Alkaloids from Marine Fungi: Promising Antimicrobials
Abstract
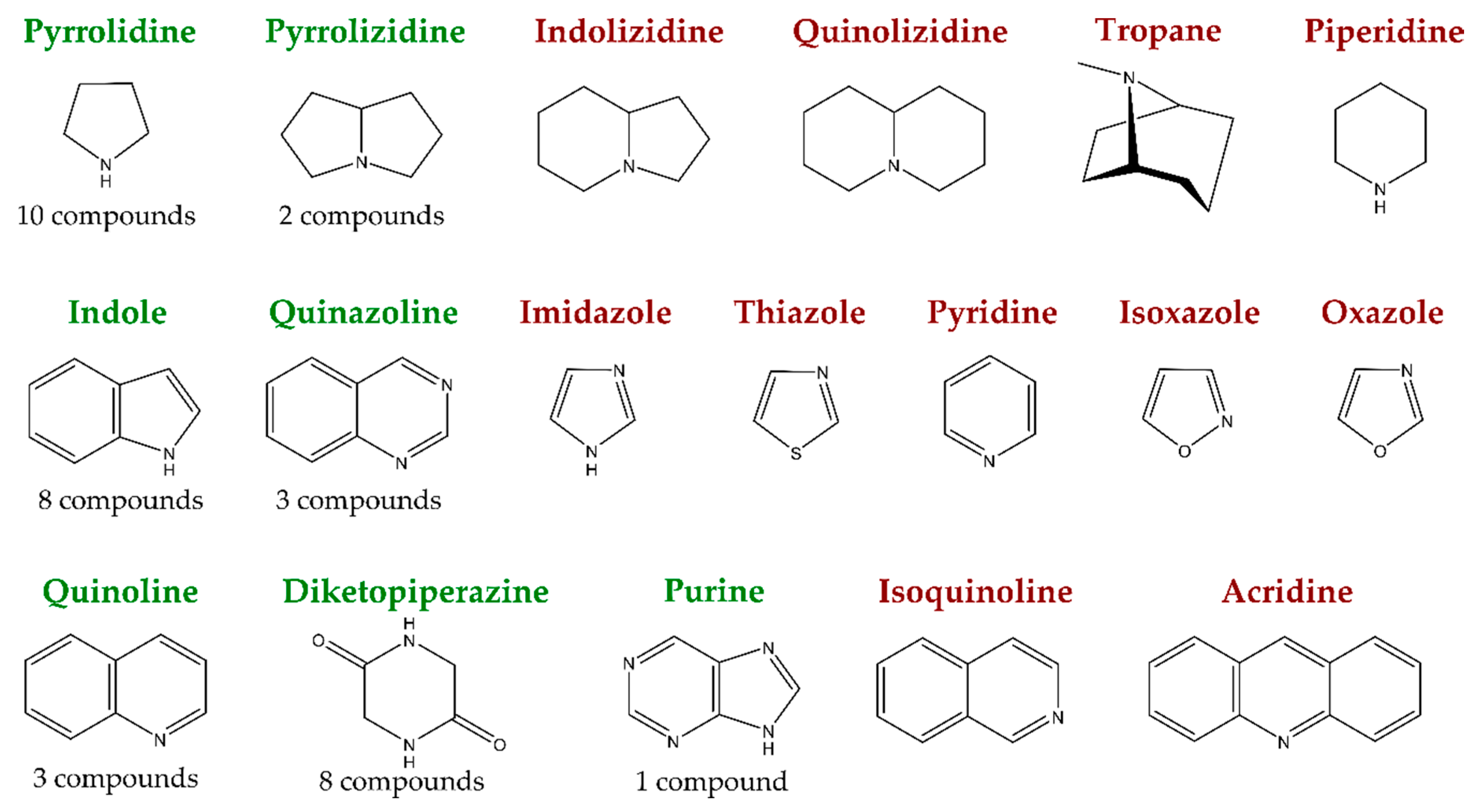
1. Introduction
2. New Antimicrobial Alkaloids from Marine Fungi
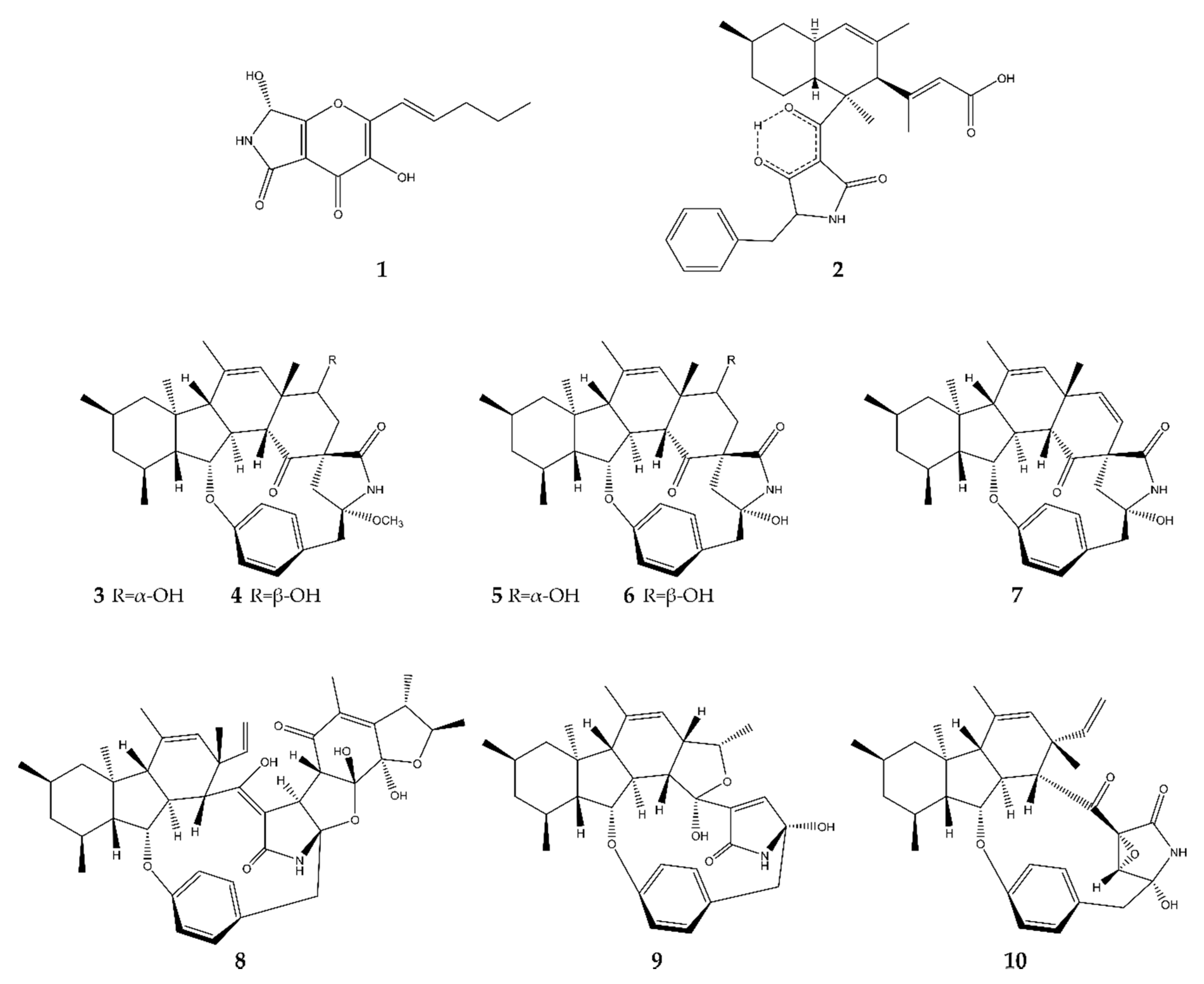
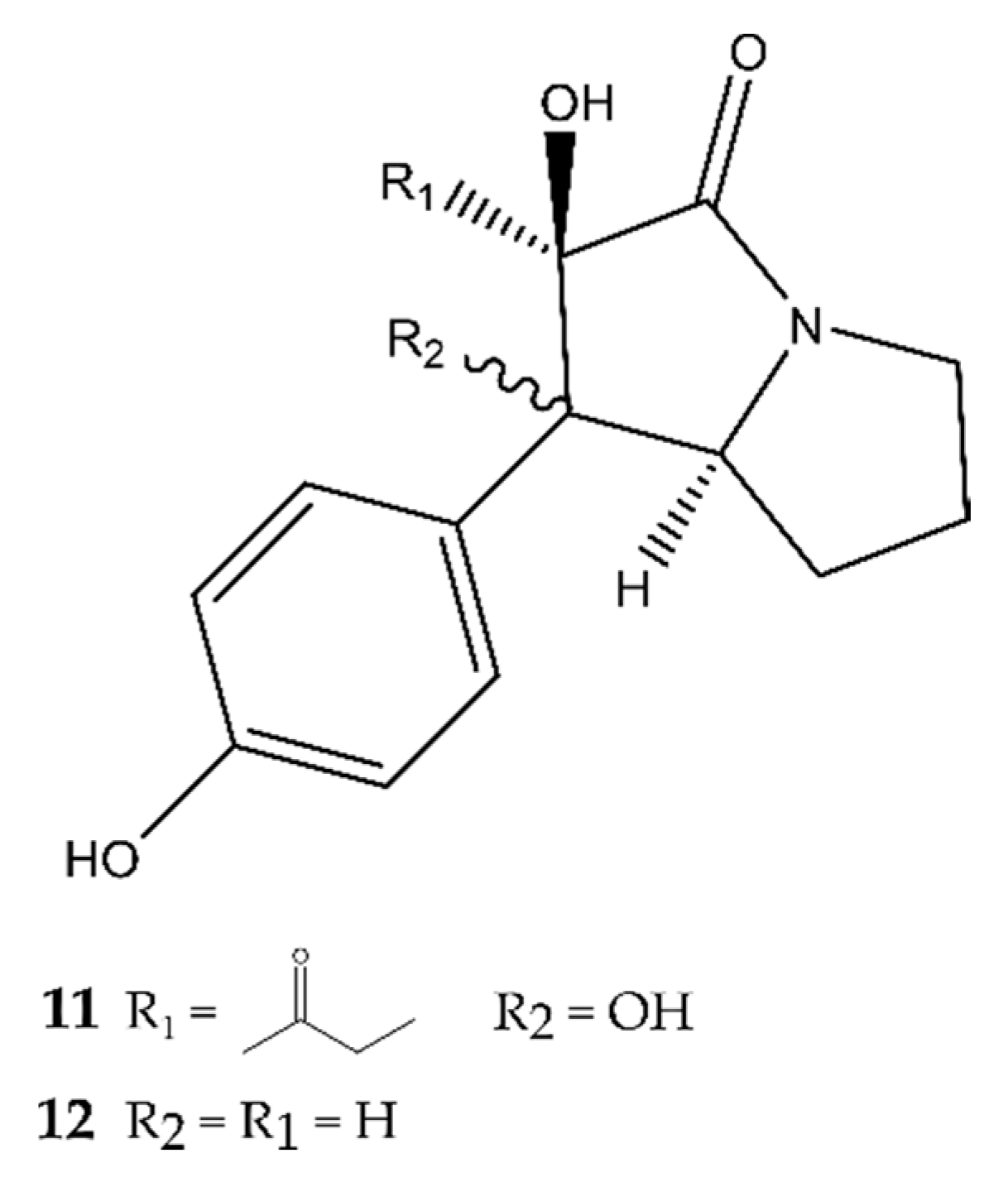
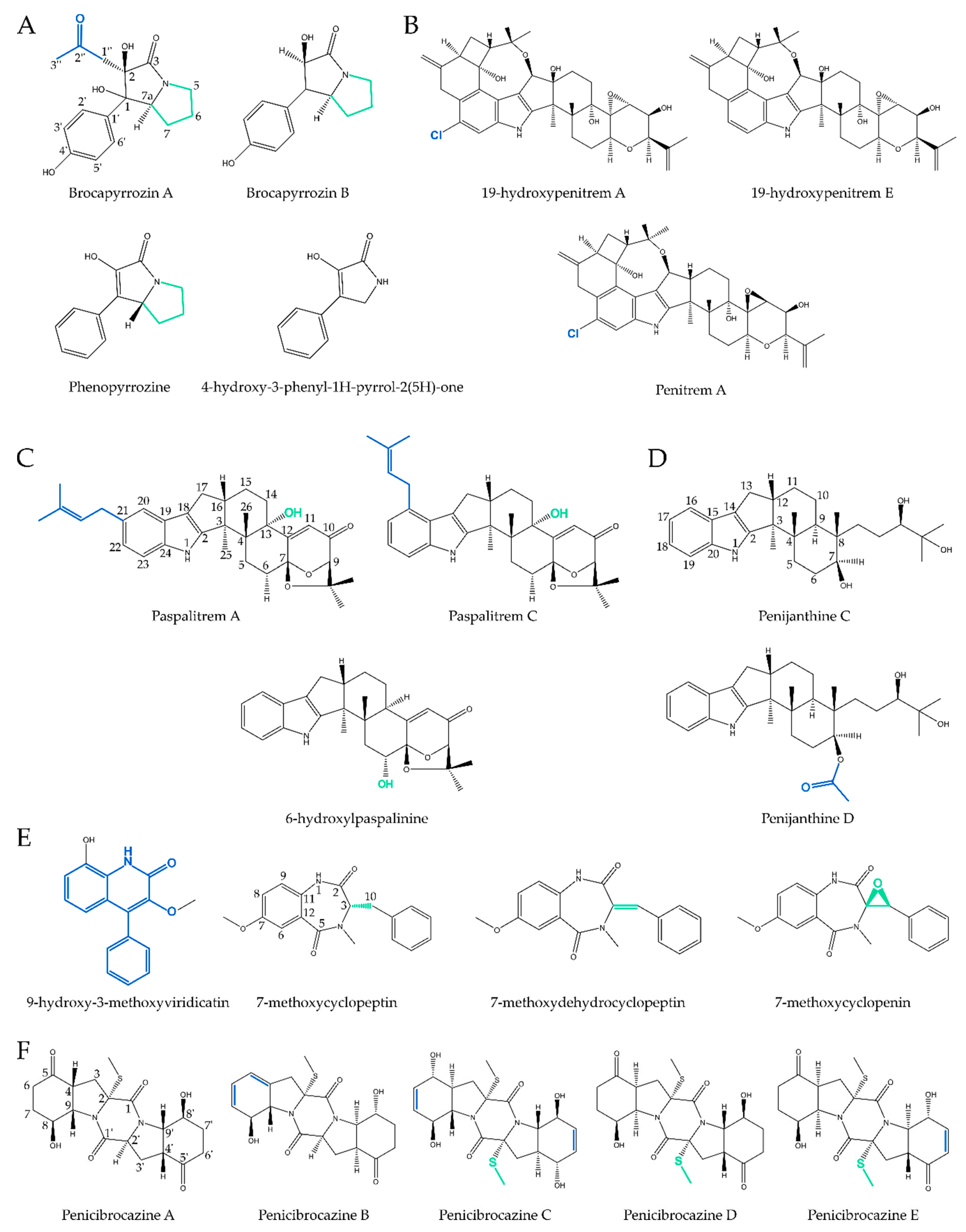
2.1. Pyrrolidines
2.2. Pyrrolizidines
2.3. Indoles
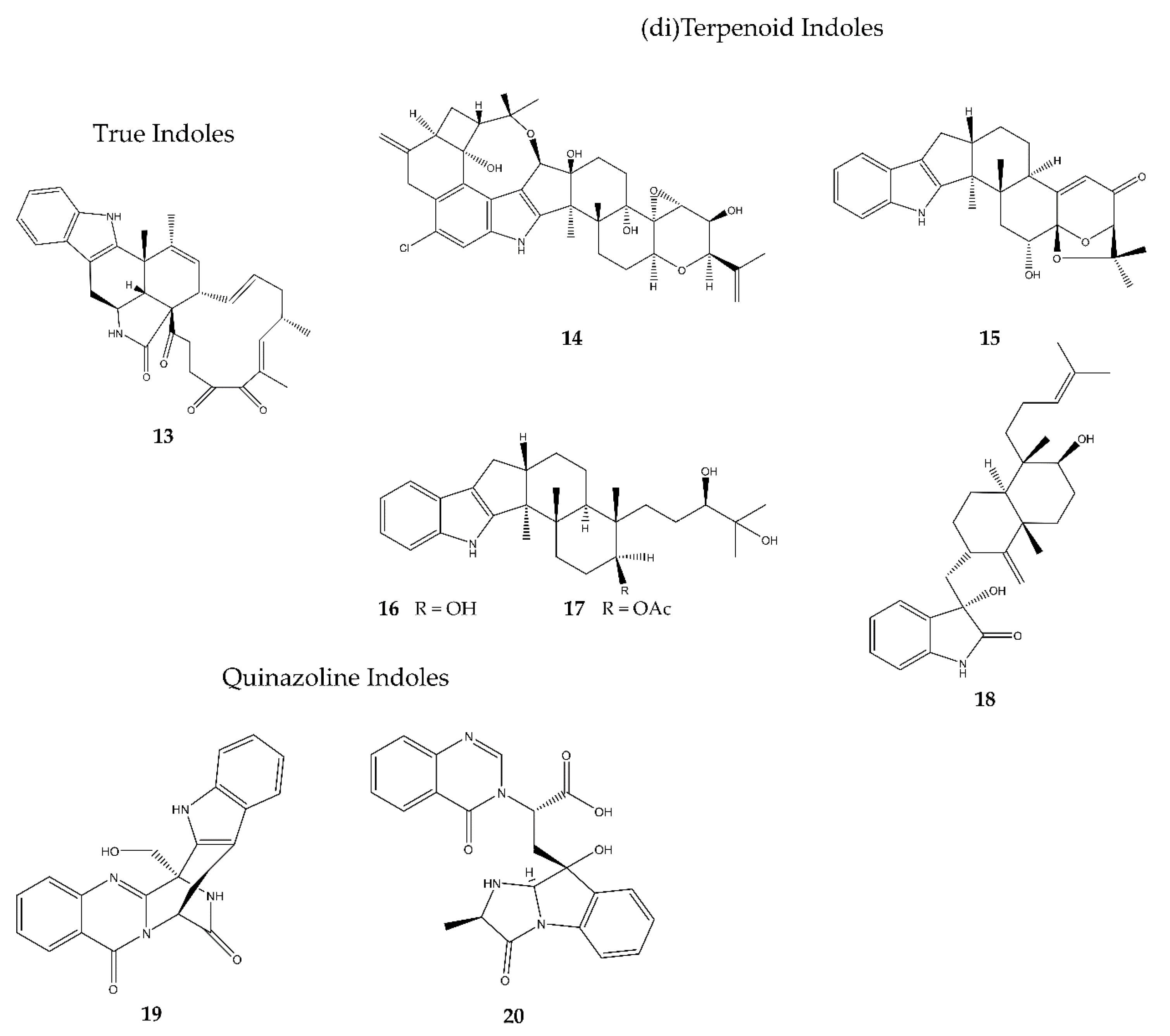
2.3.1. True Indoles
2.3.2. (di)Terpenoid Indoles
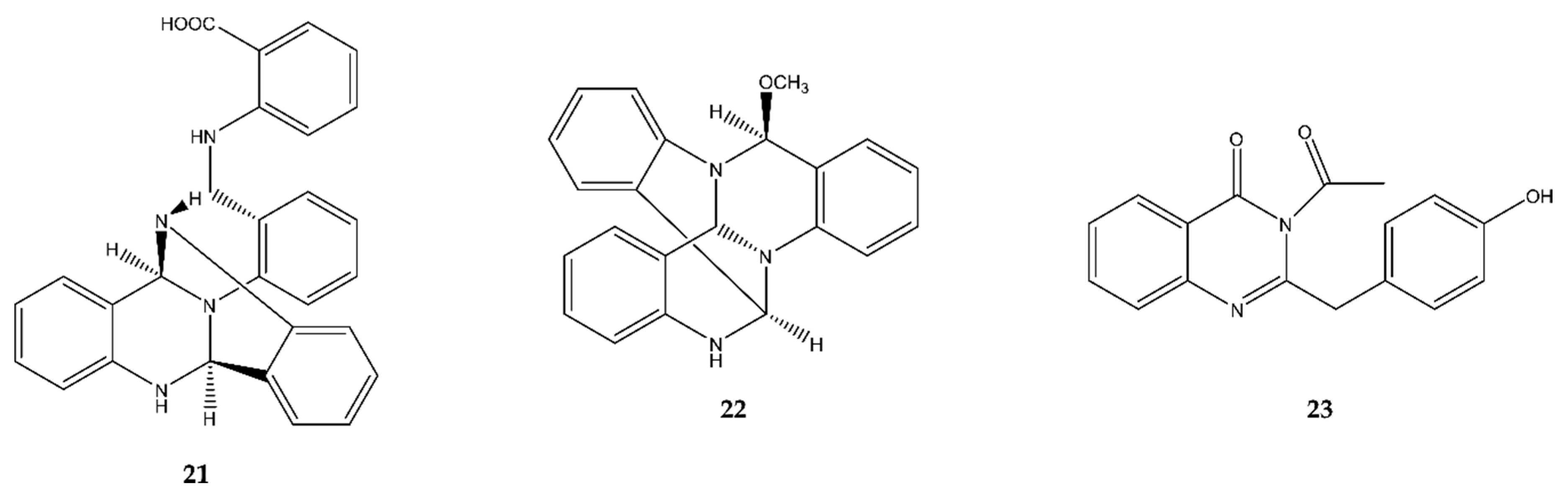
2.3.3. Quinazoline Indoles
2.4. Quinazolines
2.5. Quinolines
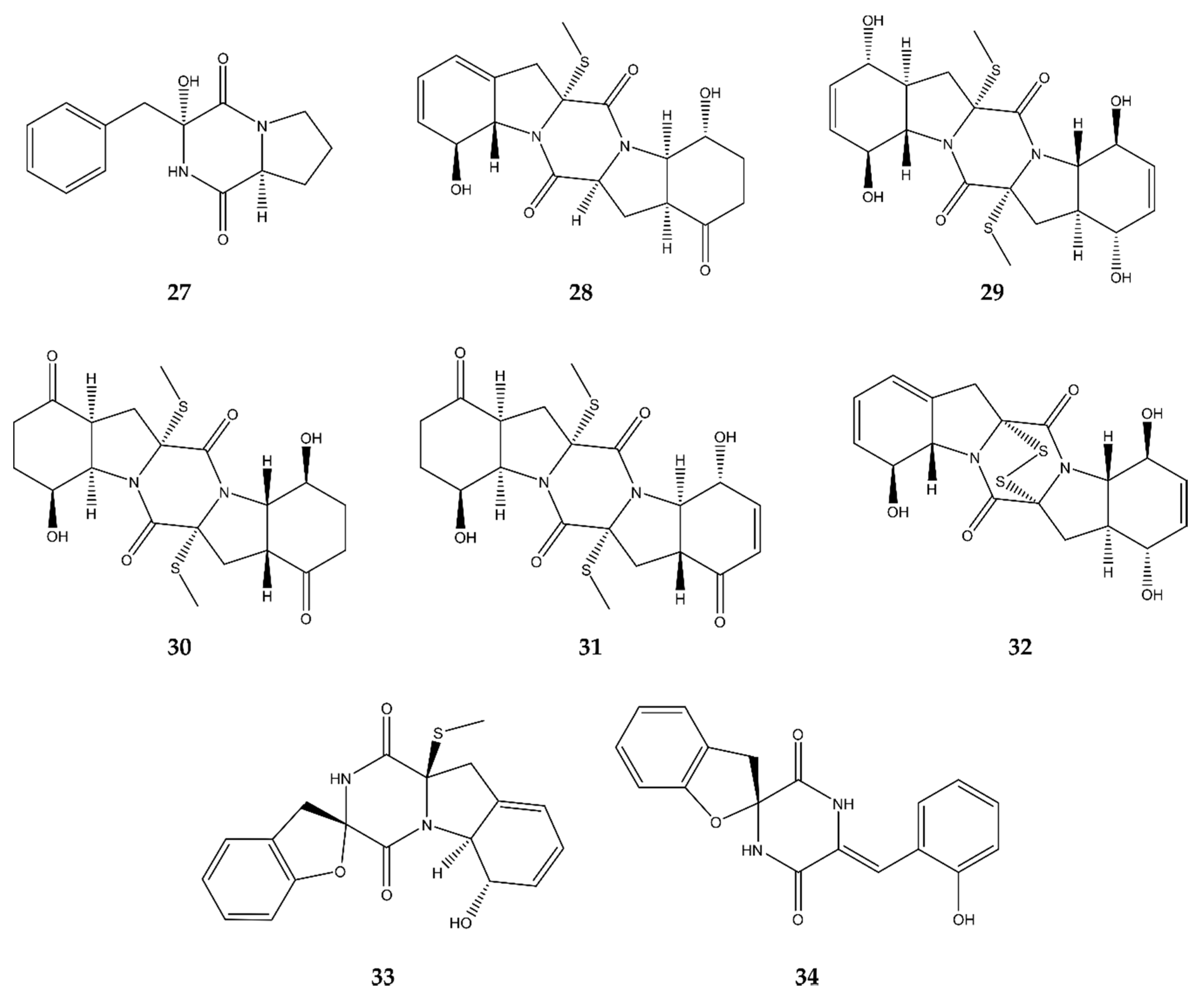
2.6. Diketopiperazines
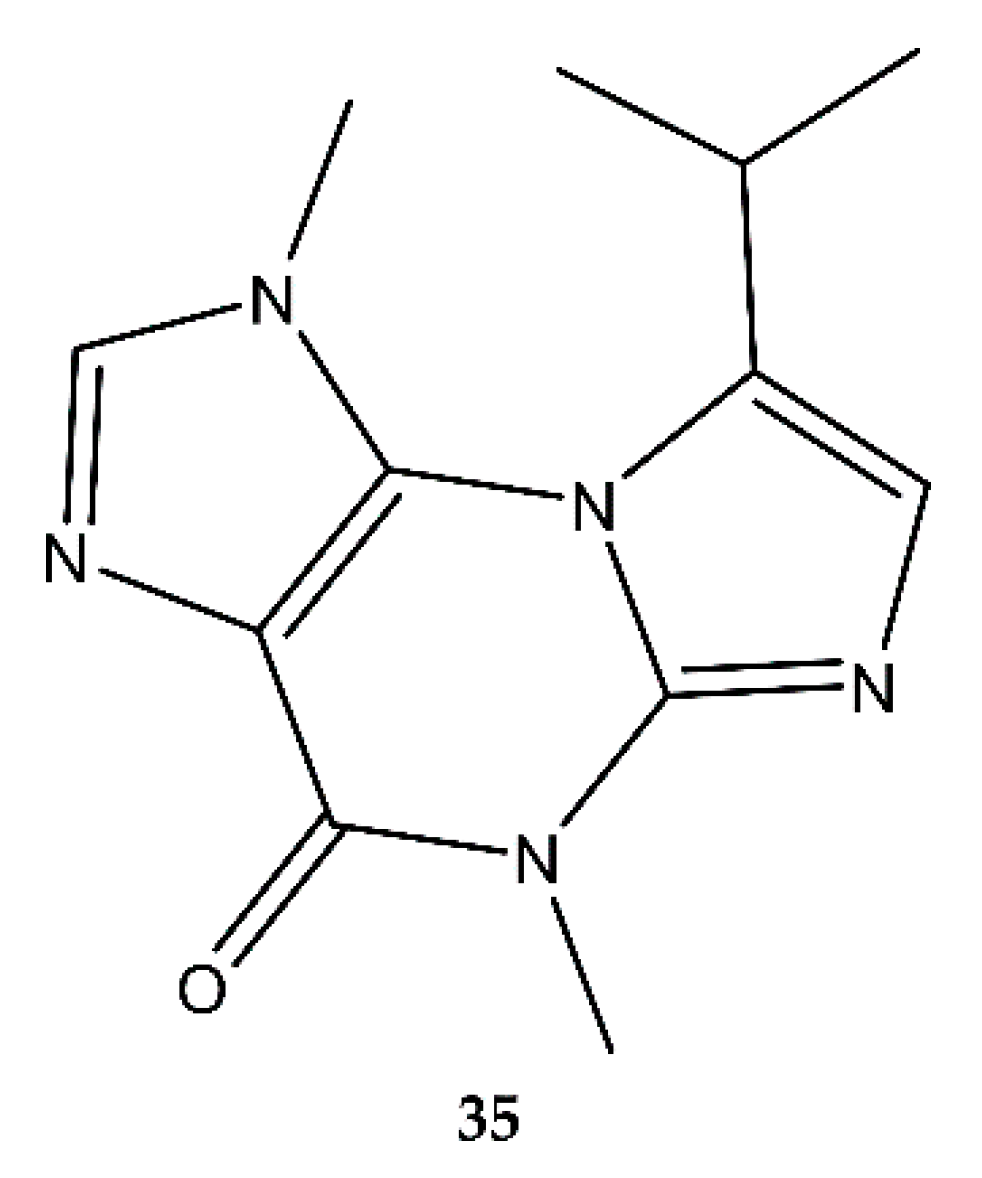
2.7. Purines
3. Natural Product Discovery Strategies for Novel Alkaloids from Marine Fungi
3.1. Isolation from Natrural Producers
3.2. Isolation from Engineered Strains
3.3. Computational Tools
3.4. Compound Identification
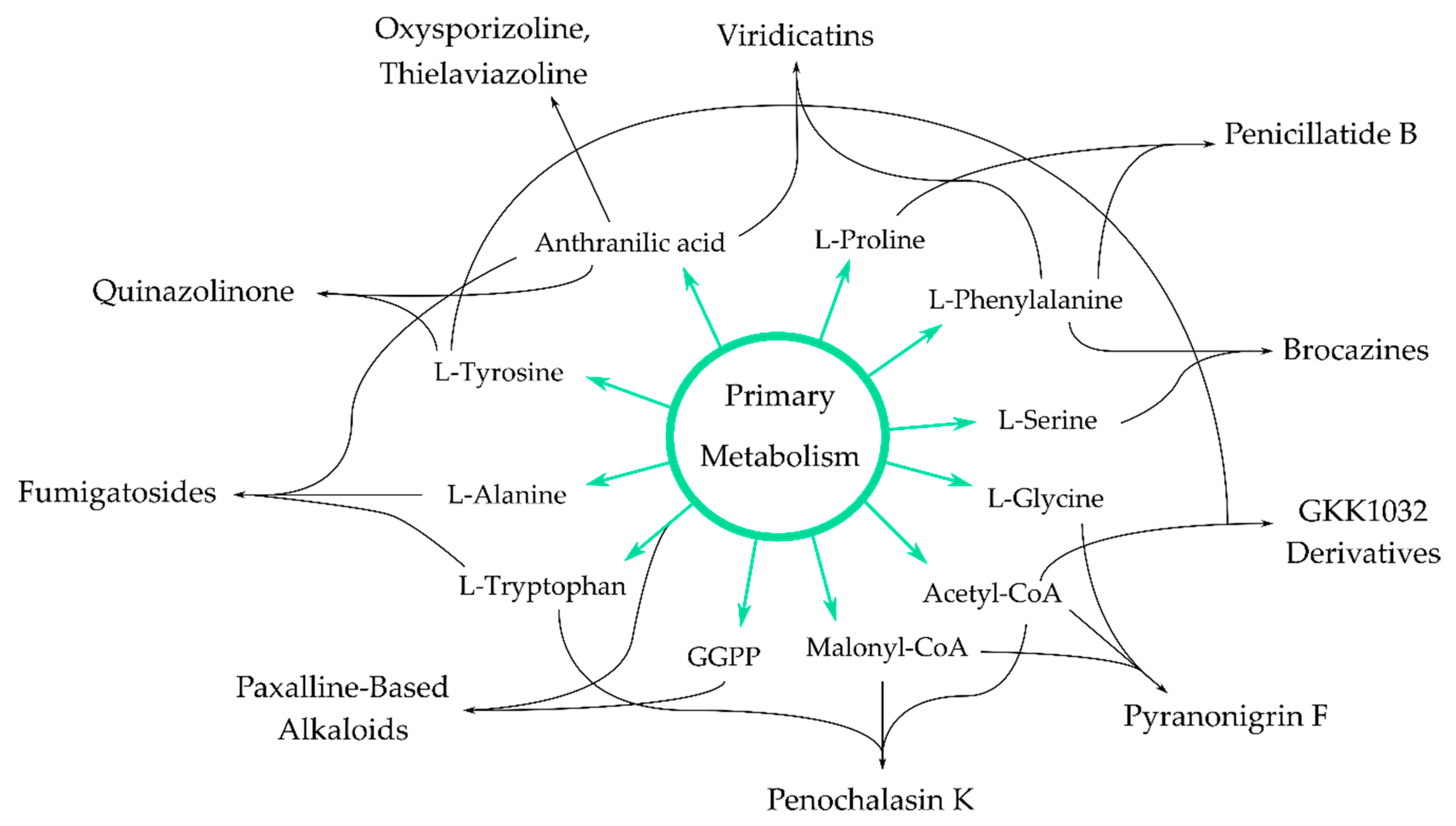
4. Antimicrobial Alkaloids from Marine Fungi and Their Proposed Biochemical Origin
4.1. Pyrrolidines
4.2. Pyrrolizidines
4.3. Indoles
4.4. Quinazolines
4.5. Quinolines
4.6. Diketopiperazines
4.7. Purines
5. Structure–Activity Analysis
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Powers, J.H. Antimicrobial drug development—The past, the present, and the future. Clin. Microbiol. Infect. 2004, 10, 23–31. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Antimicrobial Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 28 April 2020).
- De Mol, M.L.; Snoeck, N.; De Maeseneire, S.L.; Soetaert, W.K. Hidden antibiotics: Where to uncover? Biotechnol. Adv. 2018, 36, 2201–2218. [Google Scholar] [CrossRef] [PubMed]
- Demain, A.L. Importance of microbial natural products and the need to revitalize their discovery. J. Ind. Microbiol. Biotechnol. 2014, 41, 185–201. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Quan, C.; Hou, X.; Fan, S. Potential pharmacological resources: Natural bioactive compounds from marine-derived fungi. Mar. Drugs 2016, 14, 76. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Frank, M.; Yu, X.; Yu, H.; Tran-Cong, N.M.; Gao, Y.; Proksch, P. Secondary metabolites from marine-derived fungi from China. In Progress in the Chemistry of Organic Natural Products; Springer: Cham, Switzerland, 2020; Volume 111, pp. 81–150. ISBN 9783030378646. [Google Scholar]
- Xu, K.; Yuan, X.-L.; Li, C.; Li, X.-D. Recent discovery of heterocyclic alkaloids from marine-derived Aspergillus species. Mar. Drugs 2020, 18, 54. [Google Scholar] [CrossRef]
- Richter, M.F.; Drown, B.S.; Andrew, P.; Garcia, A.; Shirai, T.; Svec, R.L.; Hergenrother, P.J. Predictive compound accumulation rules yield a broad-spectrum antibiotic. Nature 2017, 545, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Mubarak, M.S. Pyrrolidine alkaloids and their promises in pharmacotherapy. Adv. Tradit. Med. 2020, 20, 13–22. [Google Scholar] [CrossRef]
- Netz, N.; Opatz, T. Marine indole alkaloids. Mar. Drugs 2015, 13, 4814–4914. [Google Scholar] [CrossRef]
- Huang, R.; Zhou, X.; Xu, T.; Yang, X.; Liu, Y. Diketopiperazines from marine organisms. Chem. Biodivers 2010, 7, 2809–2829. [Google Scholar] [CrossRef]
- Huang, R.-M.; Yi, X.-X.; Zhou, Y.; Su, X.; Peng, Y.; Gao, C.-H. An update on 2,5-diketopiperazines from marine organisms. Mar. Drugs 2014, 12, 6213–6235. [Google Scholar] [CrossRef]
- Meng, L.-H.; Li, X.-M.; Liu, Y.; Wang, B.-G. Polyoxygenated dihydropyrano[2,3-c]pyrrole-4,5-dione derivatives from the marine mangrove-derived endophytic fungus Penicillium brocae MA-231 and their antimicrobial activity. Chin. Chem. Lett. 2015, 26, 610–612. [Google Scholar] [CrossRef]
- Yamamoto, T.; Tsunematsu, Y.; Noguchi, H.; Hotta, K.; Watanabe, K. Elucidation of Pyranonigrin Biosynthetic Pathway Reveals a Mode of Tetramic Acid, Fused γ-Pyrone, and exo-Methylene Formation. Org. Lett. 2015, 17, 4992–4995. [Google Scholar] [CrossRef]
- Wu, B.; Wiese, J.; Labes, A.; Kramer, A.; Schmaljohann, R.; Imhoff, J.F. Lindgomycin, an unusual antibiotic polyketide from a marine fungus of the Lindgomycetaceae. Mar. Drugs 2015, 13, 4617–4632. [Google Scholar] [CrossRef]
- Song, T.; Chen, M.; Chai, W.; Zhang, Z.; Lian, X.-Y. New bioactive pyrrospirones C−I from a marine-derived fungus Penicillium sp. ZZ380. Tetrahedron 2018, 74, 884–891. [Google Scholar] [CrossRef]
- Song, T.; Chen, M.; Ge, Z.-W.; Chai, W.; Li, X.-C.; Zhang, Z.; Lian, X.-Y. Bioactive Penicipyrrodiether A, an Adduct of GKK1032 Analogue and Phenol A Derivative, from a Marine-Sourced Fungus Penicillium sp. ZZ380. J. Org. Chem. 2018, 83, 13395–13401. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Tang, M.; Ge, H.; Chen, M.; Lian, X.; Zhang, Z. Novel bioactive penicipyrroether A and pyrrospirone J from the marine-derived Penicillium sp. ZZ380. Mar. Drugs 2019, 17, 292. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Li, X.; Zhao, J.; He, N.; Li, Y.; Zhang, T.; Wang, S.; Yu, L.; Xie, Y. GKK1032C, a new alkaloid compound from the endophytic fungus Penicillium sp. CPCC 400817 with activity against methicillin-resistant S. aureus. J. Antibiot. 2019, 72, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.-H.; Li, X.-M.; Liu, Y.; Xu, G.-M.; Wang, B.-G. Antimicrobial alkaloids produced by the mangrove endophyte Penicillium brocae MA-231 using the OSMAC approach. RSC Adv. 2017, 7, 55026–55033. [Google Scholar] [CrossRef]
- Zhu, X.; Zhou, D.; Liang, F.; Wu, Z.; She, Z.; Li, C. Penochalasin K, a new unusual chaetoglobosin from the mangrove endophytic fungus Penicillium chrysogenum V11 and its effective semi-synthesis. Fitoterapia 2017, 123, 23–28. [Google Scholar] [CrossRef]
- Zhang, P.; Li, X.-M.; Li, X.; Wang, B.-G. New indole-diterpenoids from the algal-associated fungus Aspergillus nidulans. Phytochem. Lett. 2015, 12, 182–185. [Google Scholar] [CrossRef]
- Hu, X.-Y.; Meng, L.-H.; Li, X.; Yang, S.-Q.; Li, X.-M.; Wang, B.-G. Three new indole diterpenoids from the sea-anemone-derived fungus Penicillium sp. AS-79. Mar. Drugs 2017, 15, 137. [Google Scholar] [CrossRef]
- Guo, X.-C.; Xu, L.-L.; Yang, R.-Y.; Yang, M.-Y.; Hu, L.-D.; Zhu, H.-J.; Cao, F. Anti-vibrio indole-diterpenoids and C-25 epimeric steroids from the marine-derived fungus Penicillium janthinellum. Front. Chem. 2019, 7, 80. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yi, W.; Ge, H.; Zhang, Z.; Wu, B. A new antimicrobial indoloditerpene from a marine-sourced fungus Aspergillus versicolor ZZ761. Nat. Prod. Res. 2019, 33, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Limbadri, S.; Luo, X.; Lin, X.; Liao, S.; Wang, J.; Zhou, X.; Yang, B.; Liu, Y. Bioactive novel indole alkaloids and steroids from deep sea-derived fungus Aspergillus fumigatus SCSIO 41012. Molecules 2018, 23, 2379. [Google Scholar] [CrossRef]
- Nenkep, V.; Yun, K.; Son, B.W. Oxysporizoline, an antibacterial polycyclic quinazoline alkaloid from the marine-mudflat-derived fungus Fusarium oxysporum. J. Antibiot. 2016, 69, 709–711. [Google Scholar] [CrossRef]
- Leutou, A.S.; Yun, K.; Son, B.W. New production of antibacterial polycyclic quinazoline alkaloid, thielaviazoline, from anthranilic acid by the marine-mudflat-derived fungus Thielavia sp. Nat. Prod. Sci. 2016, 22, 216–219. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Zhang, J.-L.; Li, C.; Mu, X.-G.; Liu, X.-L.; Wang, L.; Zhao, Y.-C.; Zhang, P.; Li, X.-D.; Zhang, X.-X. Antimicrobial Secondary Metabolites from the Seawater-Derived Fungus Aspergillus sydowii SW9. Molecules 2019, 24, 4596. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Shi, Y.; Chen, X.; Chen, C.-T.A.; Tao, X.; Wu, B. New compounds from a hydrothermal vent crab-associated fungus Aspergillus versicolor XZ-4. Org. Biomol. Chem. 2017, 15, 1155–1163. [Google Scholar] [CrossRef]
- de Carvalho, M.P.; Abraham, W.-R. Antimicrobial and Biofilm Inhibiting Diketopiperazines. Curr. Med. Chem. 2012, 19, 3564–3577. [Google Scholar] [CrossRef]
- Youssef, D.T.A.; Alahdal, A.M. Cytotoxic and antimicrobial compounds from the marine-derived fungus, Penicillium species. Molecules 2018, 23, 394. [Google Scholar] [CrossRef]
- Meng, L.-H.; Zhang, P.; Li, X.-M.; Wang, B.-G. Penicibrocazines A-E, five new sulfide diketopiperazines from the marine-derived endophytic fungus Penicillium brocae. Mar. Drugs 2015, 13, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.-H.; Wang, C.-Y.; Mándi, A.; Li, X.-M.; Hu, X.-Y.; Kassack, M.U.; Kurtán, T.; Wang, B.-G. Three Diketopiperazine Alkaloids with Spirocyclic Skeletons and One Bisthiodiketopiperazine Derivative from the Mangrove-Derived Endophytic Fungus Penicillium brocae MA-231. Org. Lett. 2016, 18, 5304–5307. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Qin, X.; Lin, X.; Kaliyaperumal, K.; Zhou, X.; Liu, J.; Ju, Z.; Tu, Z.; Liu, Y. Sydoxanthone C and acremolin B produced by deep-sea-derived fungus Aspergillus sp. SCSIO Ind09F01. J. Antibiot. 2015, 68, 703–706. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Luo, D.; Huang, J.; Wang, L.; Zhang, F.; Xi, T.; Liao, J.; Lu, Y. Antibacterial constituents from Antarctic fungus, Aspergillus sydowii SP-1. Nat. Prod. Res. 2018, 32, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Banert, K.; Tantillo, D.J. A problem in the structure assignment of acremolin C, which is most probably identical with acremolin B. Nat. Prod. Res. 2019, 33, 3011–3015. [Google Scholar] [CrossRef]
- Liu, S.; Li, J.; Wu, Y.; Ren, Y.; Liu, Q.; Wang, Q.; Zhou, X.; Cai, M.; Zhang, Y. De novo transcriptome sequencing of marine-derived Aspergillus glaucus and comparative analysis of metabolic and developmental variations in response to salt stress. Genes Genom. 2017, 39, 317–329. [Google Scholar] [CrossRef]
- Gu, Y.; Ding, P.; Liang, Z.; Song, Y.; Liu, Y.; Chen, G.; Li, J.L. Activated production of silent metabolites from marine-derived fungus Penicillium citrinum. Fitoterapia 2018, 127, 207–211. [Google Scholar] [CrossRef]
- Onaka, H. Novel antibiotic screening methods to awaken silent or cryptic secondary metabolic pathways in actinomycetes. J. Antibiot. 2017, 70, 865–870. [Google Scholar] [CrossRef]
- Lodhi, A.F.; Zhang, Y.; Adil, M.; Deng, Y. Antibiotic discovery: Combining isolation chip (iChip) technology and co-culture technique. Appl. Microbiol. Biotechnol. 2018, 102, 7333–7341. [Google Scholar] [CrossRef]
- Bergmann, S.; Schümann, J.; Scherlach, K.; Lange, C.; Brakhage, A.A.; Hertweck, C. Genomics-driven discovery of PKS-NRPS hybrid metabolites from Aspergillus nidulans. Nat. Chem. Biol. 2007, 3, 213–217. [Google Scholar] [CrossRef]
- Clevenger, K.D.; Bok, J.W.; Ye, R.; Miley, G.P.; Verdan, M.H.; Velk, T.; Chen, C.; Yang, K.H.; Robey, M.T.; Gao, P.; et al. A scalable platform to identify fungal secondary metabolites and their gene clusters. Nat. Chem. Biol. 2017, 13, 895–901. [Google Scholar] [CrossRef]
- Deng, H.; Bai, Y.; Fan, T.-P.; Zheng, X.; Cai, Y. Advanced strategy for metabolite exploration in filamentous fungi. Crit. Rev. Biotechnol. 2020, 40, 180–198. [Google Scholar] [CrossRef] [PubMed]
- Overy, D.; Rämä, T.; Oosterhuis, R.; Walker, A.; Pang, K.-L. The Neglected Marine Fungi, Sensu stricto, and Their Isolation for Natural Products’ Discovery. Mar. Drugs 2019, 17, 42. [Google Scholar] [CrossRef] [PubMed]
- Van Der Hooft, J.J.J.; Mohimani, H.; Dorrestein, P.C.; Duncan, K.R.; Bauermeister, A. Linking genomics and metabolomics to chart specialized metabolic diversity. R. Soc. Chem. 2020, 49, 3297–3314. [Google Scholar] [CrossRef] [PubMed]
- Ambrosino, L.; Tangherlini, M.; Colantuono, C.; Esposito, A.; Sangiovanni, M.; Miralto, M.; Sansone, C.; Chiusano, M.L. Bioinformatics for marine products: An overview of resources, bottlenecks, and perspectives. Mar. Drugs 2019, 17, 576. [Google Scholar] [CrossRef]
- Tang, M.-C.; Zou, Y.; Yee, D.; Tang, Y. Identification of the pyranonigrin A biosynthetic gene cluster by genome mining in Penicillium thymicola IBT 5891. Aiche J. 2018, 64, 4182–4186. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Tagami, K.; Minami, A.; Matsumoto, T.; Frisvad, J.C.; Suzuki, H.; Ishikawa, J.; Gomi, K.; Oikawa, H. Reconstitution of biosynthetic machinery for the synthesis of the highly elaborated indole diterpene penitrem. Angew. Chem. Int. Ed. Engl. 2015, 54, 5748–5752. [Google Scholar] [CrossRef]
- Medema, M.H.; Fischbach, M.A. Computational approaches to natural product discovery. Nat. Chem. Biol. 2015, 11, 639–648. [Google Scholar] [CrossRef]
- Yun, C.S.; Motoyama, T.; Osada, H. Biosynthesis of the mycotoxin tenuazonic acid by a fungal NRPS-PKS hybrid enzyme. Nat. Commun. 2015, 6, 8758. [Google Scholar] [CrossRef]
- El-Gendy, B.E.D.M.; Rateb, M.E. Antibacterial activity of diketopiperazines isolated from a marine fungus using t-butoxycarbonyl group as a simple tool for purification. Bioorganic Med. Chem. Lett. 2015, 25, 3125–3128. [Google Scholar] [CrossRef]
- Aniszewski, T. Alkaloids—Secrets of Life, 1st ed.; Elsevier Science: Amsterdam, The Netherlands, 2007; ISBN 9780444527363. [Google Scholar]
- Diana, P.; Cirrincione, G. Biosynthesis of Heterocycles: From Isolation to Gene Cluster, 1st ed.; Wiley: Hoboken, NJ, USA, 2015; ISBN 9781118028674. [Google Scholar]
- Xu, W.; Tang, Y. Biosynthesis of Fungal Indole Alkaloids. Nat. Prod. Rep. 2014, 31, 1474–1487. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.; Guthridge, K.; Vassiliadis, S.; Hemsworth, J.; Hettiarachchige, I.; Spangenberg, G.; Rochfort, S. Tremorgenic mycotoxins: Structure diversity and biological activity. Toxins 2019, 11, 302. [Google Scholar] [CrossRef]
- Welch, T.R.; Williams, R.M. Epidithiodioxopiperazines occurence synthesis and biogenesis. Nat. Prod. Rep. 2014, 31, 1376–1404. [Google Scholar] [CrossRef] [PubMed]
- Awakawa, T.; Yang, X.-L.; Wakimoto, T.; Abe, I. Pyranonigrin E: A PKS-NRPS hybrid metabolite from Aspergillus niger identified by genome mining. ChemBioChem 2013, 14, 2095–2099. [Google Scholar] [CrossRef] [PubMed]
- Riko, R.; Nakamura, H.; Shindo, K. Studies on pyranonigrins-isolation of pyranonigrin E and biosynthetic studies on pyranonigrin A. J. Antibiot. 2014, 67, 179–181. [Google Scholar] [CrossRef]
- Oikawa, H. Biosynthesis of structurally unique fungal metabolite GKK1032A 2: Indication of novel carbocyclic formation mechanism in polyketide biosynthesis. J. Org. Chem. 2003, 68, 3552–3557. [Google Scholar] [CrossRef] [PubMed]
- Tadano, K.-I. Our recent progress on the intramolecular Diels-Alder reaction approach in natural products synthesis: Synthetic studies of the octahydronaphthalene substructure of versipelostatins and the A/B/C-tricyclic substructure of GKK1032s. Chem. Rec. 2014, 14, 623–640. [Google Scholar] [CrossRef]
- Nagai, S.; Yamagishi, Y.; Shimizu, Y.; Takao, K.-I.; Tadano, K.-I. An access to the 13-membered cyclophane substructure in GKK1032AS: An intramolecular 1,4-addition approach. Heterocycles 2015, 90, 819–826. [Google Scholar]
- Sugata, H.; Inagaki, K.; Ode, T.; Hayakawa, T.; Karoji, Y.; Baba, M.; Kato, R.; Hasegawa, D.; Tsubogo, T.; Uchiro, H. Total Synthesis of GKK1032A2 via Direct 13-Membered Macrocyclization Using a Nucleophilic Aromatic Substitution of an (η6-Arene)Chromium Complex. Chem. Asian J. 2017, 12, 628–632. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Sun, Y.-P.; Sarlah, D.; Zhan, W.; Wu, T.R. Bioinspired Synthesis of Hirsutellones A, B and C. Org. Lett. 2011, 13, 5708–5710. [Google Scholar] [CrossRef]
- Uchiro, H.; Kato, R.; Arai, Y.; Hasegawa, M.; Kobayakawa, Y. Total synthesis of hirsutellone B via ullmann-type direct 13-membered macrocyclization. Org. Lett. 2011, 13, 6268–6271. [Google Scholar] [CrossRef] [PubMed]
- Halvorsen, G.T.; Roush, W.R. Stereoselective synthesis of the decahydrofluorene core of the hirsutellones. Tetrahedron Lett. 2011, 52, 2072–2075. [Google Scholar] [CrossRef] [PubMed]
- Reber, K.P.; Tilley, S.D.; Carson, C.A.; Sorensen, E.J. Toward a synthesis of hirsutellone B by the concept of double cyclization. J. Org. Chem. 2013, 78, 9584–9607. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Huang, C.; Huang, M.; Liu, B. Toward the synthesis of hirsutellone B via an intramolecular Diels-Alder/ketene-trapping strategy. Tetrahedron 2015, 71, 3603–3608. [Google Scholar] [CrossRef]
- Sugata, H.; Kato, R.; Tsubogo, T.; Uchiro, H. A chirality-returning sequence for the synthesis of an unnatural isomer of hirsutellone B. Asian J. Org. Chem. 2017, 6, 609–618. [Google Scholar] [CrossRef]
- Abdelkafi, H.; Evanno, L.; Deville, A.; Dubost, L.; Chiaroni, A.; Nay, B. Synthesis of naturally occurring cyclohexene rings using stereodirected intramolecular Diels-Alder reactions through asymmetric 1,3-dioxane tethering. Eur. J. Org. Chem. 2011, 2789–2800. [Google Scholar] [CrossRef]
- Tanaka, R.; Ohishi, K.; Takanashi, N.; Nagano, T.; Suizu, H.; Suzuki, T.; Kobayashi, S. Synthetic study of pyrrocidines: First entry to the decahydrofluorene core of pyrrocidines. Org. Lett. 2012, 14, 4886–4889. [Google Scholar] [CrossRef]
- Li, X.-W.; Ear, A.; Nay, B. Hirsutellones and beyond: Figuring out the biological and synthetic logics toward chemical complexity in fungal PKS-NRPS compounds. Nat. Prod. Rep. 2013, 30, 765–782. [Google Scholar] [CrossRef]
- Shiomi, K.; Yang, H.; Xu, Q.; Arai, N.; Namiki, M.; Hayashi, M.; Inokoshi, J.; Takeshima, H.; Masuma, R.; Komiyama, K.; et al. Phenopyrrozin, a New Radical Scavenger Produced by Penicillium sp. FO-2047. J. Antibiot. 1995, 48, 1413–1418. [Google Scholar] [CrossRef]
- Kothapalli, Y.; Puthukanoori, R.K.; Alapati, S.R. Enantiospecific first total synthesis of 7a(S)-p-hydroxyphenopyrrozin. Tetrahedron Lett. 2012, 53, 1891–1893. [Google Scholar] [CrossRef]
- Cusumano, A.Q.; Boudreau, M.W.; Pierce, J.G. Direct Access to Highly Functionalized Heterocycles through the Condensation of Cyclic Imines and α-Oxoesters. J. Org. Chem. 2017, 82, 13714–13721. [Google Scholar] [CrossRef] [PubMed]
- Oikawa, H.; Murakami, Y.; Ichihara, A. 20-Ketoreductase Activity of Chaetoglobosin A and Prochaetoglobosins in a Cell-free System of Chaetomium subaffine and the Isolation of New Chaetoglobosins. Biosci. Biotechnol. Biochem. 1993, 57, 628–631. [Google Scholar] [CrossRef]
- Cui, C.-M.; Li, X.-M.; Li, C.-S.; Proksch, P.; Wang, B.-G. Cytoglobosins A-G, cytochalasans from a marine-derived endophytic Fungus, chaetomium globosum QEN-14. J. Nat. Prod. 2010, 73, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, Y.; Qin, J.; Qu, X.; Liu, J.; Li, X.; Pan, H. Antifungal Metabolites Produced by Chaetomium globosum No.04, an Endophytic Fungus Isolated from Ginkgo biloba. Indian J. Microbiol. 2013, 53, 175–180. [Google Scholar] [CrossRef]
- Schümann, J.; Hertweck, C. Molecular basis of cytochalasan biosynthesis in fungi: Gene cluster analysis and evidence for the involvement of a PKS-NRPS hybrid synthase by RNA silencing. J. Am. Chem. Soc. 2007, 129, 9564–9565. [Google Scholar] [CrossRef]
- Ishiuchi, K.; Nakazawa, T.; Yagishita, F.; Mino, T.; Noguchi, H.; Hotta, K.; Watanabe, K. Combinatorial generation of complexity by redox enzymes in the chaetoglobosin A biosynthesis. J. Am. Chem. Soc. 2013, 135, 7371–7377. [Google Scholar] [CrossRef]
- Nicholson, M.J.; Koulman, A.; Monahan, B.J.; Pritchard, B.L.; Payne, G.A.; Scott, B. Identification of two aflatrem biosynthesis gene loci in Aspergillus flavus and metabolic engineering of Penicillium paxilli to elucidate their function. Appl. Environ. Microbiol. 2009, 75, 7469–7481. [Google Scholar] [CrossRef]
- Itabashi, T.; Hosoe, T.; Wakana, D.; Fukushima, K.; Takizawa, K.; Yaguchi, T.; Okada, K.; De Campos Takaki, G.M.; Kawai, K. A new indoloditerpene derivative, penijanthine A, isolated from Penicillium janthinellum. J. Nat. Med. 2009, 63, 96–99. [Google Scholar] [CrossRef]
- Su, S.-S.; Song, A.-H.; Chen, G.; Wang, H.-F.; Li, Z.-Q.; Pei, Y.-H. Two new indole-diterpenoids from the fungus Penicillium crustosum YN-HT-15. J. Asian Nat. Prod. Res. 2014, 16, 285–289. [Google Scholar] [CrossRef]
- Kimura, Y.; Nishibe, M.; Nakajima, H.; Hamasaki, T.; Shigemitsu, N.; Sugawara, F.; Stout, T.J.; Clardy, J. Emeniveol; a new pollen growth inhibitor from the fungus Emericella nivea. Tetrahedron Lett. 1992, 33, 6987–6990. [Google Scholar] [CrossRef]
- Shimokawa, K.; Smith, A.B. Total synthesis of the pollen-growth inhibitor (-)-emeniveol. Assignement of absolute stereochemistry. Tetrahedron Lett. 1993, 34, 7383–7384. [Google Scholar] [CrossRef]
- Zou, Y.; Smith, A.B. Total synthesis of architecturally complex indole terpenoids: Strategic and tactical evolution. J. Antibiot. 2018, 71, 185–204. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wei, X.; Kim, E.L.; Lin, X.; Yang, X.-W.; Zhou, X.; Yang, B.; Jung, J.H.; Liu, Y. Fumigatosides A-D_Retracted. Org. Lett. 2014, 16, 2574. [Google Scholar] [CrossRef] [PubMed]
- Resende, D.I.S.P.; Boonpothong, P.; Sousa, E.; Kijjoa, A.; Pinto, M.M.M. Chemistry of the fumiquinazolines and structurally related alkaloids. Nat. Prod. Rep. 2019, 36, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.D.; Liu, X.; Walsh, C.T. Enzymatic Processing of Fumiquinazoline F: A Tandem Oxidative-Acylation Strategy for the Generation of Multicyclic Scaffolds in Fungal Indole Alkaloid Biosynthesis. Biochemistry 2010, 49, 8564–8576. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Chooi, Y.-H.; Ames, B.D.; Wang, P.; Walsh, C.T.; Tang, Y. Fungal Indole Alkaloid Biosynthesis: Genetic and Biochemical Investigation of Tryptoquialanine Pathway in Penicillium aethiopicum. J. Am. Chem. Soc. 2011, 133, 2729–2741. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Fanwick, P.E.; Cushman, M. Synthesis, crystal structure, and conversion of the polycyclic tris-anhydrotetramer of O-aminobenzaldehyde to Cu(TAAB)2+. Synth. Commun. 2004, 34, 3901–3907. [Google Scholar] [CrossRef]
- Ma, C.; Li, Y.; Niu, S.; Zhang, H.; Liu, X.; Che, Y. N-Hydroxypyridones, phenylhydrazones, and a quinazolinone from isaria farinosa. J. Nat. Prod. 2011, 74, 32–37. [Google Scholar] [CrossRef]
- Li, C.-S.; An, C.-Y.; Li, X.-M.; Gao, S.-S.; Cui, C.-M.; Sun, H.-F.; Wang, B.-G. Triazole and dihydroimidazole alkaloids from the marine sediment-derived fungus Penicillium paneum SD-44. J. Nat. Prod. 2011, 74, 1331–1334. [Google Scholar] [CrossRef]
- Ishikawa, N.; Tanaka, H.; Koyama, F.; Noguchi, H.; Wang, C.C.C.; Hotta, K.; Watanabe, K. Non-Heme Dioxygenase Catalyzes Atypical Oxidations of 6,7-Bicyclic Systems to Form the 6,6-Quinolone Core of Viridicatin-Type Fungal Alkaloids. Angew. Chem. Int. Ed. Engl. 2014, 53, 12880–12884. [Google Scholar] [CrossRef]
- Zou, Y.; Zhan, Z.; Li, D.; Tang, M.; Watanabe, K.; Tang, Y. Tandem Prenyltransferases Catalyze Isoprenoid Elongation and Complexity Generation in Biosynthesis of Quinolone Alkaloids. J. Am. Chem. Soc. 2015, 137, 4980–4983. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, S.; Hara, K.; Hashimoto, H.; Hirayama, Y.; Champagne, P.A.; Houk, K.N.; Tang, Y.; Watanabe, K. Enzymatic one-step ring contraction for quinolone biosynthesis. Nat. Commun. 2018, 9, 2826. [Google Scholar] [CrossRef] [PubMed]
- Scherlach, K.; Hertweck, C. Discovery of aspoquinolones A-D, prenylated quinoline-2-one alkaloids from Aspergillus nidulans, motivated by genome mining. Org. Biomol. Chem. 2006, 4, 3517–3520. [Google Scholar] [CrossRef]
- Kusano, M.; Koshino, H.; Uzawa, J.; Fujioka, S.; Kawano, T.; Kimura, Y. Nematicidal alkaloids and related compounds produced by the fungus penicillium cf. Simplicissimum. Biosci. Biotechnol. Biochem. 2000, 64, 2559–2568. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Kusano, M.; Koshino, H.; Uzawa, J.; Fujioka, S.; Tani, K. Penigequinolones A and B, pollen-growth inhibitors produced by Penicillium sp., No. 410. Tetrahedron Lett. 1996, 37, 4961–4964. [Google Scholar] [CrossRef]
- Uchida, R.; Imasato, R.; Tomoda, H.; Omura, S. Yaequinolones, new insecticidal antibiotics produced by Penicillium sp. FKI-2140: II. Structural elucidation. J. Antibiot. 2006, 59, 652–658. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Harayama, T. A concise and versatile synthesis of viridicatin alkaloids from cyanoacetanilides. Org. Lett. 2009, 11, 1603–1606. [Google Scholar] [CrossRef]
- Tangella, Y.; Manasa, K.L.; Krishna, N.H.; Sridhar, B.; Kamal, A.; Nagendra Babu, B. Regioselective Ring Expansion of Isatins with in Situ Generated α-Aryldiazomethanes: Direct Access to Viridicatin Alkaloids. Org. Lett. 2018, 20, 3639–3642. [Google Scholar] [CrossRef]
- Chahal, V.; Nirwan, S.; Kakkar, R. Isatin and its derivatives: A survey of recent syntheses, reactions, and applications. Med. Chem. Commun. 2019, 10, 351–368. [Google Scholar]
- Borthwick, A.D. 2,5-diketopiperazines: Synthesis, reactions, medicinal chemistry, and bioactive natural products. Chem. Rev. 2012, 112, 3641–3716. [Google Scholar] [CrossRef]
- Jiang, C.-S.; Guo, Y.-W. Epipolythiodioxopiperazines from Fungi: Chemistry and Bioactivities. Mini Rev. Med. Chem. 2011, 11, 728–745. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.H.; Li, X.M.; Lv, C.T.; Huang, C.G.; Wang, B.G. Brocazines A-F, cytotoxic bisthiodiketopiperazine derivatives from Penicillium brocae MA-231, an endophytic fungus derived from the marine mangrove plant Avicennia marina. J. Nat. Prod. 2014, 77, 1921–1927. [Google Scholar] [CrossRef] [PubMed]
- Hulangamuwa, C.W. Synthetic Efforts Towards the Natural Products Brocazine F and G. Ph.D. Thesis, Kansas State University, Manhattan, KS, USA, 2020. [Google Scholar]
- Gardiner, D.M.; Waring, P.; Howlett, B.J. The epipolythiodioxopiperazine (ETP) class of fungal toxins: Distribution, mode of action, functions and biosynthesis. Microbiology 2005, 151, 1021–1032. [Google Scholar] [CrossRef]
- Guo, C.-J.; Yeh, H.-H.; Chiang, Y.-M.; Sanchez, J.F.; Chang, S.-L.; Bruno, K.S.; Wang, C.C.C. Biosynthetic Pathway for Epipolythiodioxopiperazine Acetylaranotin in Aspergillus terreus Revealed by Genome- Based Deletion Analysis. J. Am. Chem. Soc. 2013, 135, 7205–7213. [Google Scholar] [CrossRef] [PubMed]
- Scharf, D.H.; Habel, A.; Heinekamp, T.; Brakhage, A.A.; Hertweck, C. Opposed effects of enzymatic gliotoxin N- And S-methylations. J. Am. Chem. Soc. 2014, 136, 11674–11679. [Google Scholar] [CrossRef]
- Julianti, E.; Oh, H.; Lee, H.-S.; Oh, D.-C.; Oh, K.-B.; Shin, J. Acremolin, a new 1H-azirine metabolite from the marine-derived fungus Acremonium strictum. Tetrahedron Lett. 2012, 53, 2885–2886. [Google Scholar] [CrossRef]
- Banert, K. Acremolin, a stable natural product with an antiaromatic 1H-azirine moiety? A structural reorientation. Tetrahedron Lett. 2012, 53, 6443–6445. [Google Scholar] [CrossRef]
- Januar, L.A.; Molinski, T.F. Acremolin from acremonium strictum is N²,3-etheno-2′-isopropyl-1-methylguanine, not a 1 H -azirine. Synthesis and structural revision. Org. Lett. 2013, 15, 2370–2373. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Willems, T.; De Mol, M.L.; De Bruycker, A.; De Maeseneire, S.L.; Soetaert, W.K. Alkaloids from Marine Fungi: Promising Antimicrobials. Antibiotics 2020, 9, 340. https://doi.org/10.3390/antibiotics9060340
Willems T, De Mol ML, De Bruycker A, De Maeseneire SL, Soetaert WK. Alkaloids from Marine Fungi: Promising Antimicrobials. Antibiotics. 2020; 9(6):340. https://doi.org/10.3390/antibiotics9060340
Chicago/Turabian StyleWillems, Thomas, Maarten L. De Mol, Aleksandar De Bruycker, Sofie L. De Maeseneire, and Wim K. Soetaert. 2020. "Alkaloids from Marine Fungi: Promising Antimicrobials" Antibiotics 9, no. 6: 340. https://doi.org/10.3390/antibiotics9060340
APA StyleWillems, T., De Mol, M. L., De Bruycker, A., De Maeseneire, S. L., & Soetaert, W. K. (2020). Alkaloids from Marine Fungi: Promising Antimicrobials. Antibiotics, 9(6), 340. https://doi.org/10.3390/antibiotics9060340






