Counting Replication Origins to Measure Growth of Pathogens
Abstract
1. Introduction
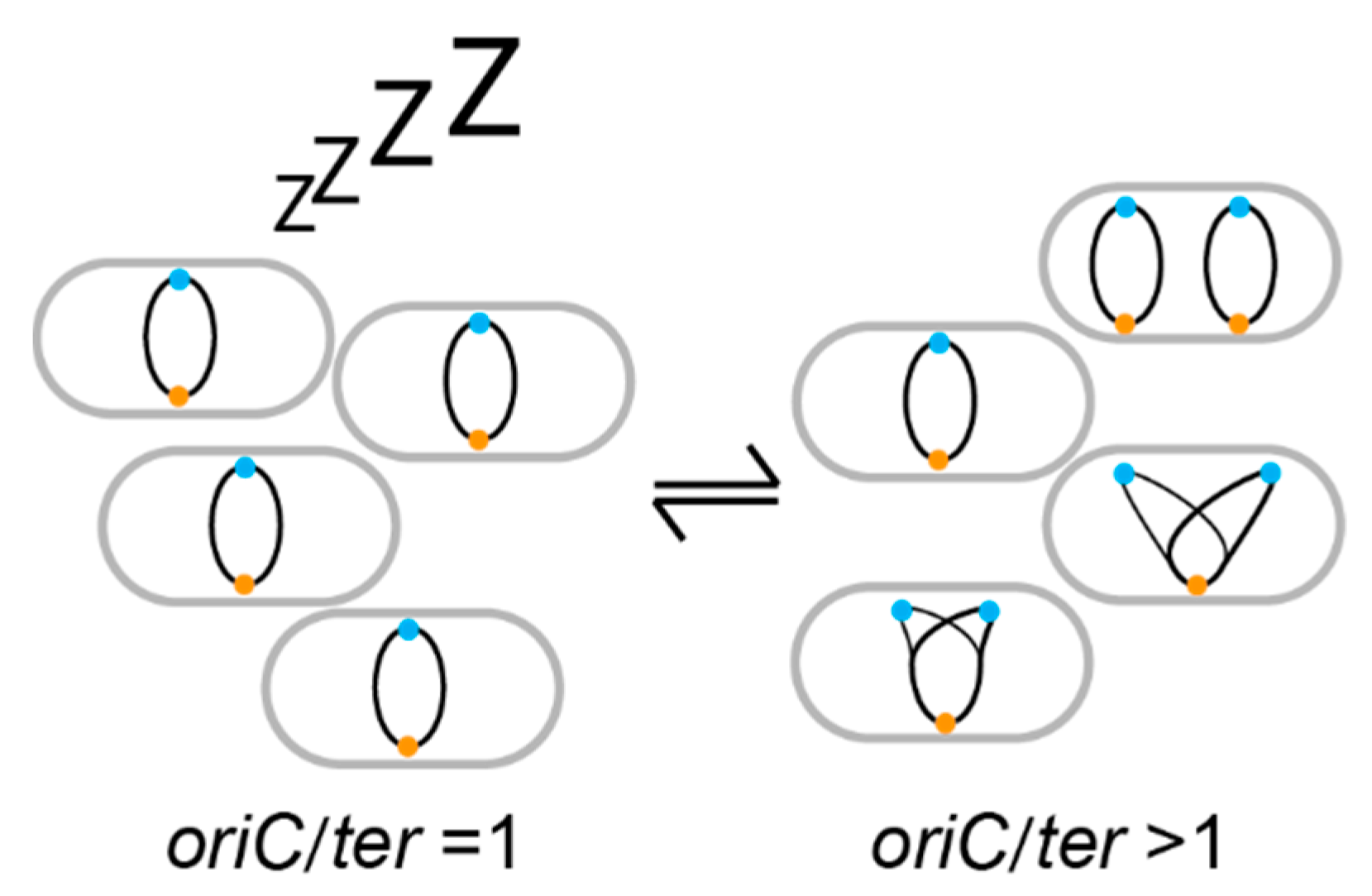
2. DNA Replication as a Growth Marker
3. Dynamics in Specific Niches
4. Dynamics During an Infection Process
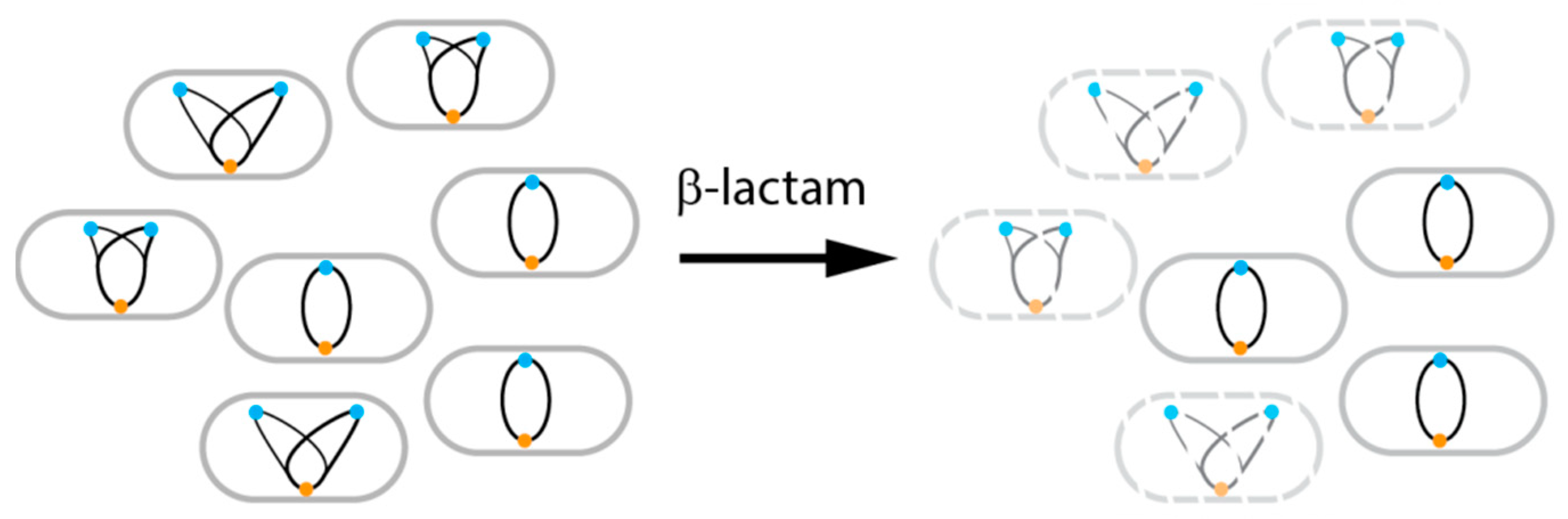
5. Dynamics During Antibiotic Therapy
6. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Riglar, D.T.; Richmond, D.L.; Potvin-Trottier, L.; Verdegaal, A.A.; Naydich, A.D.; Bakshi, S.; Leoncini, E.; Lyon, L.G.; Paulsson, J.; Silver, P.A. Bacterial variability in the mammalian gut captured by a single-cell synthetic oscillator. Nat. Commun. 2019, 10, 4665. [Google Scholar] [CrossRef] [PubMed]
- Szafranska, A.K.; Junker, V.; Steglich, M.; Nubel, U. Rapid cell division of Staphylococcus aureus during colonization of the human nose. BMC Genom. 2019, 20, 229. [Google Scholar] [CrossRef] [PubMed]
- Korem, T.; Zeevi, D.; Suez, J.; Weinberger, A.; Avnit-Sagi, T.; Pompan-Lotan, M.; Matot, E.; Jona, G.; Harmelin, A.; Cohen, N.; et al. Growth dynamics of gut microbiota in health and disease inferred from single metagenomic samples. Science 2015, 349, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, V.S.; Armbruster, C.E.; Smith, S.N.; Pirani, A.; Springman, A.C.; Walters, M.S.; Nielubowicz, G.R.; Himpsl, S.D.; Snitkin, E.S.; Mobley, H.L.T. Rapid Growth of Uropathogenic Escherichia coli during Human Urinary Tract Infection. MBio 2018, 9, e00186-18. [Google Scholar] [CrossRef] [PubMed]
- Myhrvold, C.; Kotula, J.W.; Hicks, W.M.; Conway, N.J.; Silver, P.A. A distributed cell division counter reveals growth dynamics in the gut microbiota. Nat. Commun. 2015, 6, 10039. [Google Scholar] [CrossRef] [PubMed]
- Abel, S.; Abel zur Wiesch, P.; Chang, H.H.; Davis, B.M.; Lipsitch, M.; Waldor, M.K. Sequence tag-based analysis of microbial population dynamics. Nat. Methods 2015, 12, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Rang, C.U.; Licht, T.R.; Midtvedt, T.; Conway, P.L.; Chao, L.; Krogfelt, K.A.; Cohen, P.S.; Molin, S. Estimation of growth rates of Escherichia coli BJ4 in streptomycin-treated and previously germfree mice by In Situ rRNA hybridization. Clin. Diagn. Lab. Immunol. 1999, 6, 434–436. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Haagensen, J.A.; Jelsbak, L.; Johansen, H.K.; Sternberg, C.; Hoiby, N.; Molin, S. In Situ growth rates and biofilm development of Pseudomonas aeruginosa populations in chronic lung infections. J. Bacteriol. 2008, 190, 2767–2776. [Google Scholar] [CrossRef] [PubMed]
- Cooper, S.; Helmstetter, C.E. Chromosome replication and the division cycle of Escherichia coli B/r. J. Mol. Biol. 1968, 31, 519–540. [Google Scholar] [CrossRef]
- Donachie, W.D. Relationship between cell size and time of initiation of DNA replication. Nature 1968, 219, 1077–1079. [Google Scholar] [CrossRef] [PubMed]
- Haugan, M.S.; Charbon, G.; Frimodt-Moller, N.; Lobner-Olesen, A. Chromosome replication as a measure of bacterial growth rate during Escherichia coli infection in the mouse peritonitis model. Sci. Rep. 2018, 8, 14961. [Google Scholar] [CrossRef] [PubMed]
- Haugan, M.S.; Hertz, F.B.; Charbon, G.; Sahin, B.; Lobner-Olesen, A.; Frimodt-Moller, N. Growth Rate of Escherichia coli During Human Urinary Tract Infection: Implications for Antibiotic Effect. Antibiotics (Basel) 2019, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Haugan, M.S.; Lobner-Olesen, A.; Frimodt-Moller, N. Comparative Activity of Ceftriaxone, Ciprofloxacin, and Gentamicin as a Function of Bacterial Growth Rate Probed by Escherichia coli Chromosome Replication in the Mouse Peritonitis Model. Antimicrob. Agents Chemother. 2019, 63, e02133-18. [Google Scholar] [CrossRef] [PubMed]
- Olm, M.R.; Brown, C.T.; Brooks, B.; Firek, B.; Baker, R.; Burstein, D.; Soenjoyo, K.; Thomas, B.C.; Morowitz, M.; Banfield, J.F. Identical bacterial populations colonize premature infant gut, skin, and oral microbiomes and exhibit different In Situ growth rates. Genome Res. 2017, 27, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.T.; Olm, M.R.; Thomas, B.C.; Banfield, J.F. Measurement of bacterial replication rates in microbial communities. Nat. Biotechnol. 2016, 34, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Olm, M.R.; Bhattacharya, N.; Crits-Christoph, A.; Firek, B.A.; Baker, R.; Song, Y.S.; Morowitz, M.J.; Banfield, J.F. Necrotizing enterocolitis is preceded by increased gut bacterial replication, Klebsiella, and fimbriae-encoding bacteria. Sci. Adv. 2019, 5, eaax5727. [Google Scholar] [CrossRef] [PubMed]
- Tuomanen, E.; Cozens, R.; Tosch, W.; Zak, O.; Tomasz, A. The rate of killing of Escherichia coli by beta-lactam antibiotics is strictly proportional to the rate of bacterial growth. J. Gen. Microbiol. 1986, 132, 1297–1304. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.J.; Wang, S.; Meredith, H.R.; Zhuang, B.; Dai, Z.; You, L. Robust, linear correlations between growth rates and beta-lactam-mediated lysis rates. Proc. Natl. Acad. Sci. USA 2018, 115, 4069–4074. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charbon, G.; Schei Haugan, M.; Frimodt-Møller, N.; Løbner-Olesen, A. Counting Replication Origins to Measure Growth of Pathogens. Antibiotics 2020, 9, 239. https://doi.org/10.3390/antibiotics9050239
Charbon G, Schei Haugan M, Frimodt-Møller N, Løbner-Olesen A. Counting Replication Origins to Measure Growth of Pathogens. Antibiotics. 2020; 9(5):239. https://doi.org/10.3390/antibiotics9050239
Chicago/Turabian StyleCharbon, Godefroid, Maria Schei Haugan, Niels Frimodt-Møller, and Anders Løbner-Olesen. 2020. "Counting Replication Origins to Measure Growth of Pathogens" Antibiotics 9, no. 5: 239. https://doi.org/10.3390/antibiotics9050239
APA StyleCharbon, G., Schei Haugan, M., Frimodt-Møller, N., & Løbner-Olesen, A. (2020). Counting Replication Origins to Measure Growth of Pathogens. Antibiotics, 9(5), 239. https://doi.org/10.3390/antibiotics9050239






