Urinary Pharmacokinetic and Pharmacodynamic Profiles of Fosfomycin against Extended-Spectrum β-Lactamase-Producing Escherichia coli with Canine Ex Vivo Modeling: A Pilot Study
Abstract
1. Introduction
2. Results
2.1. Safety and Laboratory Test Results
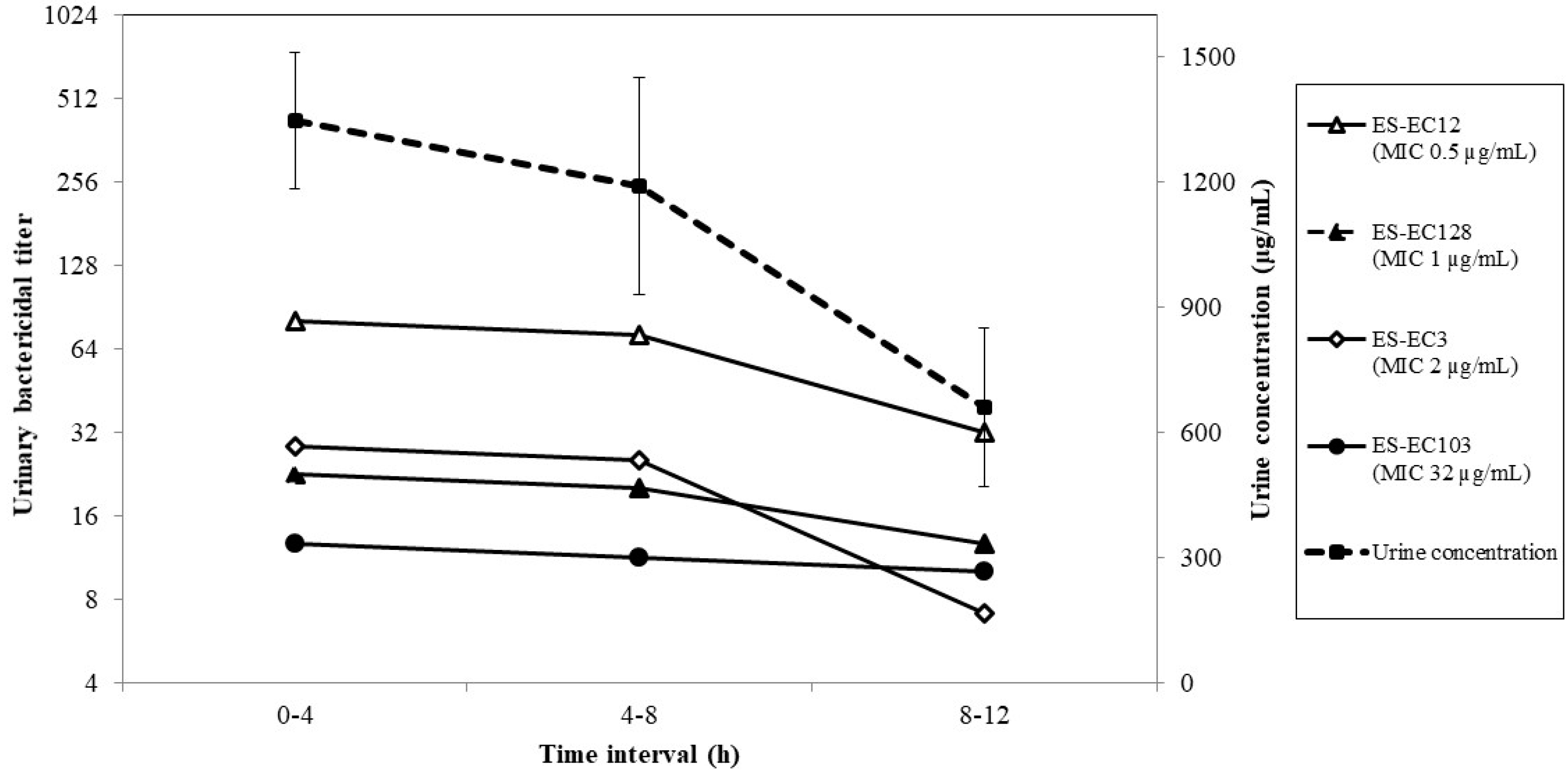
2.2. Urinary Concentration
2.3. Urinary Bactericidal Titers
3. Discussion
4. Materials and Methods
4.1. Sampling of Urine from Dogs Treated with Fosfomycin
4.2. Measurement of Urine Fosfomycin Concentration with LC–MS
4.3. Test Organisms
4.4. Determination of Urinary Bactericidal Titer
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Smee, N.; Loyd, K.; Grauer, G. UTIs in small animal patients: Part 2: Diagnosis, treatment, and complications. J. Am. Anim. Hosp. Assoc. 2013, 49, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Ling, G.V.; Norris, C.R.; Franti, C.E.; Eisele, P.H.; Johnson, D.L.; Ruby, A.L.; Jang, S.S. Interrelations of organism prevalence, specimen collection method, and host age, sex, and breed among 8,354 canine urinary tract infections (1969–1995). J. Vet. Intern. Med. 2001, 15, 341–347. [Google Scholar] [PubMed]
- Smee, N.; Loyd, K.; Grauer, G. UTIs in small animal patients: Part 1: Etiology and pathogenesis. J. Am. Anim. Hosp. Assoc. 2013, 49, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ewers, C.; Grobbel, M.; Stamm, I.; Kopp, P.A.; Diehl, I.; Semmler, T.; Fruth, A.; Beutlich, J.; Guerra, B.; Wieler, L.H.; et al. Emergence of human pandemic O25:H4-ST131 CTX-M-15 extended-spectrum-β-Lactamase-producing Escherichia coli among companion animals. J. Antimicrob. Chemother. 2010, 65, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.; Belas, A.; Franco, A.; Aboim, C.; Gama, L.T.; Pomba, C. Increase in antimicrobial resistance and emergence of major international high-risk clonal lineages in dogs and cats with urinary tract infection: 16 year retrospective study. J. Antimicrob. Chemother. 2018, 73, 377–384. [Google Scholar] [CrossRef]
- Pitout, J.D.; Laupland, K.B. Extended-spectrum β-lactamase-producing Enterobacteriaceae: An Emerging Public-Health Concern. Lancet Infect. Dis. 2008, 8, 159–166. [Google Scholar] [CrossRef]
- Michalopoulos, A.S.; Livaditis, I.G.; Gougoutas, V. The revival of fosfomycin. Int. J. Infect. Dis. 2011, 15, e732–e739. [Google Scholar] [CrossRef]
- Hendlin, D.; Stapley, E.O.; Jackson, M.; Wallick, H.; Miller, A.K.; Wolf, F.J.; Miller, T.W.; Chaiet, L.; Kahan, F.M.; Foltz, E.L.; et al. Phosphonomycin, a New Antibiotic Produced by Strains of Streptomyces. Science 1969, 166, 122–123. [Google Scholar] [CrossRef]
- Fransen, F.; Hermans, K.; Melchers, M.J.B.; Lagarde, C.C.M.; Meletiadis, J.; Mouton, J.W. Pharmacodynamics of fosfomycin against ESBL- and/or carbapenemase-producing Enterobacteriaceae. J. Antimicrob. Chemother. 2017, 72, 3374–3381. [Google Scholar] [CrossRef]
- Lepak, A.J.; Zhao, M.; VanScoy, B.; Taylor, D.S.; Ellis-Grosse, E.; Ambrose, P.G.; Andes, D.R. In vivo pharmacokinetics and pharmacodynamics of ZTI-01 (fosfomycin for Injection) in the neutropenic murine thigh infection model against Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef]
- Zykov, I.N.; Samuelsen, Ø.; Jakobsen, L.; Småbrekke, L.; Andersson, D.I.; Sundsfjord, A.; Frimodt-Møller, N. Pharmacokinetics and pharmacodynamics of fosfomycin and its activity against extended-spectrum-β-Lactamase-, plasmid-mediated AmpC-, and Carbapenemase-producing Escherichia coli in a murine urinary tract infection model. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Harada, K.; Tsuyuki, Y.; Kimura, Y.; Miyamoto, T.; Hatoya, S.; Hikasa, Y. In vitro efficacy of 16 antimicrobial drugs against a large collection of β-lactamase-producing isolates of extraintestinal pathogenic Escherichia coli from dogs and cats. J Med Microb 2017, 66, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Vouloumanou, E.K.; Samonis, G.; Vardakas, K.Z. Fosfomycin. Clin. Microbiol. Rev. 2016, 29, 321–347. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, O.L.; Ocampo, C.L.; Aguilera, J.R.; Luna, J.; Sumano, L.H. Pharmacokinetics of disodium-fosfomycin in mongrel dogs. Res. Vet. Sci. 2008, 85, 156–161. [Google Scholar] [CrossRef]
- Mazzei, T.; Cassetta, M.I.; Fallani, S.; Arrigucci, S.; Novelli, A. Pharmacokinetic and pharmacodynamic aspects of antimicrobial agents for the treatment of uncomplicated urinary tract infections. Int. J. Antimicrob. Agents 2006, 28, 35–41. [Google Scholar] [CrossRef]
- Wenzler, E.; Ellis-Grosse, E.J.; Rodvold, K.A. Pharmacokinetics, safety, and tolerability of single-dose intravenous (ZTI-01) and oral fosfomycin in healthy volunteers. Antimicrob. Agents Chemother. 2017, 61, e00775-17. [Google Scholar] [CrossRef]
- Wijma, R.A.; Koch, B.C.P.; van Gelder, T.; Mouton, J.W. High interindividual variability in urinary fosfomycin concentrations in healthy female volunteers. Clin. Microbiol. Infect. 2018, 24, 528–532. [Google Scholar] [CrossRef]
- Barger, A.; Fuhst, C.; Wiedemann, B. Pharmacological indices in antibiotic therapy. J. Antimicrob. Chemother. 2003, 52, 893–898. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Standards for Antimicrobial Susceptibility Testing, 30th ed.; CLSI Supplement M100; CLSI: Wayne, PA, USA, 2020. [Google Scholar]
- VanScoy, B.D.; McCauley, J.; Ellis-Grosse, E.J.; Okusanya, O.O.; Bhavnanl, S.M.; Forrest, A.; Ambrose, P.G. Exploration of the pharmacokinetic-pharmacodynamic relationships for fosfomycin efficacy using an in vitro infection model. Antimicrob. Agents Chemother. 2015, 59, 7170–7177. [Google Scholar] [CrossRef]
- So, W.; Crandon, J.L.; Nicolau, D.P. Effects of urine matrix and pH on the potency of delafloxacin and ciprofloxacin against urogenic Escherichia coli and Klebsiella pneumoniae. J. Urol. 2015, 194, 563–570. [Google Scholar] [CrossRef]
- Wagenlehner, F.M.E.; Wagenlehner, C.; Redman, R.; Weidner, W.; Naber, K.G. Urinary bactericidal activity of doripenem versus that of levofloxacin in patients with complicated urinary tract infections or pyelonephritis. Antimicrob. Agents Chemother. 2009, 53, 1567–1573. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bergan, T. Degree of absorption, pharmacokinetics of fosfomycin trometamol and duration of urinary antibacterial activity. Infection 1990, 18, S65–S69. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Harada, K.; Manabe, S.; Tsukamoto, T.; Ito, N.; Hikasa, Y. Assessment of urinary pharmacokinetics and pharmacodynamics of orbifloxacin in healthy dogs with ex vivo modelling. J. Med. Microb. 2017, 66, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Shimizu, T.; Miyashita, N.; Hikasa, Y. Assessment of urinary pharmacokinetic and pharmacodynamic profiles of faropenem against extended-specrtrum β-lactamase-producing Escherichia coli with canine ex vivo modelling: A pilot study. Access Microb. 2019, 1. [Google Scholar] [CrossRef]
| Strains | MIC (µg/mL) 1 | ESBL Type 1 | AUBT0-12 | AUC/MIC0-12 2 | Cmax/MIC 2 | Time above MIC0-12 (%) 2 |
|---|---|---|---|---|---|---|
| ES-EC12 | 0.5 | CTX-M-55 | 1248 | 25,607.6 | 2696.4 | 100 |
| ES-EC128 | 1 | CTX-M-2 | 578 | 12,803.8 | 1348.2 | 100 |
| ES-EC3 | 2 | CTX-M-27 | 224 | 6401.9 | 674.1 | 100 |
| ES-EC103 | 32 | CTX-M-14 | 96 | 400.12 | 42.1 | 100 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harada, K.; Shimizu, T.; Kawaguchi, K.; Furuhashi, T.; Ishihara, G. Urinary Pharmacokinetic and Pharmacodynamic Profiles of Fosfomycin against Extended-Spectrum β-Lactamase-Producing Escherichia coli with Canine Ex Vivo Modeling: A Pilot Study. Antibiotics 2020, 9, 230. https://doi.org/10.3390/antibiotics9050230
Harada K, Shimizu T, Kawaguchi K, Furuhashi T, Ishihara G. Urinary Pharmacokinetic and Pharmacodynamic Profiles of Fosfomycin against Extended-Spectrum β-Lactamase-Producing Escherichia coli with Canine Ex Vivo Modeling: A Pilot Study. Antibiotics. 2020; 9(5):230. https://doi.org/10.3390/antibiotics9050230
Chicago/Turabian StyleHarada, Kazuki, Takae Shimizu, Koji Kawaguchi, Takeshi Furuhashi, and Genki Ishihara. 2020. "Urinary Pharmacokinetic and Pharmacodynamic Profiles of Fosfomycin against Extended-Spectrum β-Lactamase-Producing Escherichia coli with Canine Ex Vivo Modeling: A Pilot Study" Antibiotics 9, no. 5: 230. https://doi.org/10.3390/antibiotics9050230
APA StyleHarada, K., Shimizu, T., Kawaguchi, K., Furuhashi, T., & Ishihara, G. (2020). Urinary Pharmacokinetic and Pharmacodynamic Profiles of Fosfomycin against Extended-Spectrum β-Lactamase-Producing Escherichia coli with Canine Ex Vivo Modeling: A Pilot Study. Antibiotics, 9(5), 230. https://doi.org/10.3390/antibiotics9050230




