky, A.I.; Pivkin, M.V.; Dmitrenok, P.C.; Elyakov, G.B. Metabolites of the Marine Fungus Humicola Fuscoatra, KMM 4629. Russ. Chem. Bull. 2004, 36, 2643–2646. [Google Scholar] [CrossRef]
- Tan, R.X.; Zou, W.X. Endophytes: A Rich Source of Functional Metabolites. Nat. Prod. Rep. 2001, 18, 448–459. [Google Scholar] [CrossRef]
- Kobayashi, H.; Meguro, S.; Yoshimoto, T.; Namikoshi, M. Absolute Structure, Biosynthesis and Anti-Microtubule Activity of Phomopsidin, Isolated from a Marine-Derived Fungus Phomopsis Sp. Tetrahedron 2003, 59, 455–459. [Google Scholar] [CrossRef]
- Proksh, P.; Edrada, R.A.; Ebel, R. Drugs From the Seas - Current Status and Microbiological Implications. Appl. Microbiol. Biotechnol. 2002, 59, 125–134. [Google Scholar]
- Weindling, R. Experimental Consideration of the Mold Toxins of Gliocladium and Trichoderma. Phytopathology 1941, 31, 991–1003. [Google Scholar]
- Garo, E.; Starks, C.M.; Jensen, P.R.; Fenical, W.; Lobkovsky, E.; Clardy, J. Trichodermamides A and B, Cytotoxic Modified Dipeptides from the Marine-Derived Fungus Trichoderma Virens. J. Nat. Prod. 2003, 66, 423–426. [Google Scholar]
- Dittmann, E.; Fewer, D.P.; Neilan, B.A. Cyanobacterial Toxins: Biosynthetic Routes and Evolutionary Roots. FEMS Microbiol. Rev. 2013, 37, 23–43. [Google Scholar] [CrossRef] [PubMed]
- Christophoridis, C.; Zervou, S.K.; Manolidi, K.; Katsiapi, M.; Moustaka-Gouni, M.; Kaloudis, T.; Triantis, T.M.; Hiskia, A. Occurrence and Diversity of Cyanotoxins in Greek Lakes. Sci. Rep. 2018, 8, 17877. [Google Scholar] [CrossRef] [PubMed]
- Gkelis, S.; Panou, M.; Konstantinou, D.; Apostolidis, P.; Kasampali, A.; Papadimitriou, S.; Kati, D.; Di Lorenzo, G.M.; Ioakeim, S.; Zervou, S.-K.; et al. Diversity, Cyanotoxin Production, and Bioactivities of Cyanobacteria Isolated from Freshwaters of Greece. Toxins 2019, 11, 436. [Google Scholar] [CrossRef]
- Singh, R.; Parihar, P.; Singh, M.; Bajguz, A.; Kumar, J.; Singh, S.; Singh, V.P.; Prasad, S.M. Uncovering Potential Applications of Cyanobacteria and Algal Metabolites in Biology, Agriculture and Medicine: Current Status and Future Prospects. Front. Microbiol. 2017, 8, 515. [Google Scholar] [CrossRef]
- Nunnery, J.K.; Mevers, E.; Gerwick, W.H. Biologically Active Secondary Metabolites from Marine Cyanobacteria. Curr. Opin. Biotechnol. 2010, 21, 787–793. [Google Scholar] [CrossRef]
- Socha, A.M.; Garcia, D.; Sheffer, R.; Rowley, D.C. Antibiotic Bisanthraquinones Produced by a Streptomycete Isolated from a Cyanobacterium Associated with Ecteinascidia Turbinata. J. Nat. Prod. 2006, 69, 1070–1073. [Google Scholar] [CrossRef]
| Bacterial Strains | MIC (μg/mL) a | Mammalian Cell Line | LC50 (μM) b | ||
|---|---|---|---|---|---|
| 27 | 28 | 27 | 28 | ||
| S. aureus | 12.5 | 12.5 | A549 | 13.8 | 13.1 |
| B. subtilis | 25 | 25 | K562 | 13.6 | 12.6 |
| M. luteus | 12.5 | 12.5 | |||
| S. typhimurium | 6.25 | 12.5 | |||
| P. vulgaris | 25 | 25 | |||
| E. coli | >100 | >100 | |||
| Compounds | MIC (μg/mL) | ||||
|---|---|---|---|---|---|
| A a | B a | C a | D a | E a | |
| cadiolide B (112) | 3.1 | 3.1 | 3.1 | >128 | >128 |
| cadiolide C (113) | 0.4 | 0.2 | 3.1 | 64 | >128 |
| cadiolide D (114) | 6.3 | 1.6 | 6.3 | >128 | >128 |
| cadiolide E (115) | 3.1 | 0.2 | 1.6 | 1.6 | 3.1 |
| cadiolide F (116) | 12.5 | 3.1 | 12.5 | - b | - b |
| cadiolide G (117) | 3.1 | 3.1 | 12.5 | 0.8 | 3.1 |
| cadiolide H (118) | 6.3 | 3.1 | 1.6 | 3.1 | 3.1 |
| cadiolide I (119) | 0.8 | 0.8 | 0.8 | 1.6 | 6.3 |
| cadiolide J (120) | 32 | - b | - b | 64 | 8 |
| cadiolide K (121) | 8 | - b | - b | 8 | 4 |
| cadiolide L (122) | 2 | - b | - b | 4 | 2 |
| cadiolide M (123) | 1 | - b | - b | - b | - b |
| cadiolide N (124) | 16 | - b | - b | 16 | 16 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casertano, M.; Menna, M.; Imperatore, C. The Ascidian-Derived Metabolites with Antimicrobial Properties. Antibiotics 2020, 9, 510. https://doi.org/10.3390/antibiotics9080510
Casertano M, Menna M, Imperatore C. The Ascidian-Derived Metabolites with Antimicrobial Properties. Antibiotics. 2020; 9(8):510. https://doi.org/10.3390/antibiotics9080510
Chicago/Turabian StyleCasertano, Marcello, Marialuisa Menna, and Concetta Imperatore. 2020. "The Ascidian-Derived Metabolites with Antimicrobial Properties" Antibiotics 9, no. 8: 510. https://doi.org/10.3390/antibiotics9080510
APA StyleCasertano, M., Menna, M., & Imperatore, C. (2020). The Ascidian-Derived Metabolites with Antimicrobial Properties. Antibiotics, 9(8), 510. https://doi.org/10.3390/antibiotics9080510







