Taurolidine Acts on Bacterial Virulence Factors and Does Not Induce Resistance in Periodontitis-Associated Bacteria—An In-Vitro Study
Abstract
1. Introduction
2. Results
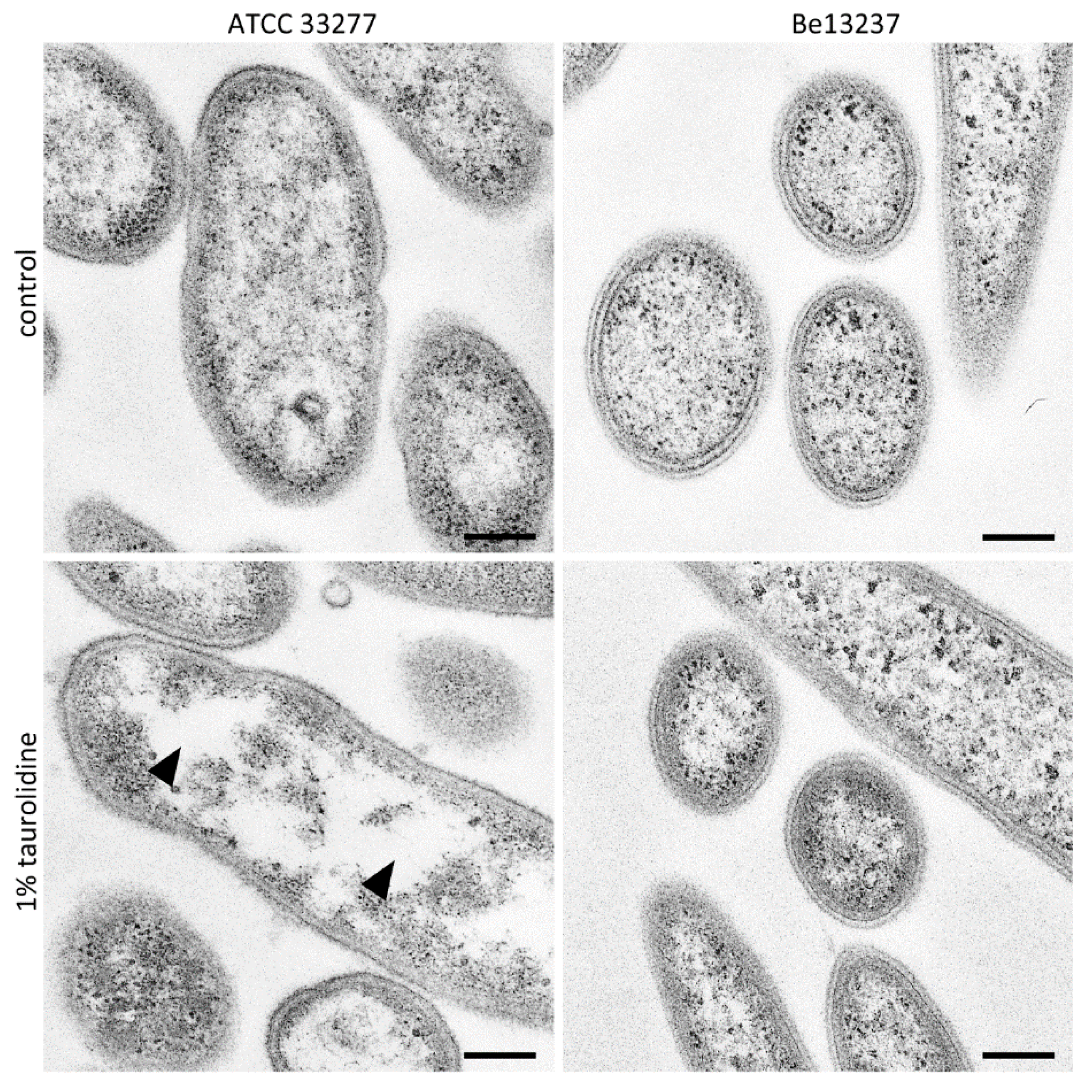
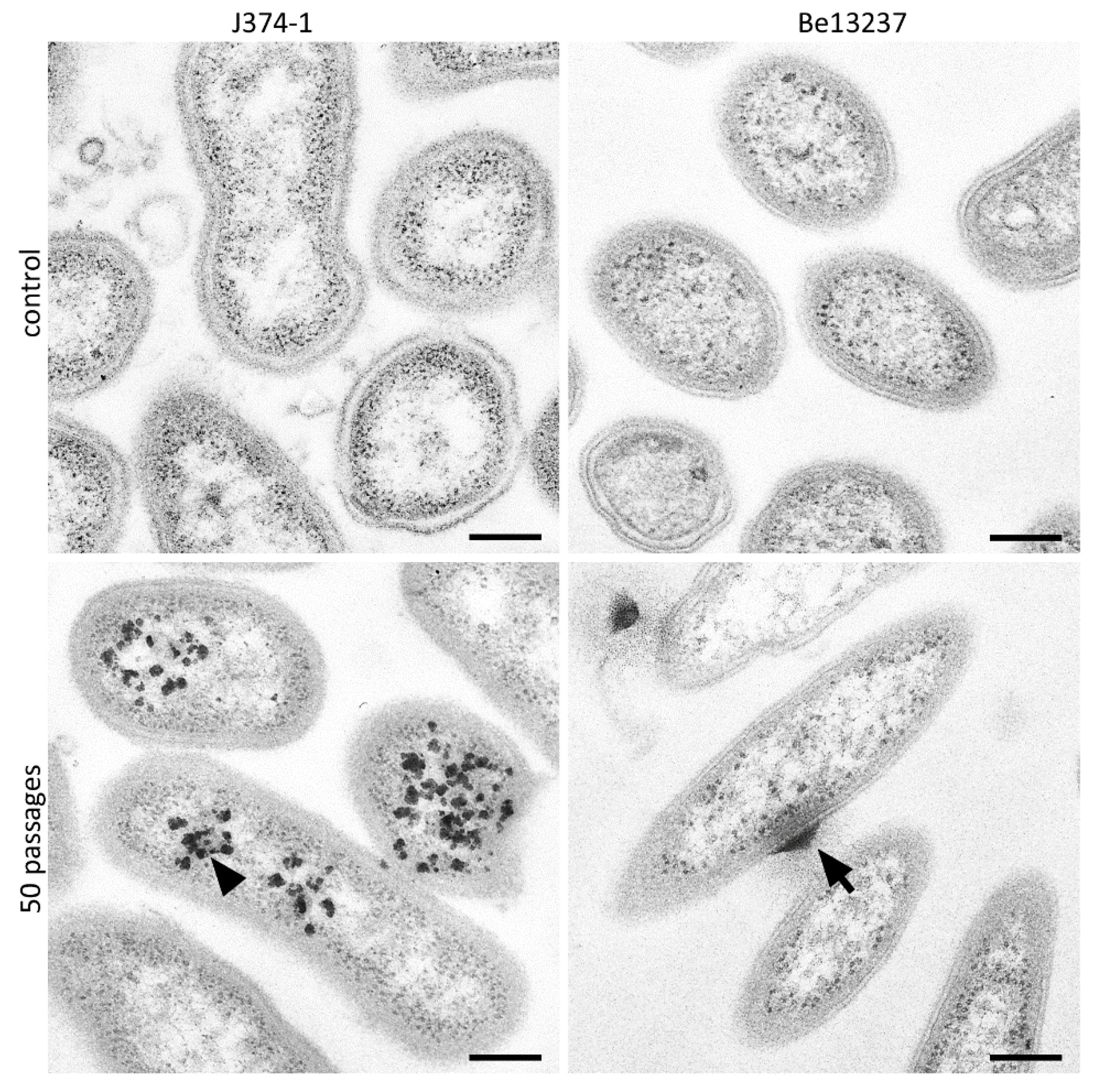
2.1. Visualization of Taurolidine Action
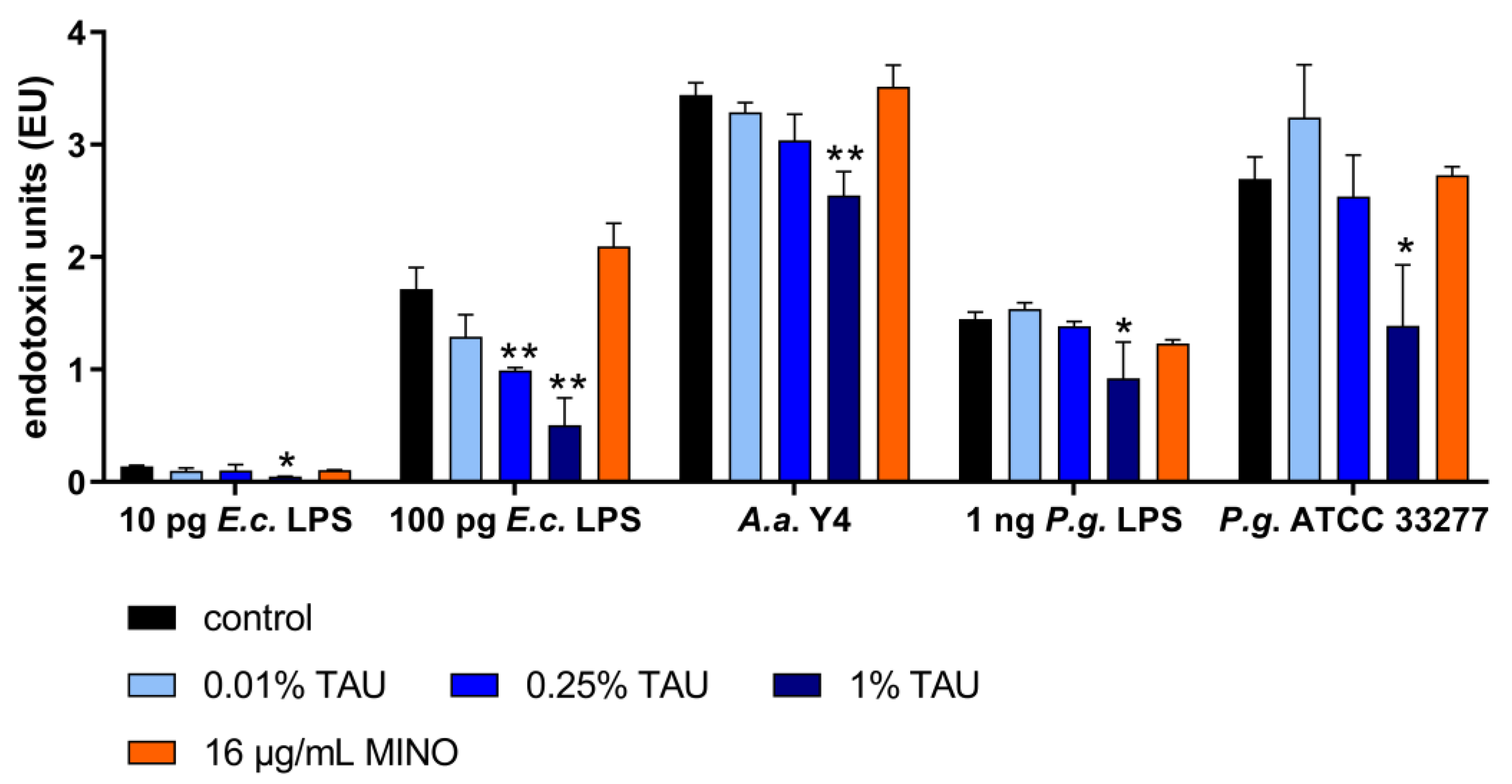
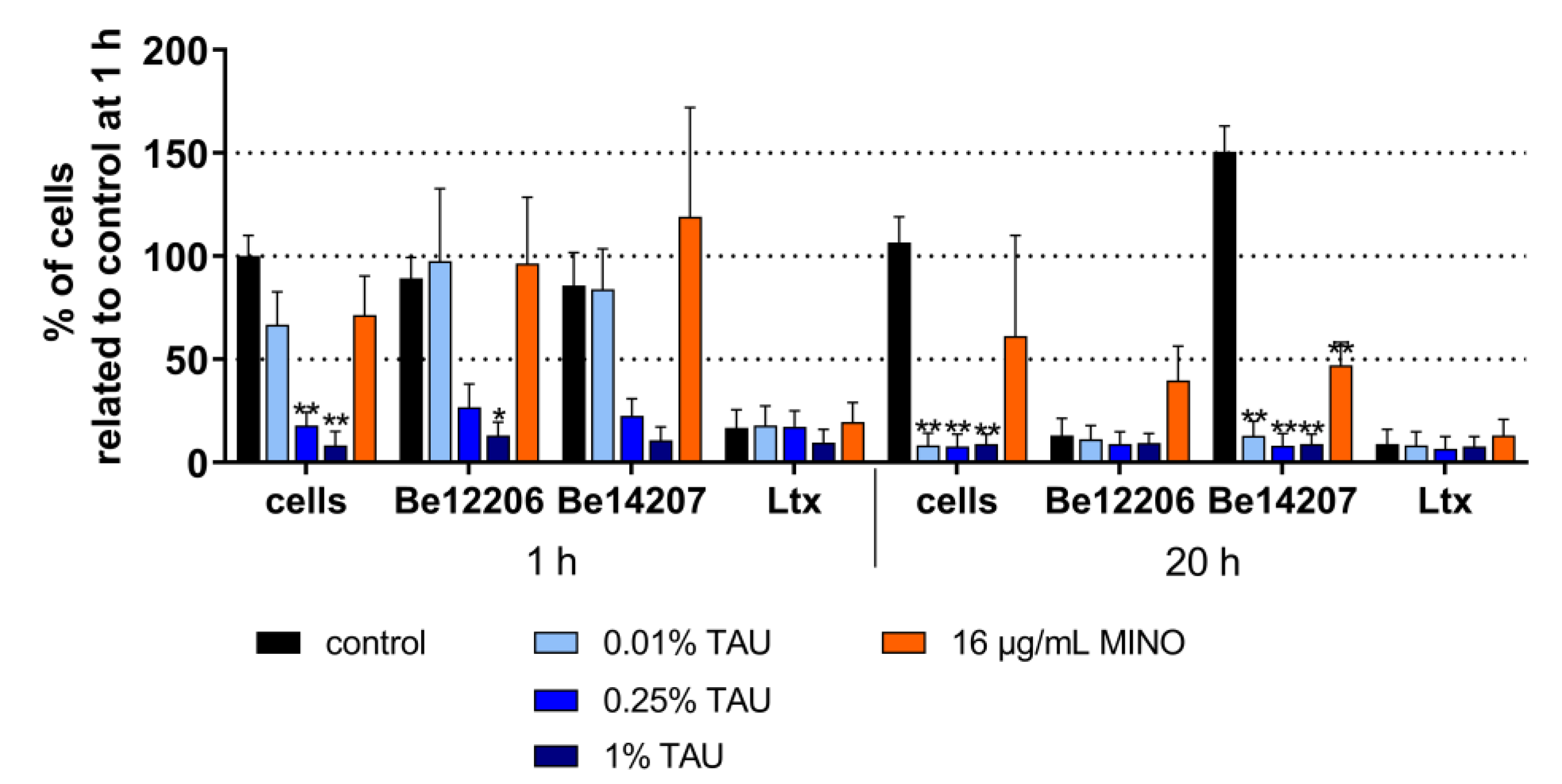
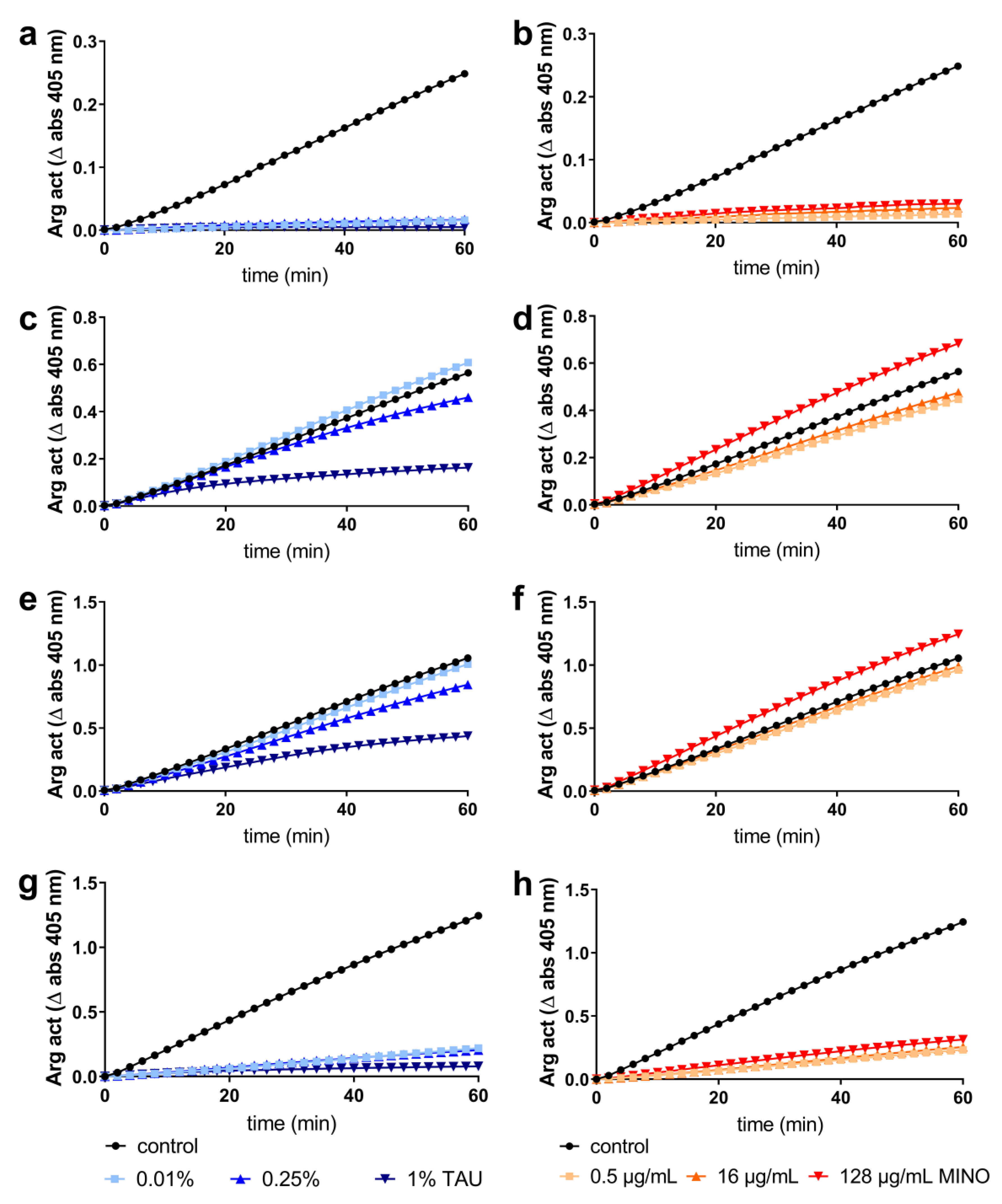
2.2. Interaction with Most Important Virulence Factors
2.3. Potential Development of Resistance
3. Discussion
4. Materials and Methods
4.1. Antimicrobials
4.2. Microorganisms
4.3. Cells
4.4. TEM Images after Exposure to 1% Taurolidine
4.5. Interaction with Bacterial Virulence Factors
4.6. Potential Development of Resistance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eke, P.I.; Thornton-Evans, G.O.; Wei, L.; Borgnakke, W.S.; Dye, B.A.; Genco, R.J. Periodontitis in US Adults: National Health and Nutrition Examination Survey 2009–2014. J. Am. Dent. Assoc. 2018, 149, 576–588.e6. [Google Scholar] [CrossRef] [PubMed]
- Mark Bartold, P.; Van Dyke, T.E. Host modulation: Controlling the inflammation to control the infection. Periodontol 2000 2017, 75, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2015, 15, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Nguyen, K.A.; Potempa, J. Dichotomy of gingipains action as virulence factors: From cleaving substrates with the precision of a surgeon’s knife to a meat chopper-like brutal degradation of proteins. Periodontol 2000 2010, 54, 15–44. [Google Scholar] [CrossRef]
- Sharma, A. Virulence mechanisms of Tannerella forsythia. Periodontol 2000 2010, 54, 106–116. [Google Scholar] [CrossRef]
- Herbert, B.A.; Novince, C.M.; Kirkwood, K.L. Aggregatibacter actinomycetemcomitans, a potent immunoregulator of the periodontal host defense system and alveolar bone homeostasis. Mol. Oral. Microbiol. 2016, 31, 207–227. [Google Scholar] [CrossRef]
- Brogan, J.M.; Lally, E.T.; Poulsen, K.; Kilian, M.; Demuth, D.R. Regulation of Actinobacillus actinomycetemcomitans leukotoxin expression: Analysis of the promoter regions of leukotoxic and minimally leukotoxic strains. Infect. Immun. 1994, 62, 501–508. [Google Scholar] [CrossRef]
- Da Costa, L.; Amaral, C.; Barbirato, D.D.S.; Leao, A.T.T.; Fogacci, M.F. Chlorhexidine mouthwash as an adjunct to mechanical therapy in chronic periodontitis: A meta-analysis. J. Am. Dent. Assoc. 2017, 148, 308–318. [Google Scholar] [CrossRef]
- Zandbergen, D.; Slot, D.E.; Niederman, R.; Van der Weijden, F.A. The concomitant administration of systemic amoxicillin and metronidazole compared to scaling and root planing alone in treating periodontitis: =a systematic review=. BMC Oral. Health 2016, 16, 27. [Google Scholar] [CrossRef]
- Harks, I.; Koch, R.; Eickholz, P.; Hoffmann, T.; Kim, T.S.; Kocher, T.; Meyle, J.; Kaner, D.; Schlagenhauf, U.; Doering, S.; et al. Is progression of periodontitis relevantly influenced by systemic antibiotics? A clinical randomized trial. J. Clin. Periodontol. 2015, 42, 832–842. [Google Scholar] [CrossRef]
- Salvi, G.E.; Mombelli, A.; Mayfield, L.; Rutar, A.; Suvan, J.; Garrett, S.; Lang, N.P. Local antimicrobial therapy after initial periodontal treatment. J. Clin. Periodontol. 2002, 29, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Wouters, Y.; Theilla, M.; Singer, P.; Tribler, S.; Jeppesen, P.B.; Pironi, L.; Vinter-Jensen, L.; Rasmussen, H.H.; Rahman, F.; Wanten, G.J.A. Randomised clinical trial: 2% taurolidine versus 0.9% saline locking in patients on home parenteral nutrition. Aliment. Pharmacol. Ther. 2018, 48, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Eick, S.; Radakovic, S.; Pfister, W.; Nietzsche, S.; Sculean, A. Efficacy of taurolidine against periodontopathic species--an in vitro study. Clin. Oral. Investig. 2012, 16, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, S.; Moran, J.; Addy, M.; Wade, W.G.; Newcombe, R. Taurolin as an oral rinse. I. Antimicrobial effects in vitro and in vivo. Clin. Prev. Dent. 1991, 13, 13–17. [Google Scholar] [PubMed]
- Muhlemann, H.R.; Strub, J.R. Inhibition of plaque growth with taurolin, vantocil and amine fluoride. Helv. Odontol. Acta 1975, 19, 57–60. [Google Scholar] [PubMed]
- Zollinger, L.; Schnyder, S.; Nietzsche, S.; Sculean, A.; Eick, S. In-vitro activity of taurolidine on single species and a multispecies population associated with periodontitis. Anaerobe 2015, 32, 18–23. [Google Scholar] [CrossRef]
- Pirracchio, L.; Joos, A.; Luder, N.; Sculean, A.; Eick, S. Activity of taurolidine gels on ex vivo periodontal biofilm. Clin. Oral. Investig. 2018, 22, 2031–2037. [Google Scholar] [CrossRef]
- Allcock, S.; Young, E.H.; Holmes, M.; Gurdasani, D.; Dougan, G.; Sandhu, M.S.; Solomon, L.; Torok, M.E. Antimicrobial resistance in human populations: Challenges and opportunities. Glob. Health Epidemiol. Genom. 2017, 2, e4. [Google Scholar] [CrossRef]
- Bell, B.G.; Schellevis, F.; Stobberingh, E.; Goossens, H.; Pringle, M. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect. Dis. 2014, 14, 13. [Google Scholar] [CrossRef]
- McDonnell, G.; Russell, A.D. Antiseptics and disinfectants: Activity, action, and resistance. Clin. Microbiol. Rev. 1999, 12, 147–179. [Google Scholar] [CrossRef]
- White, D.G.; McDermott, P.F. Biocides, drug resistance and microbial evolution. Curr. Opin. Microbiol. 2001, 4, 313–317. [Google Scholar] [CrossRef]
- Russell, A.D. Bacterial resistance to disinfectants: Present knowledge and future problems. J. Hosp. Infect. 1999, 43, S57–S68. [Google Scholar] [CrossRef]
- Russell, A.D. Mechanisms of bacterial insusceptibility to biocides. Am. J. Infect. Control. 2001, 29, 259–261. [Google Scholar] [CrossRef]
- Poole, K. Mechanisms of bacterial biocide and antibiotic resistance. J. Appl. Microbiol. 2002, 92 Suppl, 55S–64S. [Google Scholar] [CrossRef]
- Cieplik, F.; Jakubovics, N.S.; Buchalla, W.; Maisch, T.; Hellwig, E.; Al-Ahmad, A. Resistance Toward Chlorhexidine in Oral Bacteria—Is There Cause for Concern? Front. Microbiol. 2019, 10, 587. [Google Scholar] [CrossRef] [PubMed]
- Rangarajan, M.; Aduse-Opoku, J.; Paramonov, N.; Hashim, A.; Bostanci, N.; Fraser, O.P.; Tarelli, E.; Curtis, M.A. Identification of a second lipopolysaccharide in Porphyromonas gingivalis W50. J. Bacteriol. 2008, 190, 2920–2932. [Google Scholar] [CrossRef] [PubMed]
- Shah, C.B.; Mittelman, M.W.; Costerton, J.W.; Parenteau, S.; Pelak, M.; Arsenault, R.; Mermel, L.A. Antimicrobial activity of a novel catheter lock solution. Antimicrob. Agents. Chemother. 2002, 46, 1674–1679. [Google Scholar] [CrossRef] [PubMed]
- Caruso, F.; Darnowski, J.W.; Opazo, C.; Goldberg, A.; Kishore, N.; Agoston, E.S.; Rossi, M. Taurolidine antiadhesive properties on interaction with E. coli; its transformation in biological environment and interaction with bacteria cell wall. PLoS ONE 2010, 5, e8927. [Google Scholar] [CrossRef] [PubMed]
- Van Oosten, B.; Marquardt, D.; Komljenovic, I.; Bradshaw, J.P.; Sternin, E.; Harroun, T.A. Small molecule interaction with lipid bilayers: A molecular dynamics study of chlorhexidine. J. Mol. Graph. Model. 2014, 48, 96–104. [Google Scholar] [CrossRef]
- Gidley, M.J.; Sanders, J.K.; Myers, E.R.; Allwood, M.C. The mode of antibacterial action of some ‘masked’ formaldehyde compounds. FEBS Lett. 1981, 127, 225–227. [Google Scholar] [CrossRef]
- Bedrosian, I.; Sofia, R.D.; Wolff, S.M.; Dinarello, C.A. Taurolidine, an analogue of the amino acid taurine, suppresses interleukin 1 and tumor necrosis factor synthesis in human peripheral blood mononuclear cells. Cytokine 1991, 3, 568–575. [Google Scholar] [CrossRef]
- Tang, G.; Kawai, T.; Komatsuzawa, H.; Mintz, K.P. Lipopolysaccharides mediate leukotoxin secretion in Aggregatibacter actinomycetemcomitans. Mol. Oral. Microbiol. 2012, 27, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Ziegler-Heitbrock, H.W.; Thiel, E.; Futterer, A.; Herzog, V.; Wirtz, A.; Riethmuller, G. Establishment of a human cell line (Mono Mac 6) with characteristics of mature monocytes. Int. J. Cancer 1988, 41, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Saiki, K.; Konishi, K. Identification of a novel Porphyromonas gingivalis outer membrane protein, PG534, required for the production of active gingipains. FEMS Microbiol. Lett. 2010, 310, 168–174. [Google Scholar] [CrossRef][Green Version]
- Nguyen, K.A.; Travis, J.; Potempa, J. Does the importance of the C-terminal residues in the maturation of RgpB from Porphyromonas gingivalis reveal a novel mechanism for protein export in a subgroup of Gram-Negative bacteria? J. Bacteriol. 2007, 189, 833–843. [Google Scholar] [CrossRef]
- Baffone, W.; Pianetti, A.; Citterio, B.; Lombardelli, G.; Vittoria, E.; Bruscolini, F. Studies on the development and stability of resistance of Helicobacter pylori to metronidazole and clarithromycin. J. Chemother. 2001, 13, 126–132. [Google Scholar] [CrossRef]
- Eick, S.; Schmitt, A.; Sachse, S.; Schmidt, K.H.; Pfister, W. In vitro antibacterial activity of fluoroquinolones against Porphyromonas gingivalis strains. J. Antimicrob. Chemother. 2004, 54, 553–556. [Google Scholar] [CrossRef]
- Rahman, T.; Yarnall, B.; Doyle, D.A. Efflux drug transporters at the forefront of antimicrobial resistance. Eur. Biophys. J. 2017, 46, 647–653. [Google Scholar] [CrossRef]
- Poole, K. Efflux-mediated antimicrobial resistance. J. Antimicrob. Chemother. 2005, 56, 20–51. [Google Scholar] [CrossRef]
- Saleem, H.G.; Seers, C.A.; Sabri, A.N.; Reynolds, E.C. Dental plaque bacteria with reduced susceptibility to chlorhexidine are multidrug resistant. BMC Microbiol. 2016, 16, 214. [Google Scholar] [CrossRef]
- Mombeshora, M.; Mukanganyama, S. Development of an accumulation assay and evaluation of the effects of efflux pump inhibitors on the retention of chlorhexidine digluconate in Pseudomonas aeruginosa and Staphylococcus aureus. BMC Res. Notes 2017, 10, 328. [Google Scholar] [CrossRef] [PubMed]
- Duraes, F.; Pinto, M.M.M.; de Sousa, M. Medicinal Chemistry Updates on Bacterial Efflux Pump Modulators. Curr. Med. Chem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, A.; Steinke, P.; Bohnert, J.A.; Akova, M.; Jonas, D.; Kern, W.V. Effect of 1-(1-naphthylmethyl)-piperazine, a novel putative efflux pump inhibitor, on antimicrobial drug susceptibility in clinical isolates of Enterobacteriaceae other than Escherichia coli. J. Antimicrob. Chemother. 2006, 57, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Pumbwe, L.; Glass, D.; Wexler, H.M. Efflux pump overexpression in multiple-antibiotic-resistant mutants of Bacteroides fragilis. Antimicrob. Agents. Chemother. 2006, 50, 3150–3153. [Google Scholar] [CrossRef]
- Nguyen, F.; Starosta, A.L.; Arenz, S.; Sohmen, D.; Donhofer, A.; Wilson, D.N. Tetracycline antibiotics and resistance mechanisms. Biol. Chem. 2014, 395, 559–575. [Google Scholar] [CrossRef]
- Rams, T.E.; Feik, D.; Mortensen, J.E.; Degener, J.E.; van Winkelhoff, A.J. Antibiotic susceptibility of periodontal Streptococcus constellatus and Streptococcus intermedius clinical isolates. J. Periodontol. 2014, 85, 1792–1798. [Google Scholar] [CrossRef]
- Rams, T.E.; Degener, J.E.; van Winkelhoff, A.J. Antibiotic resistance in human chronic periodontitis microbiota. J. Periodontol. 2014, 85, 160–169. [Google Scholar] [CrossRef]
- Akrivopoulou, C.; Green, I.M.; Donos, N.; Nair, S.P.; Ready, D. Aggregatibacter actinomycetemcomitans serotype prevalence and antibiotic resistance in a UK population with periodontitis. J. Glob. Antimicrob. Resist. 2017, 10, 54–58. [Google Scholar] [CrossRef]
- Veloo, A.C.; Seme, K.; Raangs, E.; Rurenga, P.; Singadji, Z.; Wekema-Mulder, G.; van Winkelhoff, A.J. Antibiotic susceptibility profiles of oral pathogens. Int. J. Antimicrob. Agents. 2012, 40, 450–454. [Google Scholar] [CrossRef]
- Larsen, T.; Fiehn, N.E. Development of resistance to metronidazole and minocycline in vitro. J. Clin. Periodontol. 1997, 24, 254–259. [Google Scholar] [CrossRef]
- Eick, S.; Straube, A.; Guentsch, A.; Pfister, W.; Jentsch, H. Comparison of real-time polymerase chain reaction and DNA-strip technology in microbiological evaluation of periodontitis treatment. Diagn. Microbiol. Infect Dis. 2011, 69, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Kachlany, S.C.; Fine, D.H.; Figurski, D.H. Purification of secreted leukotoxin (LtxA) from Actinobacillus actinomycetemcomitans. Protein. Expr. Purif. 2002, 25, 465–471. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Eick, S.; Goltz, S.; Nietzsche, S.; Jentsch, H.; Pfister, W. Efficacy of chlorhexidine digluconate-containing formulations and other mouthrinses against periodontopathogenic microorganisms. Quintessence Int. 2011, 42, 687–700. [Google Scholar] [PubMed]
| Strain | Baseline | After 50 Pass. | With 20 µg/mL Reserpine | With 50 µg/mL NMP | With 10 µg/mL CCCP | With 100 µg/mL Verapamil | After 3 pass. w/o Taurolidine |
|---|---|---|---|---|---|---|---|
| P. gingivalis J374-1 | 0.025 | 0.1 | 0.013 | 0.013 | 0.006 | 0.006 | 0.025 |
| S. constellatus BeTa7-1 | 0.1 | 0.025 | n.d. | n.d. | n.d. | n.d. | 0.05 |
| T. forsythia Be13237 | 0.025 | ≤0.003 | n.d. | n.d. | n.d. | n.d. | 0.025 |
| T. forsythia Be13216 | 0.025 | ≤0.003 | n.d. | n.d. | n.d. | n.d. | 0.013 |
| Strain | Baseline | After 50 pass. | With 20 µg/mL reserpine | With 50 µg/mL NMP | With 10 µg/mL CCCP | With 100 µg/mL verapamil | After 3 pass. w/o MINO |
|---|---|---|---|---|---|---|---|
| Streptococcus oralis JM933 | 1 | 4 | 4 | 4 | 4 | 4 | 4 |
| A. actinom. OMZ 444 | 1 | 32 | 8 | ≤0.5 | ≤0.5 | 8 | 32 |
| A. actinom. Be14207 | 8 | 32 | 8 | 16 | 32 | 4 | 32 |
| Fusobacterium nucleatum BeTa9-1 | 1 | 4 | 4 | 4 | 4 | 4 | 4 |
| T. forsythia Be13216 | 0.5 | 2 | 2 | 2 | 2 | 2 | 2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radakovic, S.; Andreoli, N.; Schmid, S.; Nietzsche, S.; Zumbrunn, J.; Sculean, A.; Eick, S. Taurolidine Acts on Bacterial Virulence Factors and Does Not Induce Resistance in Periodontitis-Associated Bacteria—An In-Vitro Study. Antibiotics 2020, 9, 166. https://doi.org/10.3390/antibiotics9040166
Radakovic S, Andreoli N, Schmid S, Nietzsche S, Zumbrunn J, Sculean A, Eick S. Taurolidine Acts on Bacterial Virulence Factors and Does Not Induce Resistance in Periodontitis-Associated Bacteria—An In-Vitro Study. Antibiotics. 2020; 9(4):166. https://doi.org/10.3390/antibiotics9040166
Chicago/Turabian StyleRadakovic, Sabrina, Nicola Andreoli, Simon Schmid, Sandor Nietzsche, Jürg Zumbrunn, Anton Sculean, and Sigrun Eick. 2020. "Taurolidine Acts on Bacterial Virulence Factors and Does Not Induce Resistance in Periodontitis-Associated Bacteria—An In-Vitro Study" Antibiotics 9, no. 4: 166. https://doi.org/10.3390/antibiotics9040166
APA StyleRadakovic, S., Andreoli, N., Schmid, S., Nietzsche, S., Zumbrunn, J., Sculean, A., & Eick, S. (2020). Taurolidine Acts on Bacterial Virulence Factors and Does Not Induce Resistance in Periodontitis-Associated Bacteria—An In-Vitro Study. Antibiotics, 9(4), 166. https://doi.org/10.3390/antibiotics9040166







