Satureja montana L. and Origanum majorana L. Decoctions: Antimicrobial Activity, Mode of Action and Phenolic Characterization
Abstract
1. Introduction
2. Results
2.1. Antimicrobial Activity of Satureja montana L. and Origanum majorana L. Decoctions
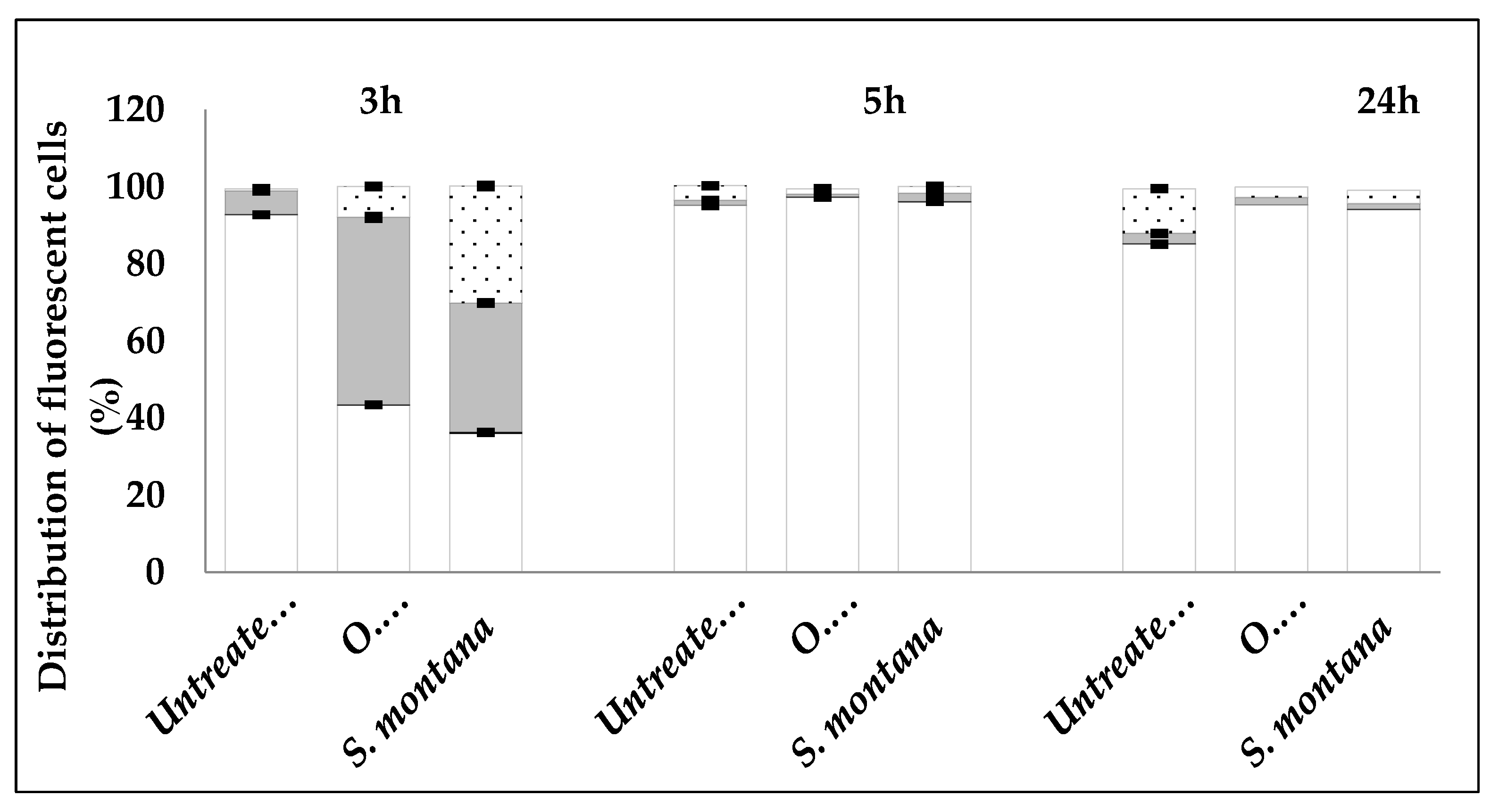
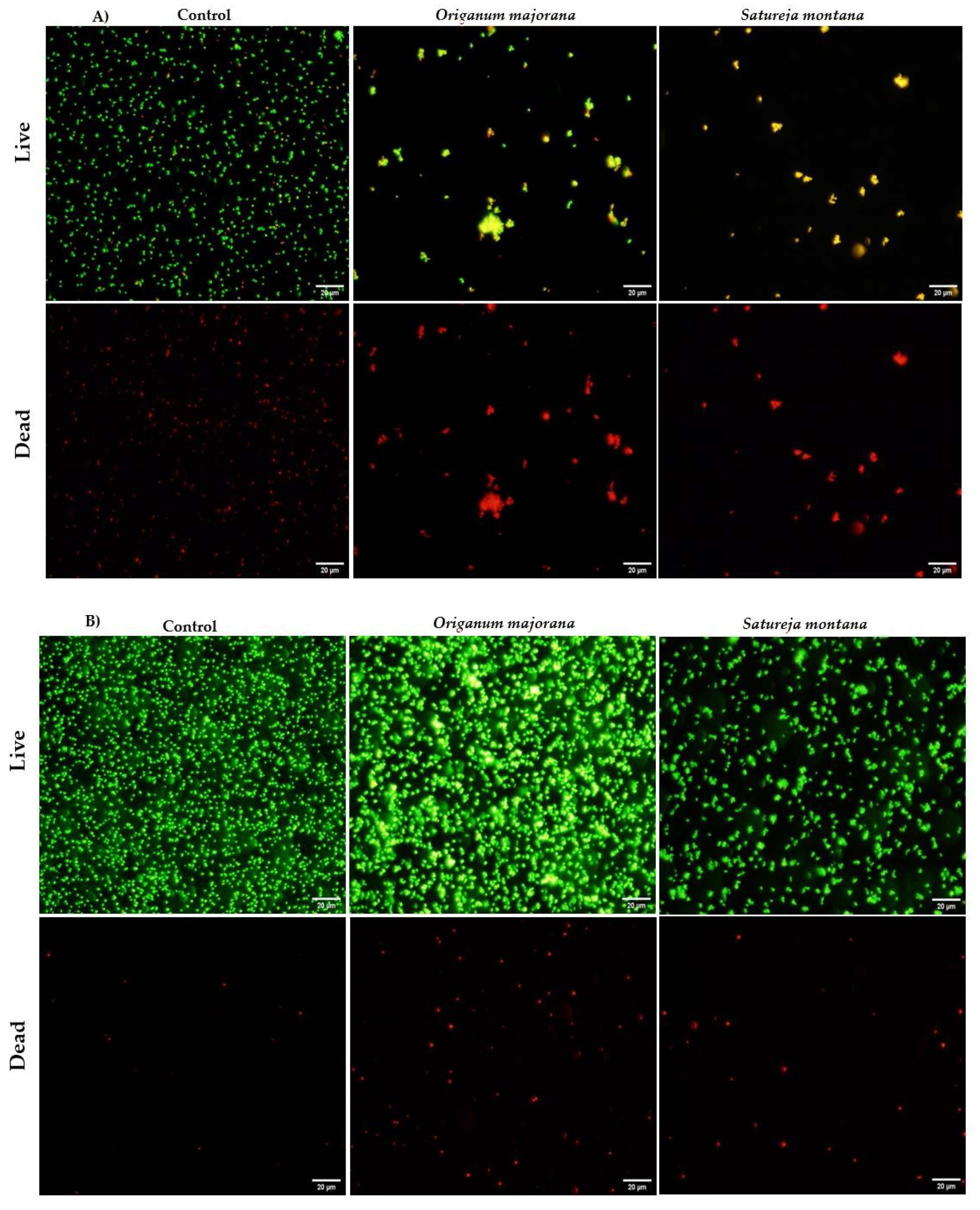
2.2. Mechanism of Action of Satureja montana and Origanum majorana Decoctions
2.3. Identification and Quantification of Phenolic Compounds
3. Discussion
4. Materials and Methods
4.1. Preparation of Plant Extracts
4.2. Evaluation of Antimicrobial Activity
4.2.1. Disc Diffusion Assay
4.2.2. Determination of MIC and MBC/MFC
4.3. Flow Cytometry
4.3.1. Sample Preparation
4.3.2. Flow Cytometry Analysis
4.3.3. Fluorescence Microscopy
4.4. Identification and Quantification of Phenolic Compounds
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, R.H. Health-promoting components of fruits and vegetables in the diet. Adv. Nutr. Int. Rev. J. 2013, 4, 384S–392S. [Google Scholar] [CrossRef] [PubMed]
- Qadir, M.A.; Shahzadi, S.K.; Bashir, A.; Munir, A.; Shahzad, S. Evaluation of phenolic compounds and antioxidant and antimicrobial activities of some common herbs. Int. J. Anal. Chem. 2017, 2017, 3475738. [Google Scholar] [CrossRef]
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic compounds in Brassica vegetables. Molecules 2011, 16, 251–280. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, R.; Sousa, M.J.; Ferreira, I.C.F.R. Contribution of essential oils and phenolics to the antioxidant properties of aromatic plants. Ind. Crops Prod. 2010, 32, 152–156. [Google Scholar] [CrossRef]
- Shakya, A.K. Medicinal plants: Future source of new drugs. Int. J. Herb. Med. IJHM 2016, 59, 59–64. [Google Scholar]
- Pour, M.A.; Sardari, S.; Eslamifar, A.; Azhar, A.; Rezvani, M.; Nazari, M. Cheminformatics-based anticoagulant study of traditionally used medicinal plants. Iran. Biomed. J. 2017, 21, 400–405. [Google Scholar] [CrossRef]
- Majumder, S.; Sigamani1, A.; Rahman, T.; Rahmatullah, M. Exploring phytochemicals as alternatives to antimicrobials-Prospects and potentials. World J. Pharm. Pharm. Sci. 2020, 9, 1802–1813 1020959/wjpps20201. [Google Scholar]
- Martins, N.; Barros, L.; Santos-Buelga, C.; Silva, S.; Henriques, M.; Ferreira, I.C.F.R. Decoction, infusion and hydroalcoholic extract of cultivated thyme: Antioxidant and antibacterial activities, and phenolic characterisation. Food Chem. 2015, 167, 131–137. [Google Scholar] [CrossRef]
- Martins, N.; Barros, L.; Santos-Buelga, C.; Henriques, M.; Silva, S.; Ferreira, I.C.F.R. Decoction, infusion and hydroalcoholic extract of Origanum vulgare L.: Different performances regarding bioactivity and phenolic compounds. Food Chem. 2014, 158, 73–80. [Google Scholar] [CrossRef]
- Martins, N.; Ferreira, I.C.F.R.; Barros, L.; Carvalho, A.M.; Henriques, M.; Silva, S.C. Plants used in folk medicine: The potential of their hydromethanolic extracts against Candida species. Ind. Crops Prod. 2015, 66, 62–67. [Google Scholar] [CrossRef]
- Banchio, E.; Bogino, P.C.; Zygadlo, J.; Giordano, W. Plant growth promoting rhizobacteria improve growth and essential oil yield in Origanum majorana L. Biochem. Syst. Ecol. 2008, 36, 766–771. [Google Scholar] [CrossRef]
- Ćetković, G.S.; Čanadanović-Brunet, J.M.; Djilas, S.M.; Tumbas, V.T.; Markov, S.L.; Cvetković, D.D. Antioxidant potential, lipid peroxidation inhibition and antimicrobial activities of Satureja montana L. subsp. kitaibelii extracts. Int. J. Mol. Sci. 2007, 8, 1013–1027. [Google Scholar] [CrossRef]
- Serrano, C.; Matos, O.; Teixeira, B.; Ramos, C.; Neng, N.; Nogueira, J.; Nunes, M.L.; Marques, A. Antioxidant and antimicrobial activity of Satureja montana L. extracts. J. Sci. Food Agric. 2011, 91, 1554–1560. [Google Scholar] [CrossRef]
- Roby, M.H.H.; Sarhan, M.A.; Selim, K.A.H.; Khalel, K.I. Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme (Thymus vulgaris L.), sage (Salvia officinalis L.), and marjoram (Origanum majorana L.) extracts. Ind. Crops Prod. 2013, 43, 827–831. [Google Scholar] [CrossRef]
- Hossain, M.B.; Camphuis, G.; Aguiló-Aguayo, I.; Gangopadhyay, N.; Rai, D.K. Antioxidant activity guided separation of major polyphenols of marjoram (Origanum majorana L.) using flash chromatography and their identification by liquid chromatography coupled with electrospray ionization tandem mass spectrometry. J. Sep. Sci. 2014, 37, 3205–3213. [Google Scholar] [CrossRef] [PubMed]
- López-Cobo, A.; Gómez-Caravaca, A.M.; Švarc-Gajić, J.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Determination of phenolic compounds and antioxidant activity of a Mediterranean plant: The case of Satureja montana subsp. kitaibelii. J. Funct. Foods 2015, 18, 1167–1178. [Google Scholar] [CrossRef]
- Kaiser, A.; Carle, R.; Kammerer, D.R. Effects of blanching on polyphenol stability of innovative paste-like parsley (Petroselinum crispum (Mill.) Nym ex A. W. Hill) and marjoram (Origanum majorana L.) products. Food Chem. 2013, 138, 1648–1656. [Google Scholar] [CrossRef] [PubMed]
- Taamalli, A.; Arráez-Román, D.; Abaza, L.; Iswaldi, I.; Fernández-Gutiérrez, A.; Zarrouk, M.; Segura-Carretero, A. LC-MS-based metabolite profiling of methanolic extracts from the medicinal and aromatic species Mentha pulegium and Origanum majorana. Phytochem. Anal. 2015, 26, 320–330. [Google Scholar] [CrossRef]
- Martins, N.; Barros, L.; Santos-Buelga, C.; Henriques, M.; Silva, S.; Ferreira, I.C.F.R. Evaluation of bioactive properties and phenolic compounds in different extracts prepared from Salvia officinalis L. Food Chem. 2015, 170, 378–385. [Google Scholar] [CrossRef]
- Cabana, R.; Silva, L.R.; Valentão, P.; Viturro, C.I.; Andrade, P.B. Effect of different extraction methodologies on the recovery of bioactive metabolites from Satureja parvifolia (Phil.) Epling (Lamiaceae). Ind. Crops Prod. 2013, 48, 49–56. [Google Scholar] [CrossRef]
- Barros, L.; Dueñas, M.; Dias, M.I.; Sousa, M.J.; Santos-Buelga, C.; Ferreira, I.C.F.R. Phenolic profiles of cultivated, in vitro cultured and commercial samples of Melissa officinalis L. infusions. Food Chem. 2013, 136, 1–8. [Google Scholar] [CrossRef]
- Kundaković, T.; Stanojković, T.; Kolundžija, B.; Marković, S.; Šukilović, B.; Milenković, M.; Lakušić, B. Cytotoxicity and antimicrobial activity of the essential oil from Satureja montana subsp. pisidica (Lamiceae). Nat. Prod. Commun. 2014, 9, 569–572. [Google Scholar]
- de Lima Marques, J.; Volcão, L.M.; Funck, G.D.; Kroning, I.S.; da Silva, W.P.; Fiorentini, Â.M.; Ribeiro, G.A. Antimicrobial activity of essential oils of Origanum vulgare L. and Origanum majorana L. against Staphylococcus aureus isolated from poultry meat. Ind. Crops Prod. 2015, 77, 444–450. [Google Scholar] [CrossRef]
- Kremer, D.; Košir, I.J.; Končić, M.Z.; Čerenak, A.; Potočnik, T.; Srečec, S.; Randić, M.; Kosalec, I. Antimicrobial and antioxidant properties of Satureja montana L. and S. subspicata Vis. (Lamiaceae). Curr. Drug Targets 2015, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Radaelli, M.; da Silva, B.P.; Weidlich, L.; Hoehne, L.; Flach, A.; da Costa, L.A.M.A.; Ethur, E.M. Antimicrobial activities of six essential oils commonly used as condiments in Brazil against Clostridium perfringens. Braz. J. Microbiol. 2016, 47, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Btissam, R.; Fatima, E.M.; Kamal, E.; Hassane, G.; Mohamed, N. Composition and antibacterial activity of hydro-alcohol and aqueous extracts obtained from the Lamiaceae family. Pharmacogn. J. 2017, 10, 81–91. [Google Scholar] [CrossRef]
- Leeja, L.; Thoppil, J.E. Antimicrobial activity of methanol extract of Origanum majorana L. (Sweet marjoram). J. Environ. Biol. 2007, 28, 145–146. [Google Scholar]
- Busatta, C.; Vidal, R.S.; Popiolski, A.S.; Mossi, A.J.; Dariva, C.; Rodrigues, M.R.A.; Corazza, F.C.; Corazza, M.L.; Vladimir Oliveira, J.; Cansian, R.L. Application of Origanum majorana L. essential oil as an antimicrobial agent in sausage. Food Microbiol. 2008, 25, 207–211. [Google Scholar] [CrossRef]
- Abdel-Massih, R.; Abdou, E.; Baydoun, E.; Daoud, Z. Antibacterial activity of the extracts obtained from Rosmarinus officinalis, Origanum majorana, and Trigonella foenum-graecum on highly drug-resistant gram negative bacilli. J. Bot. Hindawi Publ. Corp. 2010, 1–8. [Google Scholar] [CrossRef]
- Kozłowska, M.; Laudy, A.E.; Starościak, B.J.; Napiórkowski, A.; Chomicz, L.; Kazimierczuk, Z. Antimicrobial and antiprotozoal effect of sweet marjoram (Origanum majorana L.). Acta Sci. Pol. 2010, 9, 133–141. [Google Scholar]
- Mohamed, N.; Yasmen, S.; Nohir, G. Antimicrobial activity of water and ethanol marjoram (Origanum marjorana L.) extract. In The 6th Arab and 3rd International Annual Scientific Conference on: Development of Higher Specific Education Programs in Egypt and the Arab World in the Light of Knowledge Era Requirements; Faculty of Specific Education Mansoura University: Mansoura, Egypt, 2011. [Google Scholar]
- Oliveira, T.L.C.; de Araújo Soares, R.; Ramos, E.M.; das Graças Cardoso, M.; Alves, E.; Piccoli, R.H. Antimicrobial activity of Satureja montana L. essential oil against Clostridium perfringens type A inoculated in mortadella-type sausages formulated with different levels of sodium nitrite. Int. J. Food Microbiol. 2011, 144, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Freire, J.M.; Cardoso, M.G.; Batista, L.R.; Andrade, M.A. Essential oil of Origanum majorana L., Illicium verum Hook. f. and Cinnamomum zeylanicum Blume: Chemical and antimicrobial characterization. Rev. Bras. Plantas Med. 2011, 13, 209–214. [Google Scholar] [CrossRef]
- Miladi, H.; Ben Slama, R.; Mili, D.; Zouari, S.; Bakhrouf, A.; Ammar, E. Chemical composition and cytotoxic and antioxidant activities of Satureja montana L. essential oil and its antibacterial potential against Salmonella Spp. strains. J. Chem. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Blazek, A.D.; Paleo, B.J.; Weisleder, N. Plasma membrane repair: A central process for maintaining cellular homeostasis. Physiology 2015, 30, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.W.; Corrotte, M. Plasma membrane repair. Curr. Biol. 2018, 28, R392–R397. [Google Scholar] [CrossRef] [PubMed]
- Annuk, H.; Hirmo, S.; Türi, E.; Mikelsaar, M.; Arak, E.; Wadström, T. Effect on cell surface hydrophobicity and susceptibility of Helicobacter pylori to medicinal plant extracts. FEMS Microbiol. Lett. 1999, 172, 41–45. [Google Scholar] [CrossRef]
- Voravuthikunchai, S.P.; Limsuwan, S. Medicinal plant extracts as anti-Escherichia coli O157:H7 agents and their effects on bacterial cell aggregation. J. Food Prot. 2006, 69, 2336–2341. [Google Scholar] [CrossRef]
- Bessada, S.M.F.; Barreira, J.C.M.; Barros, L.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P. Phenolic profile and antioxidant activity of Coleostephus myconis (L.) Rchb. f.: An underexploited and highly disseminated species. Ind. Crops Prod. 2016, 89, 45–51. [Google Scholar] [CrossRef]
| Species | Antimicrobial Activity (Inhibition Zone, mm) | |
|---|---|---|
| Satureja montana | Origanum majorana | |
| Gram-positive Bacteria | ||
| Staphylococcus aureus | +++ | +++ |
| Enterococcus faecalis | +++ | +++ |
| Streptococcus dysgalactiae | ++ | ++ |
| Gram-negative bacteria | ||
| Escherichia coli | - | - |
| Pseudomonas aeruginosa | ++ | +++ |
| Klebsiella pneumoniae | +++ | +++ |
| Yeast | ||
| Candida albicans | - | - |
| Candida tropicalis | ++ | - |
| Candida glabrata | - | - |
| Candida parapsilosis | - | - |
| Negative control | - | - |
| Satureja montana | Origanum majorana | |||
|---|---|---|---|---|
| Antibacterial | MIC | MBC | MIC | MBC |
| Gram-positive bacterium | ||||
| S. aureus | 1.56 | 1.56 | 1.56 | 1.56 |
| Gram-negative bacterium | ||||
| K. pneumoniae | 1.56 | 1.56 | 1.56 | 1.56 |
| Antifungal | MIC | MFC | MIC | MFC |
| C. tropicalis | 6.25 | 6.25 | ND | ND |
| Peak | Rt (min) | λmax (nm) | [M − H]− (m/z) | MS2 (m/z) | Tentative Identification | Reference Used for Identification | Quantification (mg/g of Extract) | Student’s t-Test p-value | |
|---|---|---|---|---|---|---|---|---|---|
| Satureja montana | Origanum majorana | ||||||||
| 1 | 6.13 | 310 | 325 | 163(100) | p-Coumaroyl acid hexoside (A) | [17] | nd | 1.21 ± 0.003 | - |
| 2 | 7.15 | 345 | 609 | 489(100),399(10),369(6) | Luteolin-C-hexoside-C-hexoside (B) | [17,18] | nd | 2.20 ± 0.02 | - |
| 3 | 7.26 | 281 | 137 | 93(100) | p-Hydroxybenzoic acid (C) | DAD/MS | 1.21 ± 0.04 | nd | - |
| 4 | 9.55 | 336 | 593 | 503(30),473(100),383(12),353(21) | Apigenin-6,8-di-C-hexoside isomer I (D) | [16,18] | 1.7 ± 0.1 | 7.3 ± 0.2 | <0.001 |
| 5 | 9.89 | 335 | 593 | 503(31),473(100),383(11),353(22) | Apigenin-6,8-di-C-hexoside isomer II (D) | [16,18] | 4.3 ± 0.1 | nd | - |
| 6 | 11.73 | 346 | 637 | 285(100) | Luteolin-O-di-glucuronide (E) | [8,19] | nd | 3.8 ± 0.2 | - |
| 7 | 12.41 | 339 | 637 | 285(100) | Luteolin-O-di-glucuronide (E) | [8,19] | 1.5 ± 0.1 | 2.14 ± 0.02 | <0.001 |
| 8 | 14.31 | 350 | 477 | 301(100) | Quercetin-O-glucuronide (E) | DAD/MS | 1.43 ± 0.02 | nd | - |
| 9 | 14.41 | 339 | 623 | 461(100),285(18) | Luteolin-O-hexoside-O-glucuronide (E) | DAD/MS | nd | 1.58 ± 0.04 | - |
| 10 | 14.57 | 342 | 637 | 461(100),285(15) | Luteolin-O-di-glucuronide (E) | [8,19] | 1.27 ± 0.01 | nd | - |
| 11 | 15.41 | 337 | 799 | 513(100),285(15) | Luteolin derivative (E) | DAD/MS | nd | 6.874 ± 0.002 | - |
| 12 | 15.81 | 340 | 623 | 461(100),285(18) | Luteolin-O-hexoside-O-glucuronide (E) | DAD/MS | 1.36 ± 0.01 | nd | - |
| 13 | 17.25 | 340 | 579 | 285(100) | Luteolin-O-pentosyl-hexoside (E) | DAD/MS | nd | 1.53 ± 0.01 | - |
| 14 | 17.6 | 340 | 461 | 285(100) | Luteolin-O-glucuronide (E) | [16,17] | 3.8 ± 0.1 | 3.3 ± 0.1 | <0.001 |
| 15 | 18.33 | 340 | 783 | 285(100) | Luteolin-O-di-glucuronyl-deoxyhexoside (E) | DAD/MS | nd | 1.61 ± 0.03 | - |
| 16 | 20.47 | 325 | 359 | 197(28),179(35),161(100) | Rosmarinic acid (F) | DAD/MS; [15,20] | 36.3 ± 0.4 | 52.4 ± 0.2 | <0.001 |
| 17 | 23.57 | 340 | 461 | 285(100) | Luteolin-O-glucuronide (E) | [16,17] | 8.1 ± 0.1 | nd | - |
| 18 | 23.88 | 288/322 | 717 | 537(29),519(100), 493(10),359(10),339(6), 321(6),295(5),197(5), 179(5) | Salvianolic acid B isomer I (F) | [18,19] | 0.683 ± 0.001 | 5.4 ± 0.1 | <0.001 |
| 19 | 23.94 | 326 | 537 | 493(50),359(100),313(8),295(2),269(2), 197(29),179(34) | Lithospermic acid A isomer I (F) | [8,21] | 16.9 ± 0.2 | nd | - |
| 20 | 27.57 | 289/323 | 493 | 359(100),313(10),295(5), 269(5),197(5),179(5) | Salvianolic acid A isomer I (F) | [21] | 20.0 ± 0.7 | 3.4 ± 0.3 | <0.001 |
| 21 | 28.97 | 289/323 | 493 | 359(100),313(10),295(5),269(5),197(5),179) | Salvianolic acid A isomer II (F) | [21] | 2.692± 0.003 | nd | - |
| 22 | 30.01 | 323 | 537 | 493(100),359(42), 313(10),295(5),269(5), 197(5),179(5) | Lithospermic acid A isomer II (F) | [8,21] | 2.752 ± 0.001 | nd | - |
| 23 | 30.68 | 327 | 591 | 283(100),269(5) | Acacetin-O-glucuronide(G) | [16] | 4.39 ± 0.03 | nd | - |
| 24 | 32.64 | 287/321 | 717 | 537(29),519(100),493(10),359(10),339(6),321(6), 295(5),197(5),179(5) | Salvianolic acid B isomer II(F) | [18,19] | 4.7 ± 0.1 | nd | - |
| Total phenolic acids | 85.22 ± 1.03 | 72.4 ± 0.3 | <0.001 | ||||||
| Total flavonoids | 27.9 ± 0.1 | 20.4 ± 0.2 | <0.001 | ||||||
| Total phenolic compounds | 113.1 ± 0.9 | 92.8 ± 0.5 | <0.001 | ||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, F.; Dias, M.I.; Lima, Â.; Barros, L.; Rodrigues, M.E.; Ferreira, I.C.F.R.; Henriques, M. Satureja montana L. and Origanum majorana L. Decoctions: Antimicrobial Activity, Mode of Action and Phenolic Characterization. Antibiotics 2020, 9, 294. https://doi.org/10.3390/antibiotics9060294
Gomes F, Dias MI, Lima Â, Barros L, Rodrigues ME, Ferreira ICFR, Henriques M. Satureja montana L. and Origanum majorana L. Decoctions: Antimicrobial Activity, Mode of Action and Phenolic Characterization. Antibiotics. 2020; 9(6):294. https://doi.org/10.3390/antibiotics9060294
Chicago/Turabian StyleGomes, Fernanda, Maria Inês Dias, Ângela Lima, Lillian Barros, Maria Elisa Rodrigues, Isabel C.F.R. Ferreira, and Mariana Henriques. 2020. "Satureja montana L. and Origanum majorana L. Decoctions: Antimicrobial Activity, Mode of Action and Phenolic Characterization" Antibiotics 9, no. 6: 294. https://doi.org/10.3390/antibiotics9060294
APA StyleGomes, F., Dias, M. I., Lima, Â., Barros, L., Rodrigues, M. E., Ferreira, I. C. F. R., & Henriques, M. (2020). Satureja montana L. and Origanum majorana L. Decoctions: Antimicrobial Activity, Mode of Action and Phenolic Characterization. Antibiotics, 9(6), 294. https://doi.org/10.3390/antibiotics9060294








