Clinical Findings and Antimicrobial Susceptibility of Anaerobic Bacteria Isolated in Bloodstream Infections
Abstract
1. Introduction
2. Results
2.1. Characteristics of Patients
2.2. Isolated Bacteria
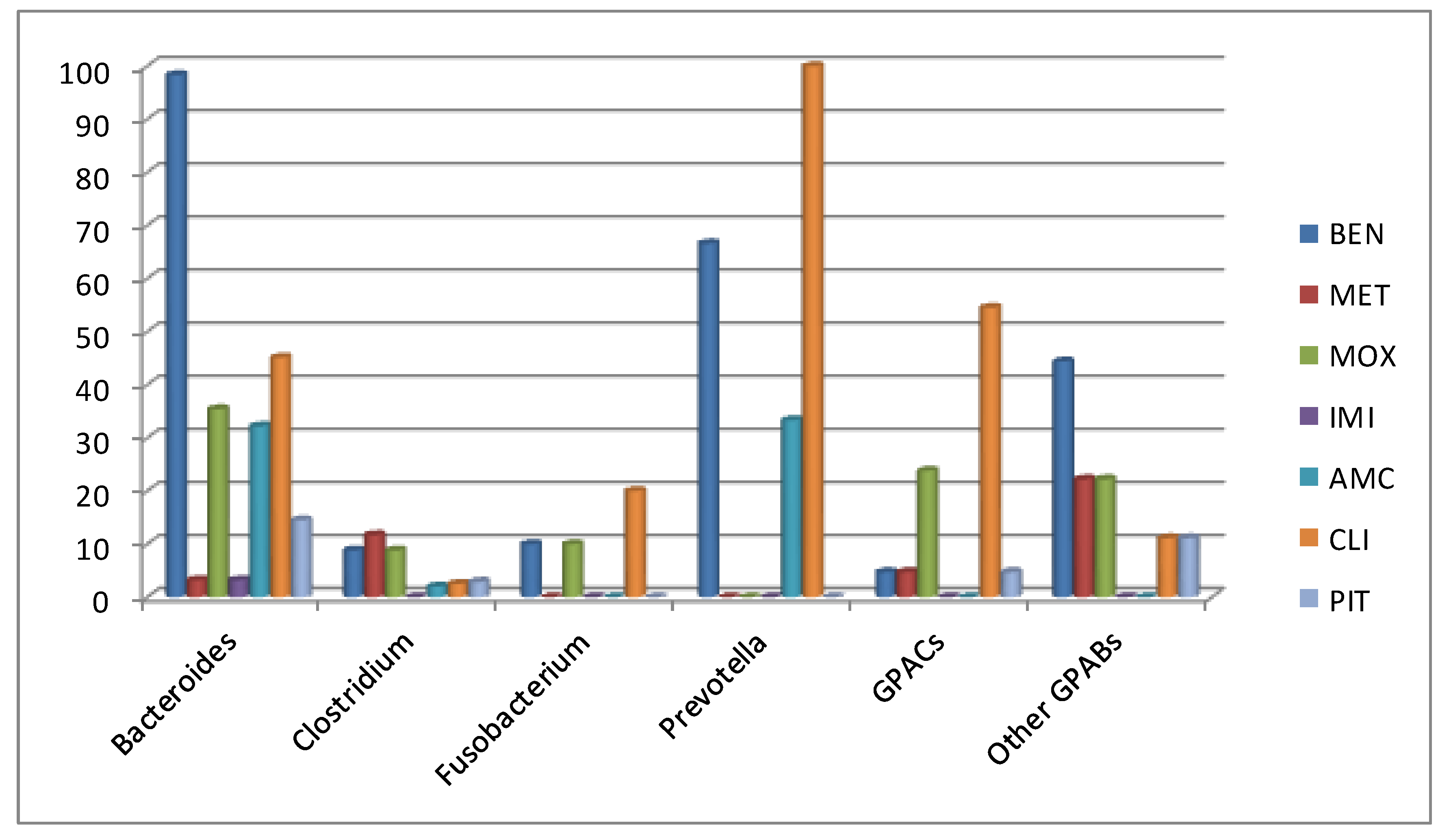
2.3. Antimicrobial Susceptibility
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Patients
5.2. Clinical Definitions
5.3. Microbiology: Isolation and Identification of Strains
5.4. Antimicrobial Susceptibility Testing
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Raymond, N.J.; Blackmore, T.K.; Humble, N.W.; Jones, M.R. Bloodstream infections in a secondary and tertiary care hospital setting. Intern. Med. J. 2006, 36, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Blairon, L.; DeGheldre, Y.; Delaere, B.; Sonet, A.; Bosly, A.; Glupczynski, Y. A 62-month retrospective epidemiological survey of anaerobic bacteremia in a university hospital. Clin. Microbiol. Infect. 2006, 12, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Arzese, A.; Trevisan, R.; Menozzi, M.G. Anaerobe-induced bacteremia in Italy: A nationwide survey. The italian anaerobe study group. Clin. Infect. Dis. 1995, 20, S230–S232. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, E.J.; Citron, D.M.; Goldman, R.J. National hospital survey of anaerobic culture and susceptibility methods: III. Anaerobe 2008, 14, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, E.J.; Citron, D.M. Resistance trends in antimicrobial susceptibility of anaerobic bacteria, part I. Clin. Microbiol. Newsl. 2011, 33, 1–8. [Google Scholar] [CrossRef]
- Smith, A.J.; Lockhart, A.; Tyers, A.; Poxton, I.R. A survey of the identification and susceptibility testing of anaerobes in diagnostic microbiology laboratories in Scotland, UK. J. Antimicrob. Chemother. 2010, 65, 805. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brook, I.; Wexler, H.M.; Goldstein, E.J.C. Antianaerobic antimicrobials: spectrum and susceptibility testing. Clin. Microbiol. Rev. 2013, 26, 526–546. [Google Scholar] [CrossRef] [PubMed]
- Schuetz, A.N. Antimicrobial resistance and susceptibility testing of anaerobic bacteria. Clin. Infect. Dis. 2014, 59, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Finegold, S.M. Anaerobic infections in humans: An overview. Anaerobe 1995, 1, 3–9. [Google Scholar] [CrossRef]
- Wang, F.D.; Liao, C.H.; Lin, Y.T.; Sheng, W.H.; Hsuch, P.R. Trends in the susceptibility of commonly encountered clinically significant anaerobes and susceptibilities of blood isolates of anaerobes to 16 antimicrobial agents, including fidaxomicin and rifaximin, 2008–2012, northern Taiwan. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 2041–2052. [Google Scholar] [CrossRef] [PubMed]
- Ng, L.S.Y.; Kwang, L.L.; Rao, S.; Tan, T.Y. Anaerobic bacteraemia revisited: species and susceptibilities. Ann. Acad. Med. Singap. 2015, 44, 13–18. [Google Scholar] [PubMed]
- Tan, T.Y.; Ng, L.S.Y.; Kwang, L.L.; Rao, S.; Eng, L.C. Clinical characteristics and antimicrobial susceptibilities of anaerobic bacteremia in an acute care hospital. Anaerobe 2017, 43, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, Y.; Park, Y.; Kim, M.; Choi, J.Y.; Yong, D.; Jeong, S.H.; Lee, K. Anaerobic bacteremia: impact of inappropriate therapy on mortality. Infect. Chemother. 2016, 48, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Dumont, Y.; Bonzon, L.; Michon, A.L.; Carriere, C.; Didelot, M.N.; Laurens, C.; Renard, B.; Veloo, A.C.M.; Godreuil, S.; Jean-Pierre, H. Epidemiology and microbiological features of anaerobic bacteremia in two French University hospitals. Anaerobe. (in press).
- Umemura, T.; Hamada, Y.; Yamagishi, Y.; Suematsu, H.; Mikamo, H. Clinical characteristics associated with mortality of patients with anaerobic bacteremia. Anaerobe 2016, 39, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Alauzet, C.; Lozniewski, H.; Marchandin, H. Metronidazole resistance and nim genes in anaerobes: A review. Anaerobe 2019, 55, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Nagy, E.; Becker, S.; Kostrzewa, M.; Barta, N.; Urbán, E. The value of MALDI-TOF MS for the identification of clinically relevant anaerobic bacteria in routine laboratories. J. Med. Microbiol. 2012, 61, 1393–1400. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria, Approved standard, 8th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012; CLSI document M11–A8. [Google Scholar]
- European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 10.0. 2020. Available online: http://eucast.org (accessed on 1 April 2020).
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; National Committee for Clinical Laboratory Standards: Wayne, PA, USA, 2020; CLSI supplement M100. [Google Scholar]
| Characteristics | n (%) |
|---|---|
| Clinical findings * | |
| Fever | 108 (76.5) |
| Digestive symptoms (abdominal pain, diarrhea, vomiting, etc.) | 54 (38.2) |
| Respiratory symptoms (chest pain, cough, dyspnea, etc.) | 18 (12.7) |
| Neurological symptoms (paralysis, loss of conscience, etc.) | 11 (7.8) |
| General symptoms (malaise, asthenia, etc.) | 17 (12) |
| Laboratory results | |
| Increased CRP level | 124 (87.9) |
| Increased Pct level | 32 (66.6) ** |
| Increased WCBC level | 71 (50.3) |
| Underlying diseases or conditions * | |
| Cancer | 54 (39.7) |
| Intestinal or abdominal | 22 (15.6) |
| Hematologic | 17 (12) |
| Other | 17 (12) |
| Surgery | 41 (29) |
| Transplantation | 8 (5.6) |
| Cytotoxic therapy | 31 (21.9) |
| Diabetes mellitus | 33 (23.4) |
| Chronic diseases | 29 (20.5) |
| CT and Atb treatment | 77 (54.6) |
| Treatment | |
| One drug | 109 (77.3) |
| More than one drug | 31 (21.9) |
| Outcome | |
| Favorable | 106 (75.1) |
| Deceased | 35 (24.8) |
| Microorganisms | n | % |
|---|---|---|
| Gram-negative bacilli | 75 | 53.1 |
| Bacteroides | 62 | 43.9 |
| B. fragilis | 42 | 29.7 |
| B. thetaiotaomicron | 8 | 5.6 |
| B. vulgatus | 6 | 4.2 |
| B. uniformis | 3 | 2.1 |
| B. ovatus | 3 | 2.1 |
| Prevotella | 3 | 2.1 |
| P. buccae | 1 | 0.7 |
| P. baroniae | 1 | 0.7 |
| P. intermedia | 1 | 0.7 |
| Fusobacterium | 10 | 7 |
| F. nucleatum | 9 | 6.3 |
| F. necrophorum | 1 | 0.7 |
| Gram-negative cocci | 1 | 0.7 |
| Veilonella parvula | 1 | 0.7 |
| Gram-positive bacilli | 43 | 30.4 |
| Clostridium | 34 | 24.1 |
| C. perfringens | 23 | 16.3 |
| C. clostridioforme | 3 | 2.1 |
| C. septicum | 2 | 1.4 |
| C. baratii | 1 | 0.7 |
| C. butyricum | 1 | 0.7 |
| C. ramosum | 3 | 2.1 |
| C. sordellii | 1 | 0.7 |
| Eggerthella lenta | 4 | 2.8 |
| Propionibacterium lymphophylum | 2 | 1.4 |
| Eggerthia catenaformis | 2 | 1.4 |
| Eubacterium limosum | 1 | 0.7 |
| Gram-positive cocci | 22 | 15.6 |
| Finegoldia magna | 4 | 2.8 |
| Peptoniphilus | 6 | 4.2 |
| P. harei | 5 | 3.5 |
| P. gorbachii | 1 | 0.7 |
| Parvimonas micra | 5 | 3.5 |
| Peptostreptococcus anaerobius | 1 | 0.7 |
| Anaerococcus | 5 | 3.5 |
| Anaerococcus spp | 2 | 1.4 |
| A. lactolyticus | 1 | 0.7 |
| A. tetradius | 1 | 0.7 |
| A. prevotii | 1 | 0.7 |
| Peptococcus niger | 1 | 0.7 |
| Total | 141 | 100 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cobo, F.; Borrego, J.; Gómez, E.; Casanovas, I.; Calatrava, E.; Foronda, C.; Navarro-Marí, J.M. Clinical Findings and Antimicrobial Susceptibility of Anaerobic Bacteria Isolated in Bloodstream Infections. Antibiotics 2020, 9, 345. https://doi.org/10.3390/antibiotics9060345
Cobo F, Borrego J, Gómez E, Casanovas I, Calatrava E, Foronda C, Navarro-Marí JM. Clinical Findings and Antimicrobial Susceptibility of Anaerobic Bacteria Isolated in Bloodstream Infections. Antibiotics. 2020; 9(6):345. https://doi.org/10.3390/antibiotics9060345
Chicago/Turabian StyleCobo, Fernando, Jaime Borrego, Esther Gómez, Isabel Casanovas, Elizabeth Calatrava, Carla Foronda, and José María Navarro-Marí. 2020. "Clinical Findings and Antimicrobial Susceptibility of Anaerobic Bacteria Isolated in Bloodstream Infections" Antibiotics 9, no. 6: 345. https://doi.org/10.3390/antibiotics9060345
APA StyleCobo, F., Borrego, J., Gómez, E., Casanovas, I., Calatrava, E., Foronda, C., & Navarro-Marí, J. M. (2020). Clinical Findings and Antimicrobial Susceptibility of Anaerobic Bacteria Isolated in Bloodstream Infections. Antibiotics, 9(6), 345. https://doi.org/10.3390/antibiotics9060345






