Solidago virgaurea L. Plant Extract Targeted against Candida albicans to Reduce Oral Microbial Biomass: A Double Blind Randomized Trial on Healthy Adults
Abstract
1. Introduction
2. Results
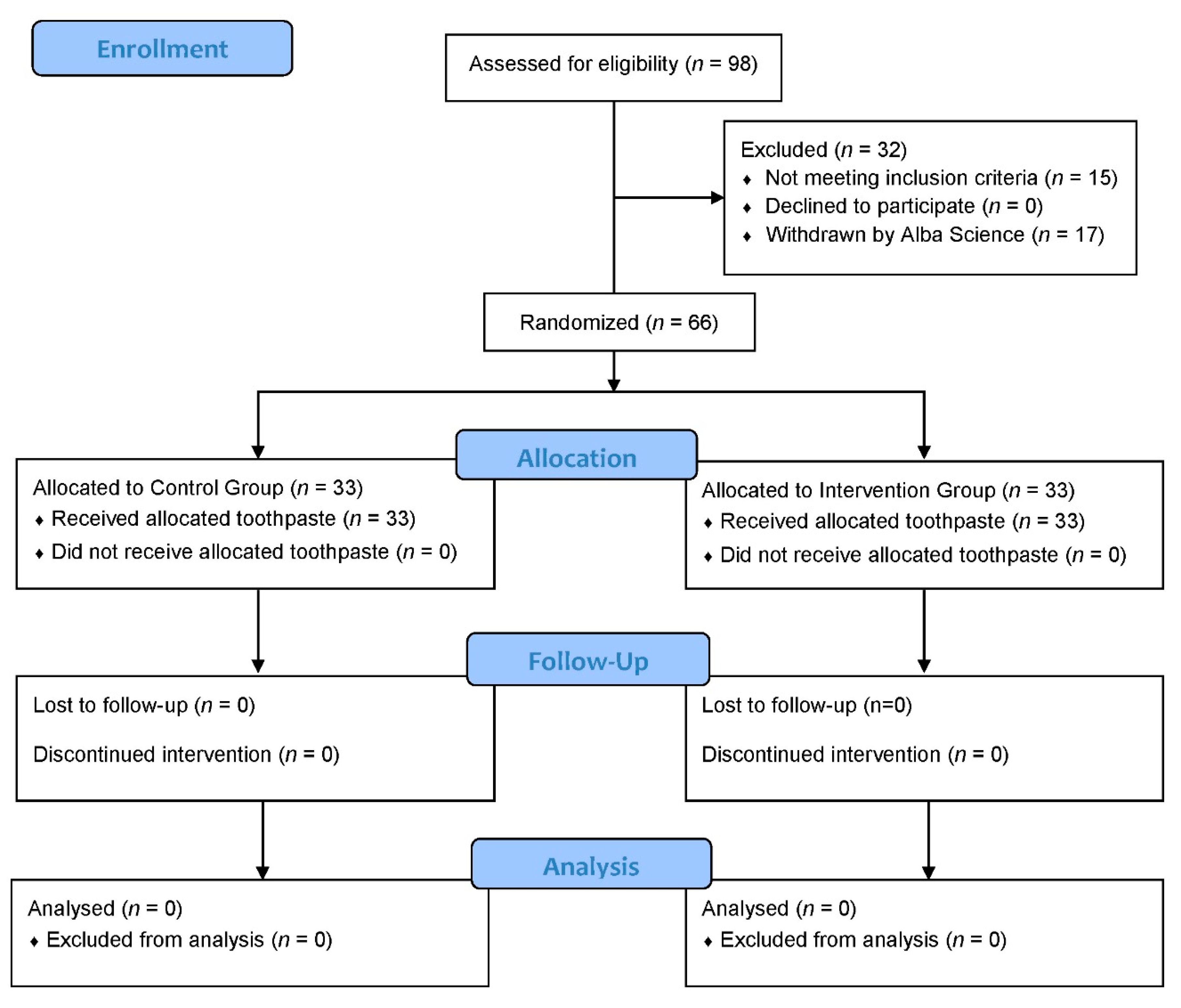
2.1. Participants
2.1.1. Flow Diagram
2.1.2. Baseline Characteristics of Participants
2.2. Microbial Numeration
2.2.1. Quantitative Polymerase Chain Reaction (qPCR) Results
2.2.2. Total Bacterial Load
2.3. Participants’s Scores and Questionnaire Answers
3. Discussion
4. Materials and Methods
4.1. Experimental Toothpastes
4.1.1. Plant Material
4.1.2. Solidago virgaurea Extraction Process
4.1.3. Toothpastes Formulation and Safety Controls
4.2. Clinical Study
4.2.1. Hypothesis, Protocol Design, and Ethics Committee
4.2.2. Endpoints
- Primary endpoint
- Secondary endpoints
4.2.3. Inclusion and Exclusion Criteria
- Inclusion criteria
- Exclusion criteria
4.2.4. Randomization
4.2.5. Dental Plaque Sampling
4.2.6. qPCR Analysis
4.2.7. Statistical Method
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marsh, P.D.; Zaura, E. Dental biofilm: Ecological interactions in health and disease. J. Clin. Periodontol. 2017, 44 (Suppl. 18), S12–S22. [Google Scholar] [CrossRef]
- Mira, A. Oral microbiome studies: Potential diagnostic and therapeutic implications. Adv. Dent. Res. 2018, 29, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.; Garg, P.K.; Dubey, A.K. Insights into the human oral microbiome. Arch. Microbiol. 2018, 200, 525–540. [Google Scholar] [CrossRef] [PubMed]
- Wade, W.G. New aspects and new concepts of maintaining “microbiological” health. J. Dent. 2010, 38, S21–S25. [Google Scholar] [CrossRef]
- Ghannoum, M.A.; Jurevic, R.J.; Mukherjee, P.K.; Cui, F.; Sikaroodi, M.; Naqvi, A.; Gillevet, P.M. Characterization of the oral fungal microbiome (mycobiome) in healthy individuals. PLoS Pathog. 2010, 6, e1000713. [Google Scholar] [CrossRef] [PubMed]
- Limon, J.J.; Skalski, J.H.; Underhill, D.M. Commensal fungi in health and disease. Cell Host Microbe 2017, 22, 156–165. [Google Scholar] [CrossRef]
- Lof, M.; Janus, M.M.; Krom, B.P. Metabolic interactions between bacteria and fungi in commensal oral biofilms. J. Fungi 2017, 3, 40. [Google Scholar] [CrossRef]
- Bowen, W.H.; Burne, R.A.; Wu, H.; Koo, H. Oral biofilms: Pathogens, matrix, and polymicrobial interactions in microenvironments. Trends Microbiol. 2018, 26, 229–242. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, Z.; Hwang, G.; Koo, H. Therapeutic strategies targeting cariogenic biofilm microenvironment. Adv. Dent. Res. 2018, 29, 86–92. [Google Scholar] [CrossRef]
- Hong, C.; Aung, M.M.; Kanagasabai, K.; Lim, C.A.; Liang, S.; Tan, K.S. The association between oral health status and respiratory pathogen colonization with pneumonia risk in institutionalized adults. Int. J. Dent. Hyg. 2018, 16, e96–e102. [Google Scholar] [CrossRef]
- Koo, H.; Andes, D.R.; Krysan, D.J. Candida-streptococcal interactions in biofilm-associated oral diseases. PLoS Pathog. 2018, 14, e1007342. [Google Scholar] [CrossRef] [PubMed]
- Falsetta, M.L.; Klein, M.I.; Colonne, P.M.; Scott-Anne, K.; Gregoire, S.; Pai, C.H.; Gonzalez-Begne, M.; Watson, G.; Krysan, D.J.; Bowen, W.H.; et al. Symbiotic relationship between Streptococcus mutans and Candida albicans synergizes virulence of plaque biofilms in vivo. Infect. Immun. 2014, 82, 1968–1981. [Google Scholar] [CrossRef] [PubMed]
- Vestby, L.K.; Gronseth, T.; Simm, R.; Nesse, L.L. Bacterial biofilm and its role in the pathogenesis of disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef]
- Blasco-Baque, V.; Garidou, L.; Pomié, C.; Escoula, Q.; Loubières, P.; Le Gall-David, S.; Lemaitre, M.; Nicolas, S.; Klopp, P.; Waget, A.; et al. Periodontitis induced by Porphyromonas gingivalis drives periodontal microbiota dysbiosis and insulin resistance via impaired adaptive immune response. Gut 2017, 66, 872–885. [Google Scholar] [CrossRef] [PubMed]
- Bunte, K.; Beikler, T. Th17 cells and the IL-23/IL-17 axis in the pathogenesis of periodontitis and immune-mediated inflammatory diseases. Int. J. Mol. Sci. 2019, 20, 3394. [Google Scholar] [CrossRef]
- Moura, M.F.; Navarro, T.P.; Silva, T.A.; Cota, L.O.M.; Soares Dutra Oliveira, A.M.; Costa, F.O. Periodontal and endothelial dysfunction: Periodontal clinical parameters and levels of salivary markers interleukin-1β, tumor necrosis factor-α, matrix metalloproteinases-2, tissue inhibitor of metalloproteinases-2 compls, and nitric oxide. J. Periodontol. 2017, 88, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Isola, G.; Polizzi, A.; Alibrandi, A.; Indelicato, F.; Ferlito, S. Analysis of Endothelin-1 Concentrations in individuals with periodontitis. Sci. Rep. 2020, 10, 1652. [Google Scholar] [CrossRef]
- Gutierrez Gossweiler, A.; Martinez-Mier, E.A. Chapter 6: Vitamins and oral health. Monogrph. Oral. Sci. 2020, 28, 59–67. [Google Scholar] [CrossRef]
- Varela-Lopez, A.; Navarro-Hortal, M.D.; Giampieri, F.; Bullon, P.; Battino, M.; Ouiles, J.L. Nutriceuticals in periodontal health: A systematic review on the role of vitamins in periodontal health maintenance. Molecules 2018, 23, 1226. [Google Scholar] [CrossRef]
- Isola, G. Current evidence of natural agents in oral and periodontal health. Nutrients 2020, 12, 585. [Google Scholar] [CrossRef]
- Park, J.A.; Lee, J.H.; Lee, H.J.; Jin, B.H.; Bae, K.H. Association of some vitamins and minerals with periodontitis in a nationally representative sample of Korean Young adults. Biol. Trace Elem. Res. 2017, 178, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Dommisch, H.; Kuzmanova, D.; Jonsson, D.; Grant, M.; Chapple, I. Effect of micronutrient malnutrition on periodontal disease and periodontal therapy. Periodontology 2000 2018, 87, 129–153. [Google Scholar] [CrossRef] [PubMed]
- Gunsolley, J.C. Clinical efficacy of antimicrobial mouthrinses. J. Dent. 2010, 38, S6–S10. [Google Scholar] [CrossRef]
- Riley, P.; Lamont, T. Triclosan/copolymer containing toothpastes for oral health (Review). Cochrane Database Syst. Rev. 2013, 12, CD010514. [Google Scholar] [CrossRef]
- Tezel, U.; Pavlosthathis, S.G. Quaternary ammonium disinfectants: Microbial adaptation, degradation and ecology. Curr. Opin. Biotechnol. 2015, 33, 296–304. [Google Scholar] [CrossRef]
- Zhang, C.; Cui, F.; Zeng, G.M.; Jiang, M.; Yang, Z.Z.; Zhu, M.Y.; Shen, L.Q. Quaternary ammonium compounds (QACs): A review on occurrence, fate and toxicity in the environment. Sci. Total Environ. 2015, 518–519, 352–362. [Google Scholar] [CrossRef]
- James, P.; Worthington, H.V.; Parnell, C.; Harding, M.; Lamont, T.; Cheung, A.; Whelton, H.; Riley, P. Chlorhexidine mouthrinse as an adjunctive treatment for gingival health. Cochrane Database Syst. Rev. 2017, 3, CD008676. [Google Scholar] [CrossRef]
- Wessels, S.; Ingmer, H. Modes of action of three disinfectant active substances: A review. Regul. Toxicol. Pharm. 2013, 67, 456–467. [Google Scholar] [CrossRef]
- Wassenaar, T.M.; Ussery, D.; Nielsen, L.N.; Ingmer, H. Review and phylogenetic analysis of qac genes that reduce susceptibility to quaternary ammonium compounds in Staphylococcus species. Eur. J. Microbiol. Immunol. 2015, 1, 44–61. [Google Scholar] [CrossRef]
- Buffet-Bataillon, S.; Tattevin, P.; Maillard, J.Y.; Bonnaure-Mallet, M.; Jolivet-Gougeon, A. Efflux pump induction by quaternary ammonium compounds and fluoroquinolone resistance in bacteria. Future Microbiol. 2016, 11, 81–92. [Google Scholar] [CrossRef]
- Von der Ohe, P.C.; Schmitt-Jansen, M.; Slobodnik, J.; Brack, W. Triclosan—The forgotten priory substance? Environ. Sci. Pollut. Res. Int. 2012, 19, 585–591. [Google Scholar] [CrossRef]
- Manamsa, K.; Crane, E.; Stuart, M.; Talbot, J.; Lapworth, D.; Hart, A. A national-scale assessment of micro-organic contaminants in groundwater of England and Wales. Sci. Total Environ. 2016, 568, 712–726. [Google Scholar] [CrossRef]
- Clarke, B.O.; Smith, S.R. Review of “emerging” organic contaminants in biosolids and assessment of international research priorities for the agricultural use of biosolids. Environ. Int. 2011, 37, 226–247. [Google Scholar] [CrossRef]
- Dann, A.B.; Hontela, A. Triclosan: Environmental exposure, toxicity and mechanisms of action. J. Appl. Toxicol. 2011, 31, 285–311. [Google Scholar] [CrossRef]
- Bedoux, G.; Roig, B.; Thomas, O.; Dupont, V.; Le Bot, B. Occurrence and toxicity of antimicrobial triclosan and by-products in the environment. Environ. Sci. Pollut. Res. Int. 2012, 19, 1044–1065. [Google Scholar] [CrossRef]
- Dinwiddie, M.T.; Terry, P.D.; Chen, J. Recent evidence regarding triclosan and cancer risk. Int. J. Environ. Res. Public Health 2014, 11, 2209–2217. [Google Scholar] [CrossRef] [PubMed]
- Witorsch, R.J. Critical analysis of endocrine disruptive activity of triclosan and its relevance to human exposure through the use of personal care products. Crit. Rev. Toxicol. 2014, 44, 535–555. [Google Scholar] [CrossRef]
- Salehi, B.; Lopez-Jornet, P.; Pons-Fuster Lopez, E.; Calinia, D.; Sharifi-Rad, M.; Ramirez-Alarcon, K.; Forman, K.; Fernandez, M.; Martorell, M.; Setzer, W.N.; et al. Plant-derived bioactives in oral mucosal lesions: A key emphasis to curcumin, lycopene, chamomile, Aloe vera, green tea and coffee properties. Biomolecules 2019, 9, 106. [Google Scholar] [CrossRef] [PubMed]
- Haas, A.N.; Wagner, T.P.; Muniz, F.W.; Fiorini, T.; Cavagni, J.; Celeste, R.K. Essential oils-containing mouthwashes for gingivitis and plaque: Meta-analyses and meta-regression. J. Dent. 2016, 55, 7–15. [Google Scholar] [CrossRef]
- Lombardo Bedran, T.B.; Palomari Spolidorio, D.; Grenier, D. Green tea polyphenol epigallocatechin-3-gallate and cranberry proanthocyanidins act in synergy with cathelicidin (LL-37) to reduce the LPS-induced inflammatory response in a three-dimensional co-culture model of gingival epithelial cells and fibroblasts. Arch. Oral. Biol. 2015, 60, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Radafshar, G.; Ghotbizadeh, M.; Saadat, F.; Mirfarhadi, N. Effects of green tea (Camellia sinensis) mouthwash containing 1% tannin on dental plaque and chronic gingivitis: A double-blind, randomized, controlled trial. J. Investig. Clin. Dent. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Adami, G.R.; Tangney, C.C.; Tang, J.L.; Zhou, Y.; Ghaffari, S.; Naqib, A.; Sinha, S.; Green, S.J.; Schwartz, J.L. Effects of green tea on miRNA and microbiome of oral epithelium. Sci. Rep. 2018, 8, 5873. [Google Scholar] [CrossRef] [PubMed]
- Isola, G.; Matarese, M.; Ramaglia, L.; Iorio-Siciliano, V.; Cordasco, G.; Matarese, G. Efficacy of a drug composed of herbal extracts on postoperative discomfort after surgical removal of impacted mandibular third molar: A randomized, triple blind, controlled clinical trial. Clin. Oral. Investig. 2019, 23, 2443–2453. [Google Scholar] [CrossRef] [PubMed]
- Unban, K.; Khatthongngam, N.; Shetty, K.; Khanongnuch, C. Nutritional biotransformation in traditional fermented tea (Miang) from north Thailand and its impact on antioxidant and antimicrobial activities. J. Food Sci. Technol. 2019, 56, 2687–2699. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.C.; Son, Y.O.; Hwang, J.M.; Kim, B.T.; Chae, M.; Lee, J.C. Antioxidant, anti-inflammatory and anti-septic potential of phenolic acids and flavonoid fractions isolated form Lilium multiflorum. Pharm. Biol. 2017, 55, 611–619. [Google Scholar] [CrossRef]
- Mugita, N.; Nambu, T.; Takahashi, K.; Wang, P.L.; Komasa, Y. Proteases, actinidin, papain and trypsin reduce oral biofilm on the tongue in elderly subjects and in vitro. Arch. Oral Biol. 2017, 82, 233–240. [Google Scholar] [CrossRef]
- Seleem, D.; Pardi, V.; Murata, R.M. Review of flavonoids: A diverse group of natural compounds with anti-Candida albicans activity in vitro. Arch. Oral Biol. 2017, 76, 76–83. [Google Scholar] [CrossRef]
- De Souza, G.M.; Fernandes, I.A.; Dos Santos, C.R.R.; Falci, S.G.M. Is bromelain effective in controlling the inflammatory parameters of pain, edema, and trismus after lower third molar surgery? A systematic review and meta-analysis. Phytother. Res. 2019, 33, 473–481. [Google Scholar] [CrossRef]
- Woelber, J.P.; Bremer, K.; Vach, K.; Konig, D.; Hellwig, E.; Ratka-Kruger, P.; Al-Ahmad, A.; Tennert, C. An oral health optimized diet can reduce gingival and periodontal inflammation in humans—A randomized controlled pilot study. BMC Oral Health 2016, 17, 28. [Google Scholar] [CrossRef]
- Isola, G.; Polizzi, A.; Muraglie, S.; Leonardi, R.; Lo Giudice, A. Assessment of vitamin C and antioxidant profiles in saliva and serum in patients with periodontitis and ischemic heart disease. Nutrients 2019, 11, 2956. [Google Scholar] [CrossRef]
- Chevalier, M.; Médioni, E.; Prêcheur, I. Inhibition of Candida albicans yeast-hyphal transition and biofilm formation by Solidago virgaurea water extract. J. Med. Microbiol. 2012, 61, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Laurençon, L.; Sarrazin, E.; Chevalier, M.; Prêcheur, I.; Herbette, G.; Fernandez, X. Inhibition of Candida albicans yeast-hyphal conversion by triterpenoid saponins from the aerial parts of Solidago virgaurea alpestris. Phytochemistry 2013, 86, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, M.; Doglio, A.; Rajendran, R.; Ramage, G.; Prêcheur, I.; Ranque, S. Acute inhibition of adhesion-specific genes by Solidago virgaurea plant extract causes loss of Candida albicans biofilm integrity. J. Appl. Microbiol. 2019, 23. [Google Scholar] [CrossRef]
- Abusleme, L.; Dupuyn, A.K.; Dutzan, N.; Silva, N.; Burleson, J.A.; Strausbaugh, L.D.; Gamonal, J.; Diaz, P.I. The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation. ISME J. 2013, 7, 1016–1025. [Google Scholar] [CrossRef]
- Stsepetova, J.; Truu, J.; Runne, R.; Nommela, R.; Saag, M.; Olak, J.H.; Nolvak, H.; Preem, J.K.; Oopkaup, K.; Krjutskow, K.; et al. Impact of polyols on oral microbiome of Estonian schoolchildren. BMC Oral Health 2019, 19, 60. [Google Scholar] [CrossRef]
- Chen, Z.; Saxena, D.; Caufield, P.W.; Ge, Y.; Wang, M.; Li, Y. Development of species-specific primers for detection of Streptococcus mutans in mixed bacterial samples. FEMS Microbiol. Lett. 2007, 272, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Lin, C.T.; Wu, C.Y.; Peng, W.S.; Lee, M.J.; Tsai, Y.C. Inhibitory effect of Lactobacillus salivarius on Streptococcus mutans biofilm formation. Mol. Oral Microbiol. 2015, 30, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Crisea, G.; Scordino, F.; Romeo, O. Current methods for identifying clinically important cryptic Candida species. J. Microbiol. Methods 2015, 111, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, M.; Ranjan, A.; Thompson, A.; Diaz, P.I.; Sobue, T.; Maas, K.; Dongari-Bagtzoglou, A. Candida albicans induces mucosal bacterial dysbiosis that promotes invasive infection. PLoS Pathog. 2019, 15, e1007717. [Google Scholar] [CrossRef]
- Adams, S.E.; Arnold, D.; Murphy, B.; Carroll, P.; Green, A.K.; Smith, A.M.; Marsh, P.D.; Chen, T.; Marriott, R.E.; Brading, M.G. A randomised clinical study to determine the effect of a toothpaste containing enzymes and proteins on plaque oral microbiome ecology. Sci. Rep. 2017, 7, 43344. [Google Scholar] [CrossRef]
- Edlund, A.; Yang, Y.; Yooseph, S.; He, X.; Wenyuan, S.; McLean, J.S. Uncovering complex microbiome activities via metatranscriptomics during 24 hours of oral biofilm assembly and maturation. Microbiome 2018, 6, 217. [Google Scholar] [CrossRef] [PubMed]
- Peterson, S.N.; Meissner, T.; Su, A.I.; Snesrud, E.; Ong, A.C.; Schork, N.J.; Bretz, W.A. Functional expression of dental plaque microbiota. Front. Cell. Infect. Microbiol. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Palmer, S.R.; Miller, J.H.; Abranches, J.; Zeng, L.; Lefebure, T.; Richards, V.P.; Lemos, J.A.; Stanhope, M.J.; Burne, R.A. Phenotypic heterogeneity of genomically-diverse isolates of Streptococcus mutans. PLoS ONE 2013, 8, e61358. [Google Scholar] [CrossRef]
- Bedoya-Correa, C.M.; Rincón Rodríguez, R.J.; Parada-Sanchez, M.T. Genomic and phenotypic diversity of Streptococcus mutans. J. Oral Biosci. 2019, 61, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Ai, D.; Huang, R.; Wen, J.; Li, C.; Zhu, J.; Xia, L.C. Integrated metagenomics data analysis demonstrates that a loss of diversity in oral microbiota is associated with periodontitis. BMC Genom. 2017, 18, 1041. [Google Scholar] [CrossRef]
- Velsko, I.M.; Fellows Yates, J.A.; Aron, F.; Hagan, R.W.; Frantz, L.A.F.; Loe, L.; Martinez, J.B.R.; Chaves, E.; Gosden, C.; Larson, G.; et al. Microbial differences between dental paque and historic dental calculus are related to oral biofilm maturation stage. Microbiome 2019, 7, 102. [Google Scholar] [CrossRef]
- Deng, Z.L.; Szafranski, S.P.; Jarek, M.; Bhuju, S.; Wagner-Dobler, I. Dysbiosis in chronic periodontitis: Key microbial players and interactions with the human host. Sci. Rep. 2017, 7, 43344. [Google Scholar] [CrossRef]
- Mun, M.; Yap, T.; Alnuaimi, A.D.; Adams, G.G.; McCullough, M.J. Oral candidal carriage in asymptomatic patients. Aust. Dent. J. 2016, 61, 190–195. [Google Scholar] [CrossRef]
- Bader, G.; Wray, V.; Hiller, K. The main saponins from the aerial parts and the roots of Solidago virgaurea subsp. virgaurea. Planta Med. 1995, 61, 158–161. [Google Scholar] [CrossRef]
- Dupuy, A.K.; David, M.S.; Li, L.; Heider, T.N.; Peterson, J.D.; Montano, E.A.; Dongari-Bagtzoglou, A.; Diaz, P.I.; Strausbaugh, L.D. Redefining the human oral mycobiome with improved practices in amplicon-based taxonomy: Discovery of Malassezia as a prominent commensal. PLoS ONE 2014, 9, e90899. [Google Scholar] [CrossRef]
- Chinnici, J.; Yerke, L.; Tsou, C.; Busarajan, S.; Mancuso, R.; Sadhak, N.D.; Kim, J.; Maddi, A. Candida albicans cell wall integrity transcription factors regulate polymicrobial biofilm formation with Streptococcus gordonii. PeerJ 2019, 7, e7870. [Google Scholar] [CrossRef] [PubMed]
- Tyszkiewicz, E.W. Assessment Report on Solidago virgaurea L., Herba; Evaluation of medicines for human use; EMEA/HMPC/285759/2007; European Medicines Agency: Amsterdam, The Netherlands, 2008; Available online: http://www.emea.europa.eu (accessed on 2 February 2020).
- Sälzer, S.; Rosema, N.A.; Martin, E.C.; Slot, D.E.; Timmer, C.J.; Dörfer, C.E.; van der Weijden, G.A. The effectiveness of dentifrices without and with sodium lauryl sulfate on plaque, gingivitis and gingival abrasion—A randomized clinical trial. Clin. Oral Investig. 2016, 20, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Athuluru, D.; Reddy, C.; Sudhir, K.M.; Kumar, K.; Gomasani, S.; Nagarakanti, S. Evaluation and comparison of efficacy of three desensitizing dentifrices on dentinal hypersensitivity and salivary biochemical characteristics: A randomized controlled trial. Dent. Res. J. 2017, 14, 150–157. [Google Scholar]
- Straks, C.M.; Williams, R.B.; Goering, M.G.; O’Neil-Johnson, M.; Norman, V.L.; Hu, J.F.; garo, E.; Hough, G.W.; Rice, S.M.; Eldridge, G.R. Antibacterial clerodane diterpenes from Goldenrod (Solidago virgaurea). Phytochemistry 2010, 71, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Demir, H.; Acik, L.; Burcu Bali, E.; Yasemin Koc, L.; Kaynak, G. Antixoxidant and antimicrobial activities of Solidago virgaurea extracts. Afr. J. Biotechnol. 2009, 8, 274–279. [Google Scholar]
- Chevalier, M.; Ranque, S.; Prêcheur, I. Oral fungal-bacterial biofilm models in vitro: A review. Med. Mycol. 2018, 56, 653–667. [Google Scholar] [CrossRef] [PubMed]
- Nobile, V. Guidelines on cosmetic efficacy testing on humans. Ethical, technical, and regulatory requirements in the main cosmetics markets. J. Cosmo. Trichol. 2016, 2, 1. [Google Scholar] [CrossRef]
- Rebelo, M.A.B.; Correra de Queiroz, A. Gingival Indices: State of Art, Gingival Diseases—Their Aetiology, Prevention and Treatment; Panagakos, F.S., Davies, R.P., Eds.; InTech: Rijeka, Croatia, 2011; ISBN 978-953-307-376-7. Available online: http://www.intechopen.com/books/gingival-diseases-their-aetiology-prevention-and-treatment/gingival-indices-state-of-art (accessed on 31 October 2019). [CrossRef]

| Microbial Load | D0 | D14 | D28 | ΔD0D14 | ΔD0D28 |
|---|---|---|---|---|---|
| (SE 1) | p-Value | p-Value | |||
| Intervention group (n 2) | |||||
| Total bacterial load | 1.7 × 109 | 1.6 × 109 | 1.2 × 109 | 0.996 | 0.005 |
| (6.2 × 108) | (6.3 × 108) | (5.9 × 108) | |||
| A. a3 (4) | 9.9 × 104 | 1.4 × 104 | 5.3 × 104 | 0.099 | 0.100 |
| (8.1 × 104) | (1.7 × 104) | (8.5 × 104) | |||
| F. nucleatum (21) | 1.5 × 106 | 1.5 × 106 | 1.0 × 106 | 0.509 | 0.370 |
| (2.6 × 106) | (2.3 × 106) | (1.7 × 106) | |||
| P. gingivalis (8) | 1.2 × 107 | 1.0 × 107 | 9.6 × 106 | 0.624 | 0.262 |
| (1.7 × 107) | (1.9 × 107) | (1.4 × 107) | |||
| P. intermedia (21) | 3.7 × 106 | 3.8 × 106 | 5.2 × 106 | 1 | 0.651 |
| (4.5 × 106) | (5.0 × 106) | (8.6 × 106) | |||
| T. denticola (32) | 6.7 × 105 | 3.2 × 106 | 2.9 × 106 | 0.633 | 0.743 |
| (4.8 × 106) | (4.0 × 106) | (4.2 × 106) | |||
| T. forsythia (20) | 2.2 × 106 | 1.4 × 106 | 2.1 × 106 | 0.145 | 0.232 |
| (3.8 × 106) | (3.5 × 106) | (4.4 × 106) | |||
| S. mutans (32) | 4.3 × 106 | 2.6 × 106 | 2.9 × 106 | 0.024 | 0.090 |
| (8.0 × 106) | (5.9 × 106) | (8.8 × 106) | |||
| C. albicans (7) | 6.8 × 104 | 8.9 × 103 | 1.3 × 103 | 0.446 | 0.022 |
| (1.1 × 105) | (1.0 × 104) | (2.6 × 103) | |||
| Control group (n) | |||||
| Total bacterial load | 1.5 × 109 | 1.6 × 109 | 1.1 × 109 | 0.987 | 0.062 |
| (6.0 × 108) | (5.3 × 108) | (6.8 × 108) | |||
| A. a3 (2) | 2.2 × 105 | 5.6 × 104 | 7.5 × 105 | ||
| (3.0 × 105) | (7.5 × 104) | (1.0 × 106) | - | NA 4 | |
| F. nucleatum (17) | 2.0 × 106 | 2.4 × 106 | 2.4 × 106 | 0.129 | 0.754 |
| (4.6 × 106) | (4.8 × 106) | (4.3 × 106) | |||
| P. gingivalis (8) | 4.8 × 106 | 4.7 × 106 | 6.8 × 106 | 0.944 | 0.362 |
| (6.5 × 106) | (1.1 × 107) | (9.3 × 106) | |||
| P. intermedia (8) | 6.8 × 106 | 6.9 × 106 | 8.6 × 106 | 0.293 | 0.441 |
| (7.1 × 106) | (8.6 × 106) | (9.5 × 106) | |||
| T. denticola (31) | 2.8 × 106 | 2.1 × 106 | 2.9 × 106 | 0.259 | 0.906 |
| (3.8 ×106) | (2.3 × 106) | (4.6 × 106) | |||
| T. forsythia (21) | 1.1 × 106 | 8.0 × 105 | 1.9 × 106 | 0.130 | 0.651 |
| (1.9 × 106) | (1.5 × 106) | (3.9 × 106) | |||
| S. mutans (32) | 7.4 × 106 | 7.2 × 107 | 4.9 × 106 | 1 | 0.326 |
| (1.3 × 107) | (1.3 × 107) | (1.3 × 107) | |||
| C. albicans (6) | 3.1 × 104 | 5.9 × 104 | 1.4 × 104 | 0.674 | 0.833 |
| (4.9 × 104) | (6.7 × 104) | (1.3 × 106) |
| Total Bacterial Load | Difference | CI 1-Lower | CI 1-Higher | p-Value |
|---|---|---|---|---|
| Intergroup comparison | ||||
| Intervention D0 vs. Control D0 | −1.7 × 108 | −6.1 × 108 | 2.7 × 108 | 0.869 |
| Intervention D14 vs. Control D14 | −1.5 × 107 | −4.5 × 108 | 4.2 × 108 | 0.999 |
| Intervention D28 vs. Control D28 | −6.2 × 107 | −4.9 × 108 | 3.7 × 108 | 0.998 |
| Intragroup comparison | ||||
| Intervention D0 vs. Intervention D14 | 6.7 × 107 | −3.3 × 108 | 4.7 × 108 | 0.996 |
| Intervention D0 vs. Intervention D28 | 4.9 × 108 | 9.8 × 107 | 9 × 108 | 0.005 |
| Intervention D14 vs. Intervention D28 | 4.3 × 108 | 3.1 × 107 | 8.3 × 108 | 0.026 |
| Control D0 vs. Control D14 | −8.9 × 107 | −4.9 × 108 | 3.1 × 108 | 0.987 |
| Control D0 vs. Control D28 | 3.9 × 108 | −1.1 × 107 | 7.9 × 108 | 0.062 |
| Control D14 vs. Control D28 | 4.8 × 108 | 7.8 × 107 | 8.8 × 108 | 0.009 |
| Mean Value (SE 1) | D0 | D28 | p |
|---|---|---|---|
| Intervention group (n = 33) | |||
| Plaque Index | 2.4 (0.4) | 2 (0.6) | <0.001 |
| Gingival Index | 1.6 (0.3) | 1.1 (0.6) | <0.001 |
| Halitosis score | 3.5 (0.8) | 2 (1.2) | <0.001 |
| Control group (n = 33) | |||
| Plaque Index | 2.3 (0.3) | 1.8 (0.5) | <0.001 |
| Gingival Index | 1.5 (0.2) | 0.9 (0.6) | <0.001 |
| Halitosis score | 3.1 (1.1) | 1.5 (0.9) | <0.001 |
| Questionnaire Responses at D28 | Yes | No | |||
|---|---|---|---|---|---|
| No. | Question | Control group | Intervention group | Control group | Intervention group |
| 1 | The tested product improves dry mouth? | 8 | 7 | 25 | 26 |
| 2 | The tested product gives clean feeling in mouth? | 23 | 22 | 10 | 11 |
| 3 | If the tested product gives clean feeling in mouth, how long? 1 | NA 2 | NA 2 | NA 2 | NA2 |
| 4 | The tested product delays bad breath occurring? | 20 | 15 | 13 | 18 |
| 5 | The tested product soothes irritated gum? | 11 | 9 | 22 | 24 |
| 6 | The tested product reduces gum redness and swelling? | 11 | 12 | 22 | 21 |
| 7 | The tested product reduces gum bleeding? | 14 | 11 | 19 | 22 |
| 8 | The tested product reduces biofilm formation (plaque) on teeth? | 20 | 18 | 13 | 15 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prêcheur, I.; Rolland, Y.; Hasseine, L.; Orange, F.; Morisot, A.; Landreau, A. Solidago virgaurea L. Plant Extract Targeted against Candida albicans to Reduce Oral Microbial Biomass: A Double Blind Randomized Trial on Healthy Adults. Antibiotics 2020, 9, 137. https://doi.org/10.3390/antibiotics9040137
Prêcheur I, Rolland Y, Hasseine L, Orange F, Morisot A, Landreau A. Solidago virgaurea L. Plant Extract Targeted against Candida albicans to Reduce Oral Microbial Biomass: A Double Blind Randomized Trial on Healthy Adults. Antibiotics. 2020; 9(4):137. https://doi.org/10.3390/antibiotics9040137
Chicago/Turabian StylePrêcheur, Isabelle, Yohan Rolland, Lilia Hasseine, François Orange, Adeline Morisot, and Anne Landreau. 2020. "Solidago virgaurea L. Plant Extract Targeted against Candida albicans to Reduce Oral Microbial Biomass: A Double Blind Randomized Trial on Healthy Adults" Antibiotics 9, no. 4: 137. https://doi.org/10.3390/antibiotics9040137
APA StylePrêcheur, I., Rolland, Y., Hasseine, L., Orange, F., Morisot, A., & Landreau, A. (2020). Solidago virgaurea L. Plant Extract Targeted against Candida albicans to Reduce Oral Microbial Biomass: A Double Blind Randomized Trial on Healthy Adults. Antibiotics, 9(4), 137. https://doi.org/10.3390/antibiotics9040137






