Antimicrobial Spectrum of Titroleane™: A New Potent Anti-Infective Agent
Abstract
1. Introduction
2. Results
2.1. Comparative Compounds of Standard TTO and Titroleane™
2.2. Homogeneity between Batches
2.3. MIC of Titroleane™ and Fraction 2
2.4. Antibacterial Spectrum of Titroleane™
2.5. Activity of Titroleane™ versus TTO
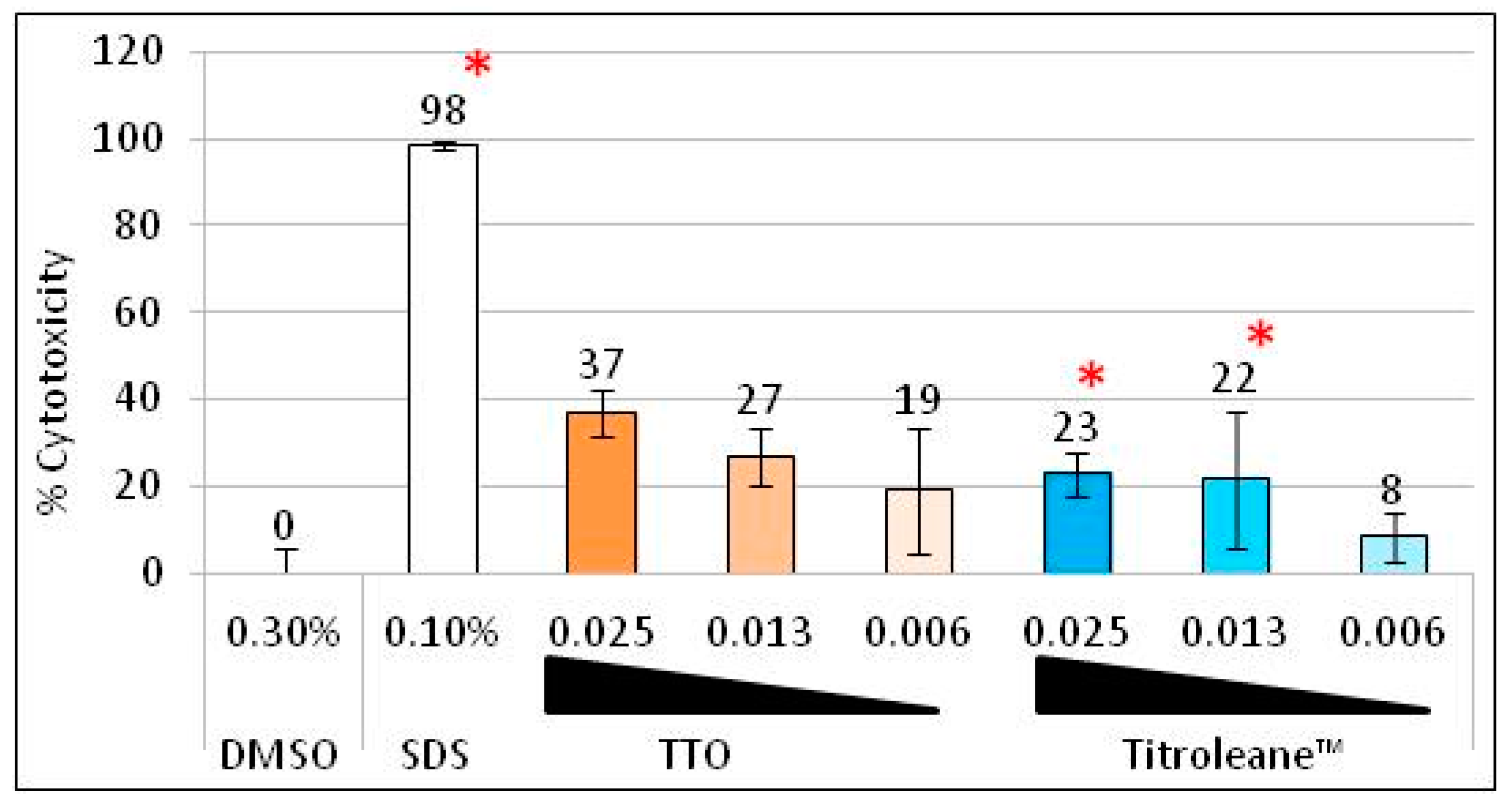
2.6. Cytotoxicity of TTO versus Titroleane™
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.1.1. Active Compounds
4.1.2. TTO Fractionation
4.1.3. Industrial Batches
4.1.4. Samples Preparation
4.2. Antimicrobial Activities
4.2.1. Bacteria and Fungi
4.2.2. Growth Conditions and Inoculum Preparation
4.2.3. Bacillus atrophaeus Spore’s Suspension Preparation
4.2.4. Minimum Inhibitory Concentration (MIC) Determination
4.3. In Vitro Cytotoxicity
4.4. Statistical Analysis
5. Patents
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ATCC | American Type Culture Collection |
| BMD | Broth Microdilution |
| CFU | Colony forming unit |
| CIP | Pasteur Institute Collection |
| CLSI | Clinical and Laboratory Standards Institute |
| DMSO | Dimethyl sulfoxide |
| GC-FID | Gas Chromatography with Flame Ionization Detector |
| MEM | Minimum Essential Medium |
| MIC | Minimal Inhibitory Concentration |
| MRSA | Methicillin-resistant S. aureus |
| PBS | Phosphate Buffered Saline |
| SDB | Sabouraud Dextrose Broth |
| SDS | Sodium Dodecyl Sulfate |
| SSTI | Skin and soft tissue infection |
| SSI | Skin structure infection |
| TPS | Tryptone salt solution |
| TTO | Tea Tree oil |
| UMIP | Pasteur Institute Collection of Fungi |
References
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca alternifolia (Tea Tree) oil: A review of antimicrobial and other medicinal properties. Clin. Microbiol. Rev. 2006, 19, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Ramage, G.; Milligan, S.; Lappin, D.F.; Sherry, L.; Sweeney, P.; Williams, C.; Bagg, J.; Culshaw, S. Antifungal, cytotoxic, and immunomodulatory properties of Tea Tree oil and its derivative components: Potential role in management of oral candidosis in cancer patients. Front. Microbiol. 2012, 3, 220. [Google Scholar] [CrossRef]
- James, P.J.; Callander, J.T. Bioactivity of Tea Tree oil from Melaleuca alternifolia against sheep lice (Bovicola ovis Schrank) in vitro. Vet. Parasitol. 2012, 187, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Mao, X.; Luo, Y. Study on antifungal mechanism of α-pinene. Hunan Yi Ke Da Xue Xue Bao. 1999, 24, 507–509. [Google Scholar] [PubMed]
- Dorman, H.J.D.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Antifungal activity of the components of Melaleuca alternifolia (tea tree) oil. J. Appl. Microbiol. 2003, 95, 853–860. [Google Scholar] [CrossRef]
- Kim, K.J.; Kim, Y.H.; Yu, H.H.; Jeong, S.I.; Cha, J.D.; Kil, B.S.; You, Y.O. Antibacterial activity and chemical composition of essential oil of Chrysanthemum boreale. Planta Med. 2003, 69, 274–277. [Google Scholar] [CrossRef]
- Bagamboula, C.F.; Uyttendaele, M.; Debevere, J. Inhibitory effect of thyme and basil essential oils, carvacrol, thymol, estragol, linalool and p-cymene towards Shigella sonnei and S. flexneri. Food Microbiol. 2004, 21, 33–42. [Google Scholar] [CrossRef]
- Santoyo, S.; Cavero, S.; Jaime, L.; Ibañez, E.; Señoráns, F.J.; Reglero, G. Chemical composition and antimicrobial activity of Rosmarinus officinalis L. essential oil obtained via supercritical fluid extraction. J. Food Prot. 2005, 68, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Pichette, A.; Larouche, P.L.; Lebrun, M.; Legault, J. Composition and antibacterial activity of Abies balsamea essential oil. Phytother. Res. 2006, 20, 371–373. [Google Scholar] [CrossRef]
- Cristani, M.; D’Arrigo, M.; Mandalari, G.; Castelli, F.; Sarpietro, M.G.; Micieli, D.; Venuti, V.; Bisignano, G.; Saija, A.; Trombetta, D. Interaction of four monoterpenes contained in essential oils with model membranes: Implications for their antibacterial activity. J. Agric. Food Chem. 2007, 55, 6300–6308. [Google Scholar] [CrossRef]
- Rattanachaikunsopon, P.; Phumkhachorn, P. Assessment of factors influencing antimicrobial activity of carvacrol and cymene against Vibrio cholerae in food. J. Biosci. Bioeng. 2010, 110, 614–619. [Google Scholar] [CrossRef]
- Jiang, Y.; Wu, N.; Fu, Y.J.; Wang, W.; Luo, M.; Zhao, C.J.; Zu, Y.G.; Liu, X.L. Chemical composition and antimicrobial activity of the essential oil of Rosemary. Environ. Toxicol. Pharmacol. 2011, 32, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Tighe, S.; Gao, Y.Y.; Tseng, S.C.G. Terpinen-4-ol is the Most Active Ingredient of Tea Tree oil to Kill Demodex Mites. Transl. Vis. Sci. Technol. 2013, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Hausen, B.M.; Reichling, J.; Harkenthal, M. Degradation products of monoterpenes are the sensitizing agents in Tea Tree oil. Am. J. Contact Dermat. 1999, 10, 68–77. [Google Scholar] [CrossRef]
- Meesters, R.J.W.; Duisken, M.; Hollender, J. Cytochrome P450-catalysed arene-epoxidation of the bioactive Tea Tree oil ingredient p-cymene: Indication for the formation of a reactive allergenic intermediate? Xenobiotica 2009, 39, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Perry, N.B.; Brennan, N.J.; Van Klink, J.W.; Harris, W.; Douglas, M.H.; McGimpsey, J.A.; Smallfield, B.M.; Anderson, R.E. Essential oils from New Zealand manuka and kanuka: Chamotaxonomy of Leptospermum. Phytochemistry 1997, 44, 1485–1494. [Google Scholar] [CrossRef]
- Johansen, B.; Sergere, J.C. Activité Antimicrobienne D’un Mélange Terpénique D’origine Végétale. Patent Application FR2977799–A1, 2016. [Google Scholar]
- Southwell, I.A.; Hayes, A.J.; Markham, J.; Leach, D.N. The search for optimally bioactive Australian Tea Tree oil. Acta Hortic. 1993, 334, 265–275. [Google Scholar] [CrossRef]
- Carson, C.F.; Riley, T.V. Antimicrobial activity of the major components of the essential oil of Melaleuca alternifolia. J. Appl. Bacteriol. 1995, 78, 264–269. [Google Scholar] [CrossRef]
- Abreu, A.C.; McBain, A.J.; Simões, M. Plants as sources of new antimicrobials and resistance-modifying agents. Nat. Prod. Rep. 2012, 29, 1007–1021. [Google Scholar] [CrossRef]
- Duval, R.E.; Grare, M.; Demoré, B. Fight against antimicrobial resistance: We always need new antibacterials but for right bacteria. Molecules 2019, 24, 3152. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils-Present Status and Future Perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef]
- Ford, R.A.; Letizia, C.; Api, A.M. Monographs on fragrance raw materials. Food Chem. Toxicol. 1988, 26, 273–415. [Google Scholar] [CrossRef] [PubMed]
- Greig, J.E.; Carson, C.F.; Stuckey, M.S.; Riley, T.V. Safety of Tea Tree Oil—Second Stage; Rural industries research and Development Corporation (UWA-51A): Wagga Wagga, Australia, 2002. [Google Scholar]
- Hendry, E.R.; Worthington, T.; Conway, B.R.; Lambert, P.A. Antimicrobial efficacy of eucalyptus oil and 1,8-cineole alone and in combination with chlorhexidine digluconate against microorganisms grown in planktonic and biofilm cultures. J. Antimicrob. Chemother. 2009, 64, 1219–1225. [Google Scholar] [CrossRef]
- Li, L.; Li, Z.W.; Yin, Z.Q.; Wei, Q.; Jia, R.Y.; Zhou, L.J.; Xu, J.; Song, X.; Zhou, Y.; Du, Y.H.; et al. Antibacterial activity of leaf essential oil and its constituents from Cinnamomum longepaniculatum. Int. J. Clin. Exp. Med. 2014, 7, 1721–1727. [Google Scholar] [PubMed]
- Vilela, G.R.; Gloria, E.M.; Almeida, G.S.; D’Arce, M.A.B.R.; Moraes, M.H.D.; Brito, J.O.; Silva, M.F.G.F.; Silva, S.C.; Piedade, S.M.L.S.; Calori-Domingues, M.A. Activity of essential oil and its major compound, 1,8-cineole, from Eucalyptus globulus Labill., against the storage fungi Aspergillus flavus Link and Aspergillus parasiticus Speare. J. Stored Prod. Res. 2009, 45, 108–111. [Google Scholar] [CrossRef]
- Randrianariveloa, R.; Sarterb, S.; Odouxc, E.; Bratc, P.; Lebrunc, M.; Romestandd, B.; Menute, C.; Andrianoelisoaf, H.S.; Raherimandimbyg, M.; Danthu, P. Composition and antimicrobial activity of essential oils of Cinnamosma fragrans. Food Chem. 2009, 114, 680–684. [Google Scholar] [CrossRef]
- Raman, A.; Weir, U.; Bloomfield, S.F. Antimicrobial effects of tea-tree oil and its major components on Staphylococcus aureus, Staph. epidermidis and Propionibacterium acnes. Lett. Appl. Microbiol. 1995, 21, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Leite, A.M.; Lima, E.O.; Souza, E.L.; Diniz, M.F.F.M.; Trajano, V.N.; Medeiros, I.A. Inhibitory effect of B-pinene and eugenol on the growth of potential infectious endocarditis causing gram-positive bacteria. Braz. J. Pharm. Sci. 2007, 43, 121–126. [Google Scholar] [CrossRef]
- Rivas da Silva, A.C.; Lopes, P.M.; Barros de Azevedo, M.M.; Costa, D.C.; Alviano, C.S.; Alviano, D.S. Biological activities of α-pinene and β-pinene enantiomers. Molecules 2012, 17, 6305–6316. [Google Scholar] [CrossRef]
- Griffin, S.G.; Wyllie, S.G.; Markhan, J.L.; Leach, D.N. The role of structure and molecular properties of terpenoids in determining their antimicrobial activity. Flavour. Fragr. J. 1999, 14, 322–332. [Google Scholar] [CrossRef]
- Cox, S.D.; Mann, C.M.; Markham, J.L. Interactions between components of the essential oil of Melaleuca alternifolia. J. Appl. Microbiol. 2001, 91, 492–497. [Google Scholar] [CrossRef]
- Longbottom, C.J.; Carson, C.F.; Hammer, K.A.; Mee, B.J.; Riley, T.V. Tolerance of Pseudomonas aeruginosa to Melaleuca alternifolia (tea tree) oil is associated with the outer membrane and energy-dependent cellular processes. J. Antimicrob. Chemother. 2004, 54, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Carson, C.F.; Mee, B.J.; Riley, T.V. Mechanism of Action of Melaleuca alternifolia (Tea Tree) Oil on Staphylococcus aureus Determined by Time-Kill, Lysis, Leakage, and Salt Tolerance Assays and Electron Microscopy. Antimicrob. Agents Chemother. 2002, 46, 1914–1920. [Google Scholar] [CrossRef] [PubMed]
- Loughlin, R.; Gilmore, B.F.; McCarron, P.A.; Tunney, M.M. Comparison of the cidal activity of Tea Tree oil and terpinen-4-ol against clinical bacterial skin isolates and human fibroblast cells. Lett. Appl. Microbiol. 2008, 46, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Weant, K.A.; Bailey, A.M.; Fleishaker, E.L.; Justice, S.B. Being prepared: Bioterrorism and mass prophylaxis: Part I. Adv. Emerg Nurs. J. 2014, 36, 226–238. [Google Scholar] [CrossRef]
- Sartelli, M.; Catena, F.; Ansaloni, L.; Leppaniemi, A.; Taviloglu, K.; van Goor, H.; Viale, P.; Lazzareschi, D.V.; Coccolini, F.; Corbella, D.; et al. Complicated intra-abdominal infections in Europe: A comprehensive review of the CIAO study. World J. Emerg. Surg. 2012, 7, 36. [Google Scholar] [CrossRef]
- Lamont, R.F.; Sobel, J.D.; Akins, R.A.; Hassan, S.S.; Chaiworapongsa, T.; Kusanovic, J.P.; Romero, R. The vaginal microbiome: New information about genital tract flora using molecular based techniques. BJOG 2011, 118, 533–549. [Google Scholar] [CrossRef]
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.M.; Topping, D.L.; Suzuki, T.; et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 2011, 469, 543–547. [Google Scholar] [CrossRef]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. In vitro susceptibilities of lactobacilli and organisms associated with bacterial vaginosis to Melaleuca alternifolia (tea tree) oil. Antimicrob. Agents Chemother. 1999, 43, 196. [Google Scholar] [CrossRef]
- Tucker, J.; Lau, J. Antibiotics and resistance, Drugs, Bugs and the patients who get them Part 1 to 3: A Scientific Overview. BioMedTracker 2014, 1, 16–17. [Google Scholar]
- Thiolet, J.M.; Vaux, S.; Lamy, M.; Gautier, A.; Barret, A.S.; Léon, L.; Coignard, B. Enquête Nationale de Prévalence Des Infections Nosocomiales et Des Traitements Anti-Infectieux en Etablissements de Santé, France, Mai-juin 2012. Available online: http://www.invs.sante.fr/Publications-et-outils/Rapports-et-syntheses/Maladies-infectieuses/2013/Enquete-nationale-de-prevalence-des-infections-nosocomiales-et-des-traitements-anti-infectieux-en-etablissements-de-sante-France-mai-juin-2012 (accessed on 26 April 2016).
- Loesche, W.J. Medical Microbiology: Microbiology of Dental Decay and Periodontal Disease, 4th ed.; University of Texas Medical Branch: Galveston, TX, USA, 1996. [Google Scholar]
- Guggenheim, B.; Giertsen, E.; Schüpbach, P.; Shapiro, S. Validation of an in vitro biofilm model of supragingival plaque. J. Dent. Res. 2001, 80, 363–370. [Google Scholar] [CrossRef]
- Nishijima, S.; Kurokawa, I.; Katoh, N.; Watanabe, K. The bacteriology of acne vulgaris and antimicrobial susceptibility of Propionibacterium acnes and Staphylococcus epidermidis isolated from acne lesions. J. Dermatol. 2000, 27, 318–323. [Google Scholar] [CrossRef] [PubMed]
- D’Auria, F.D.; Laino, L.; Strippoli, V.; Tecca, M.; Salvatore, G.; Battinelli, L.; Mazzanti, G. In vitro activity of Tea Tree oil against Candida albicans mycelial conversion and other pathogenic fungi. J. Chemother. 2001, 13, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Baker-Austin, C.; Olivier, J.D.; Alam, M.; Ali, A.; Waldor, M.K.; Qadri, F.; Martinez-Urtaza, J. Vibrio spp. Infections. Nat. Rev. Dis. Primers. 2018, 4, 8. [Google Scholar] [CrossRef]
- Lawrence, H.A.; Palombo, E.A. Activity of essential oils against Bacillus subtilis spores. J. Microbiol. Biotechnol. 2009, 19, 1590–1595. [Google Scholar] [CrossRef]
- Takarada, K.; Kimizuka, R.; Takahashi, N.; Honma, K.; Okuda, K.; Kato, T. A comparison of the antibacterial efficacies of essential oils against oral pathogens. Oral Microbiol. Immunol. 2004, 19, 61–64. [Google Scholar] [CrossRef]
- Thomsen, P.S.; Jensen, T.M.; Hammer, K.A.; Carson, C.F.; Mølgaard, P.; Riley, T.V. Survey of the antimicrobial activity of commercially available Australian tea tree (Melaleuca alternifolia) essential oil products in vitro. J. Altern. Complement. Med. 2011, 17, 835–841. [Google Scholar] [CrossRef]
- Lee, C.J.; Chen, L.W.; Chen, L.G.; Chang, T.L.; Huang, C.W.; Huang, M.C.; Wang, C.C. Correlation of the components of Tea Tree oil with its antibacterial effects and skin irritation. J. Food Drug Ana. 2013, 21, 169–176. [Google Scholar] [CrossRef]
- Bassett, I.B.; Pannowitz, D.L.; Barnetson, R.S. A comparative study of tea-tree oil versus benzoylperoxide in the treatment of acne. Med. J. Aust. 1990, 153, 455–458. [Google Scholar] [CrossRef]
- Elgendy, E.A.; Ali, S.A.; Zineldeen, D.H. Effect of local application of tea tree (Melaleuca alternifolia) oil gel on long pentraxin level used as an adjunctive treatment of chronic periodontitis: A randomized controlled clinical study. J. Indian Soc. Periodontol. 2013, 17, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Groppo, F.C.; Ramacciato, J.C.; Simões, R.P.; Flório, F.M.; Sartoratto, A. Antimicrobial activity of garlic, Tea Tree oil, and chlorhexidine against oral microorganisms. Int. Dent. J. 2002, 52, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, M., Jr.; Petermann, K.D.; Vedovello, S.A.; Degan, V.; Lucato, A.; Franzini, C.M. Antimicrobial effect of Melaleuca alternifolia dental gel in orthodontic patients. Am. J. Orthod. Dentofac. Orthop. 2014, 145, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, M.; Newall, N.; Carville, K.; Smith, J.; Riley, T.V.; Carson, C.F. Uncontrolled, open-label, pilot study of tea tree (Melaleuca alternifolia) oil solution in the decolonisation of methicillin-resistant Staphylococcus aureus positive wounds and its influence on wound healing. Int. Wound J. 2011, 8, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.B.; Cordell, B. The effect of Tea Tree oil (Melaleuca alternifolia) on wound healing using a dressing model. J. Altern. Complement. Med. 2013, 19, 942–945. [Google Scholar] [CrossRef]
- Cross, S.; Roberts, M. In-vitro Human Epidermal Membrane Penetration of Tea Tree oil Components from Pure oil and a 20% Formulation; Rural industries research and Development Corporation: Canberra, Australia, 2006. [Google Scholar]
- Hammer, K.A. Treatment of acne with Tea Tree oil (melaleuca) products: A review of efficacy, tolerability and potential modes of action. Int. J. Antimicrob. Agents 2015, 45, 106–110. [Google Scholar] [CrossRef]
- Mikus, J.; Harkenthal, M.; Steverding, D.; Reichling, J. In vitro effect of essential oils and isolated mono- and sesquiterpenes on Leishmania major and Trypanosoma brucei. Planta Med. 2000, 66, 366–368. [Google Scholar] [CrossRef]
- Priestley, C.M.; Burgess, I.F.; Williamson, E.M. Lethality of essential oil constituents towards the human louse, Pediculus humanus, and its eggs. Fitoterapia 2006, 77, 303–309. [Google Scholar] [CrossRef]
- Yang, Y.C.; Lee, S.H.; Clark, J.M.; Ahn, Y.J. Ovicidal and adulticidal activities of Origanum majorana essential oil constituents against insecticide-susceptible and pyrethroid/malathion-resistant Pediculus humanus capitis (Anoplura: Pediculidae). J. Agric. Food Chem. 2009, 57, 2282–2287. [Google Scholar] [CrossRef]
- Gu, H.J.; Cheng, S.S.; Lin, C.Y.; Huang, C.G.; Chen, W.J.; Chang, S.T. Repellency of essential oils of Cryptomeria japonica (Pinaceae) against adults of the mosquitoes Aedes aegypti and Aedes albopictus (Diptera:Culicidae). J. Agric. Food Chem. 2009, 57, 11127–11133. [Google Scholar] [CrossRef]
- Walton, S.F.; McKinnon, M.; Pizzutto, S.; Dougall, A.; Williams, E.; Currie, B.J. Acaricidal activity of Melaleuca alternifolia (tea tree) oil: In vitro sensitivity of Sarcoptes scabiei var hominis to terpinen-4-ol. Arch. Dermatol. 2004, 140, 563–566. [Google Scholar] [CrossRef]
- Attia, S.; Grissa, K.L.; Lognay, G.; Heuskin, S.; Mailleux, A.C.; Hance, T. Chemical composition and acaricidal properties of Deverra scoparia essential oil (Araliales: Apiaceae) and blends of its major constituents against Tetranychus urticae (Acari:Tetranychidae). J. Econ. Entomol. 2011, 104, 1220–1228. [Google Scholar] [CrossRef] [PubMed]
- Garozzo, A.; Timpanaro, R.; Bisignano, B.; Furneri, P.M.; Bisignano, G.; Castro, A. In vitro antiviral activity of Melaleuca alternifolia essential oil. Lett. Appl. Microbiol. 2009, 49, 806–808. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Duan, S.; Chu, C.; Xu, J.; Zeng, G.; Lam, A.K.; Zhou, J.; Yin, Y.; Fang, D.; Reynolds, M.J.; et al. Melaleuca alternifolia concentrate inhibits in vitro entry of influenza virus into host cells. Molecules 2013, 18, 9550–9566. [Google Scholar] [CrossRef]
- Brand, C.; Ferrante, A.; Prager, R.H.; Riley, T.V.; Carson, C.F.; Finlay-Jones, J.J.; Hart, P.H. The water-soluble components of the essential oil of Melaleuca alternifolia (Tea Tree oil) suppress the production of superoxide by human monocytes, but not neutrophils, activated in vitro. Inflamm. Res. 2001, 50, 213–219. [Google Scholar] [CrossRef]
- Rodriguez, S.A.; Murray, A.P. Antioxidant activity and chemical composition of essential oil from Atriplex undulata. Nat. Prod. Commun. 2010, 5, 1841–1844. [Google Scholar] [CrossRef] [PubMed]
- Trinh, H.T.; Lee, I.A.; Hyun, Y.J.; Kim, D.H. Artemisia princeps Pamp. Artemisia princeps Pamp. Essential oil and its constituent eucalyptol and α-terpineol ameliorate bacterial vaginosis and vulvovaginal candidiasis in mice by inhibiting bacterial growth and NF-κB activation. Planta Med. 2011, 77, 1996–2002. [Google Scholar] [CrossRef]
- Nogueira, M.N.; Aquino, S.G.; Rossa Junior, C.; Spolidorio, D.M. Terpinen-4-ol and alpha-terpineol (Tea Tree oil components) inhibit the production of IL-1β, IL-6 and IL-10 on human macrophages. Inflamm. Res. 2014, 63, 769–778. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard, 8th ed.; CLSI document M07-A8; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2009. [Google Scholar]
- Iatta, R.; Figueredo, L.A.; Montagna, M.T.; Otranto, D.; Cafarchia, C. In vitro antifungal susceptibility of Malassezia furfur from bloodstream infections. J. Med. Microbiol. 2014, 63 Pt 11, 1467–1473. [Google Scholar] [CrossRef]
- Söderberg, T.A.; Johansson, A.; Gref, R. Toxic effects of some conifer resin acids and tea tree oil on human epithelial and fibroblast cells. Toxicology 1997, 107, 99–109. [Google Scholar] [CrossRef]
- Brackman, G.; Forier, K.; Al Quntar, A.A.; De Canck, E.; Enk, C.D.; Srebnik, M.; Braeckmans, K.; Coenye, T. Thiazolidinedione derivatives as novel agents against Propionibacterium acnes biofilms. J. Appl. Microbiol. 2014, 116, 492–501. [Google Scholar] [CrossRef] [PubMed]
| Terpenes Family | Component | Tea Tree oil (%) Min/Max | Titroleane™ (%) Min/Max | Fraction 2 (%) |
|---|---|---|---|---|
| Monoterpenes | α-Pinene | 1/4 | Trace | 5.1 |
| Sabinene | Trace/3.5 | Trace | 0.2 | |
| α-Terpinene | 6/12 | 0.3/0.4 | 16.9 | |
| Limonene | 0.5/1.5 | 0.03/0.07 | 2.9 | |
| p-Cymene | 0.5/8 | 0.2/0.4 | 8.2 | |
| 1,8-Cineol (Eucalyptol) | Trace/10 | Trace/0.3 | 4.2 | |
| γ-Terpinene | 14/28 | 1.7/2.8 | 40.4 | |
| Terpinolene | 1.5/5 | 0.5/0.8 | 5.3 | |
| Monoterpenes alcohol | Terpinen-4-ol | 35/48 | 71/75 | 9.6 |
| α-Terpineol | 2/5 | 5/9 | 0.2 | |
| Sesquiterpenes | Aromadendrene | 0.2/3 | 1.9/3.3 | Trace |
| Leden (Viridiflorene) | 0.1/3 | 0.9/2.8 | Trace | |
| δ-Cadinene | 0.2/3 | 0.6/2.2 | Trace | |
| Sesquiterpenes alcohol | Globulol | Trace/1 | 0.4/0.9 | Trace |
| Viridiflarol | Trace/1 | 0.2/0.4 | Trace |
| Strains | Batch Number | TitroleaneTM (%) |
|---|---|---|
| E. coli | B1 | 1.25 |
| B2 | 1.25 | |
| B3 | 2.5 | |
| B4 | 2.5 | |
| S. aureus | B1 | 1.25 |
| B2 | 1.25 | |
| B3 | 2.5 | |
| B4 | 2.5 | |
| Y. enterocolitica | B1 | 2.5 |
| B2 | 1.25 | |
| B3 | 2.5 | |
| B4 | 2.5 |
| B2 (%) | B3 (%) | |||
|---|---|---|---|---|
| Strain | TitroleaneTM | Fraction 2 | TitroleaneTM | Fraction 2 |
| E. coli | 1.25 | 2.5 | 2.5 | - |
| S. aureus | 1.25 | 2.5 | 2.5 | 2.5 |
| Y. enterocolitica | 1.25 | 1.25 | 2.5 | 2.5 |
| Order | Genus | Species | TitroleaneTM (%) |
|---|---|---|---|
| GRAM-POSITIVE BACTERIA | |||
| Bacillales | Bacillus | B. cereus | 2.50 |
| B. subtilis | 1.25 | ||
| B. atrophaeus | 1.25 | ||
| B. atrophaeus spores | 0.62 | ||
| Staphylococcus | S. aureus | 0.62–1.25 | |
| MRSA | 1.25 | ||
| S. epidermidis | 0.62–1.25 | ||
| Listeria | L. monocytogenes | 1.25 | |
| Lactobacillales | Enterococcus | E. faecalis | 2.50 |
| E. faecium | 1.25 | ||
| E. hirae | 2.50 | ||
| Lactobacillus | L. acidophilus | 1.25 | |
| L. gasseri | 2.50 | ||
| L. paracasei | 2.50 | ||
| L. rhamnosus | 2.50 | ||
| Streptococcus | S. mutans | 1.25 | |
| S. oralis | 2.50 | ||
| S. sobrinus | 1.25 | ||
| Selenomonadales | Veillonella | V. dispar | 0.02 |
| Clostridiales | Clostridium | C. difficile | 0.62 |
| C. sporogenes | 1.25–2.50 | ||
| Bifidobacteriales | Bifidobacterium | B. bifidum | 0.62 |
| B. breve | 1.25 | ||
| B. longum infantis | 0.62 | ||
| B. longum longum | 0.62 | ||
| B. lactis | 1.25 | ||
| Actinomycetales | Actinomyces | A. naeslundii | 0.15–0.62 |
| Corynebacterium | C. xerosis | 1.25 | |
| Propionibacterium | P. acnes | 0.62 | |
| GRAM-NEGATIVE BACTERIA | |||
| Enterobacteriales | Klebsiella | K. oxytoca | 1.25 |
| Salmonella | S. enterica enterica typhimurium | 0.62 | |
| Escherichia | E. coli | 0.62–1.25 | |
| Shigella | S. boydii | 0.62 | |
| Yersinia | Y. enterocolitica | 1.25 | |
| Vibrionales | Vibrio | V. alginolyticus | 0.62 |
| V. anguillarum | 0.62 | ||
| V. cholerae | 1.25 | ||
| V. harveyi | 0.31 | ||
| V. nigripulchritudo | 1.25 | ||
| V. parahaemolyticus | 1.25 | ||
| V. vulnificus | 0.62 | ||
| Pseudomonadales | Acinetobacter | A. baumannii | 1.25 |
| Pseudomonas | P. aeruginosa | 1.25–2.50 | |
| Campylobacteriales | Campylobacter | C. jejuni | - |
| Helicobacter | H. pylori | 2.50 | |
| Fusobacteriales | Fusobacterium | F. nucleatum | 0.019 |
| Bacteroidales | Bacteroides | B. fragilis | 0.08 |
| B. ovatus | 0.62 | ||
| B. thetaiotaomicron | 0.31 | ||
| FUNGI | |||
| Saccharomycetales | Candida | C. albicans | 1.25 |
| C. kefyr | 1.25 | ||
| C. tropicalis | 0.62–1.25 | ||
| Eurotiales | Aspergillus | A. niger | - |
| Malasseziales | Malassezia | M. furfur | 1.25 |
| Order | Genus | Species | TitroleaneTM (%) | TTO (%) |
|---|---|---|---|---|
| GRAM-POSITIVE BACTERIA | ||||
| Bacillales | Bacillus | B. cereus | 2.50 | 1.25 |
| Staphylococcus | S. aureus | 0.62–1.25 | 0.62 | |
| MRSA | 1.25 | 0.62 | ||
| S. epidermidis | 0.62–1.25 | 0.62 | ||
| Listeria | L. monocytogenes | 1.25 | 1.25 | |
| Lactobacillales | Enterococcus | E. faecalis | 2.50 | 2.50 |
| E. faecium | 1.25 | 2.50 | ||
| E. hirae | 2.50 | 2.50 | ||
| Lactobacillus | L. acidophilus | 1.25 | 1.25 | |
| L. casei | 2.50 | 2.50 | ||
| L. gasseri | 2.50 | 2.50 | ||
| L. rhamnosus | 2.50 | 2.50 | ||
| Clostridiales | Clostridium | C. difficile | 0.62 | 1.25 |
| C. sporogenes | 1.25–2.50 | 2.50 | ||
| Bifidobacteriales | Bifidobacterium | B. bifidum | 0.62 | 1.25 |
| B. longum infantis | 0.62 | 0.62 | ||
| B. longum longum | 0.62–1.25 | 2.50 | ||
| B. lactis | 1.25 | 0.62 | ||
| Actinomycetales | Corynebacterium | C. xerosis | 1.25 | 1.25 |
| GRAM-NEGATIVE BACTERIA | ||||
| Enterobacteriales | Salmonella | S. enterica enterica typhimurium | 0.31–0.62 | 0.31 |
| Escherichia | E. coli | 0.62–1.25 | 1.25 | |
| Yersinia | Y. enterocolitica | 1.25 | 0.31 | |
| Vibrionales | Vibrio | V. alginolyticus | 0.62 | 0.31 |
| V. anguillarum | 0.62 | 0.15 | ||
| V. cholerae | 1.25 | 0.62 | ||
| V. harveyi | 0.31 | 0.31 | ||
| V. nigripulchritudo | 1.25 | 0.15 | ||
| V. parahaemolyticus | 1.25 | 1.25 | ||
| V. vulnificus | 0.62 | 0.62 | ||
| Pseudomonadales | Acinetobacter | A. baumannii | 1.25 | 2.50 |
| Pseudomonas | P. aeruginosa | 1.25–2.50 | 2.50 | |
| FUNGI | ||||
| Saccharomycetales | Candida | C. kefyr | 1.25 | 1.25 |
| C. tropicalis | 0.62–1.25 | 0.62 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johansen, B.; Duval, R.E.; Sergere, J.-C. Antimicrobial Spectrum of Titroleane™: A New Potent Anti-Infective Agent. Antibiotics 2020, 9, 391. https://doi.org/10.3390/antibiotics9070391
Johansen B, Duval RE, Sergere J-C. Antimicrobial Spectrum of Titroleane™: A New Potent Anti-Infective Agent. Antibiotics. 2020; 9(7):391. https://doi.org/10.3390/antibiotics9070391
Chicago/Turabian StyleJohansen, Bianca, Raphaël E. Duval, and Jean-Christophe Sergere. 2020. "Antimicrobial Spectrum of Titroleane™: A New Potent Anti-Infective Agent" Antibiotics 9, no. 7: 391. https://doi.org/10.3390/antibiotics9070391
APA StyleJohansen, B., Duval, R. E., & Sergere, J.-C. (2020). Antimicrobial Spectrum of Titroleane™: A New Potent Anti-Infective Agent. Antibiotics, 9(7), 391. https://doi.org/10.3390/antibiotics9070391






