The Iron-chelator, N,N’-bis (2-hydroxybenzyl) Ethylenediamine-N,N’-diacetic acid is an Effective Colistin Adjunct against Clinical Strains of Biofilm-Dwelling Pseudomonas aeruginosa
Abstract
1. Introduction
2. Results
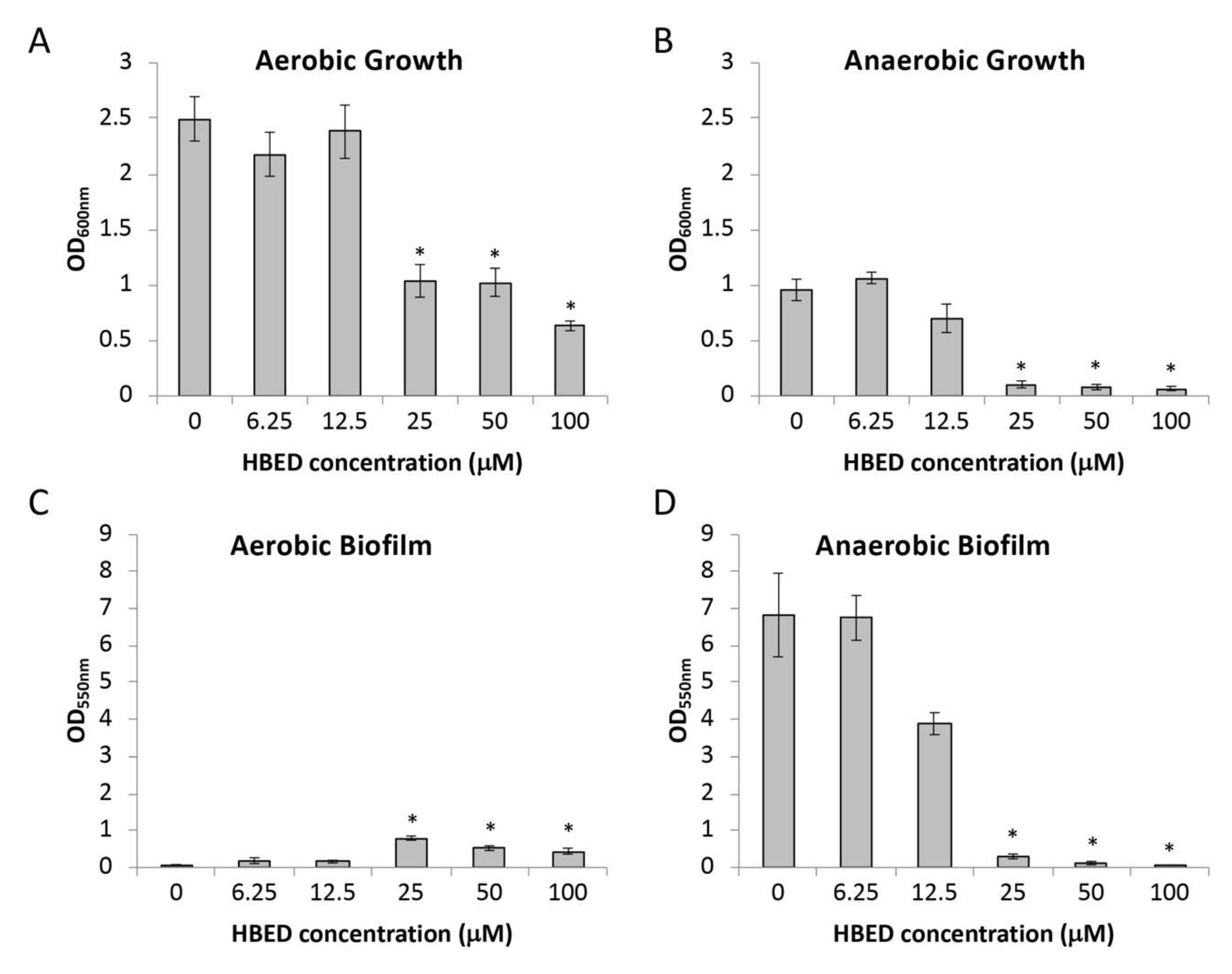
2.1. HBED Decreases Aerobic and Anaerobic Biofilm Formation of P. aeruginosa
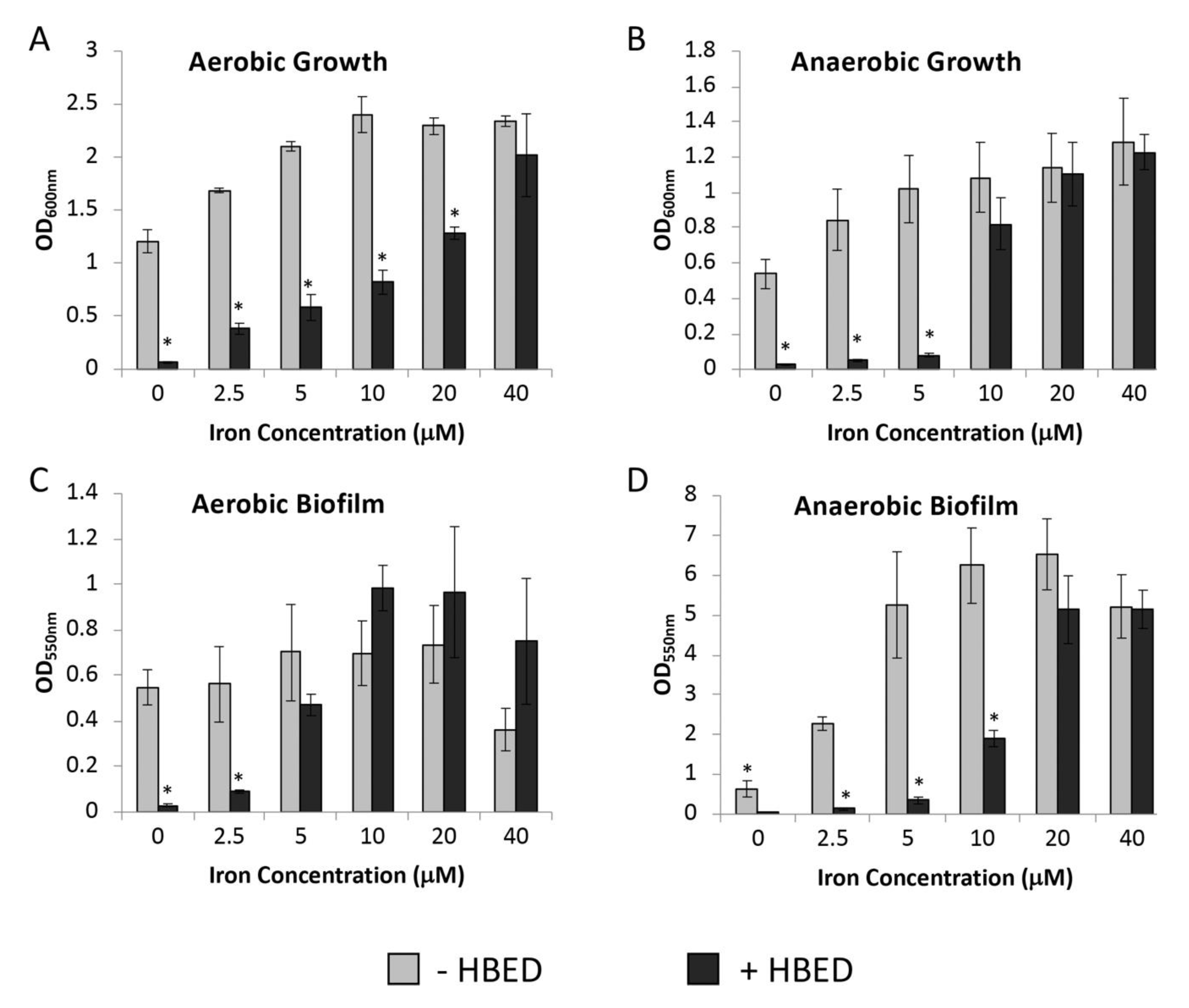
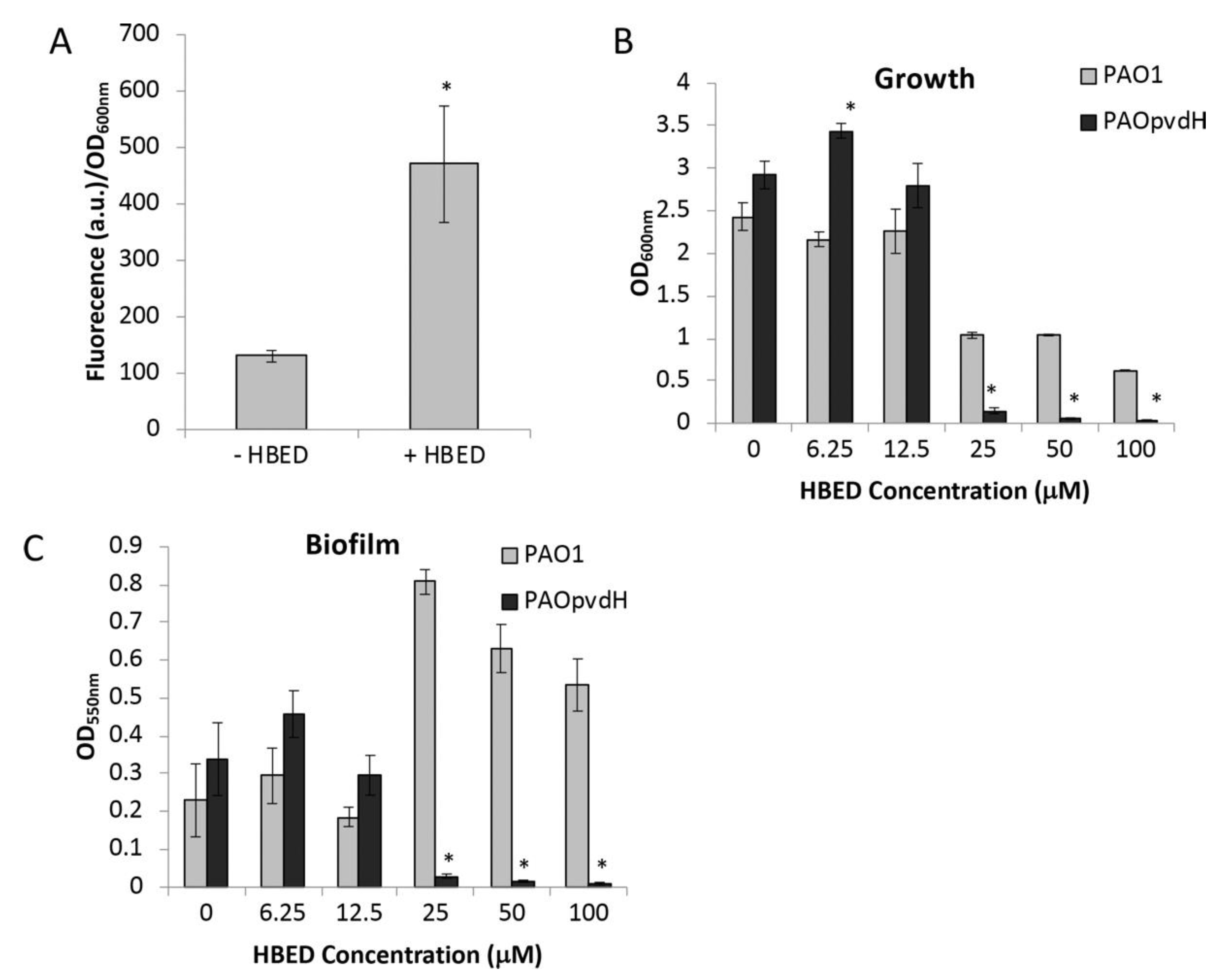
2.2. Inhibition of Growth and Biofilm Formation by HBED is Iron-Dependent
2.3. HBED Decreases Growth of P. aeruginosa in Sub-inhibitory Concentrations of Antibiotics
2.4. HBED Decreases Growth and Biofilm Formation of P. aeruginosa Clinical Isolates
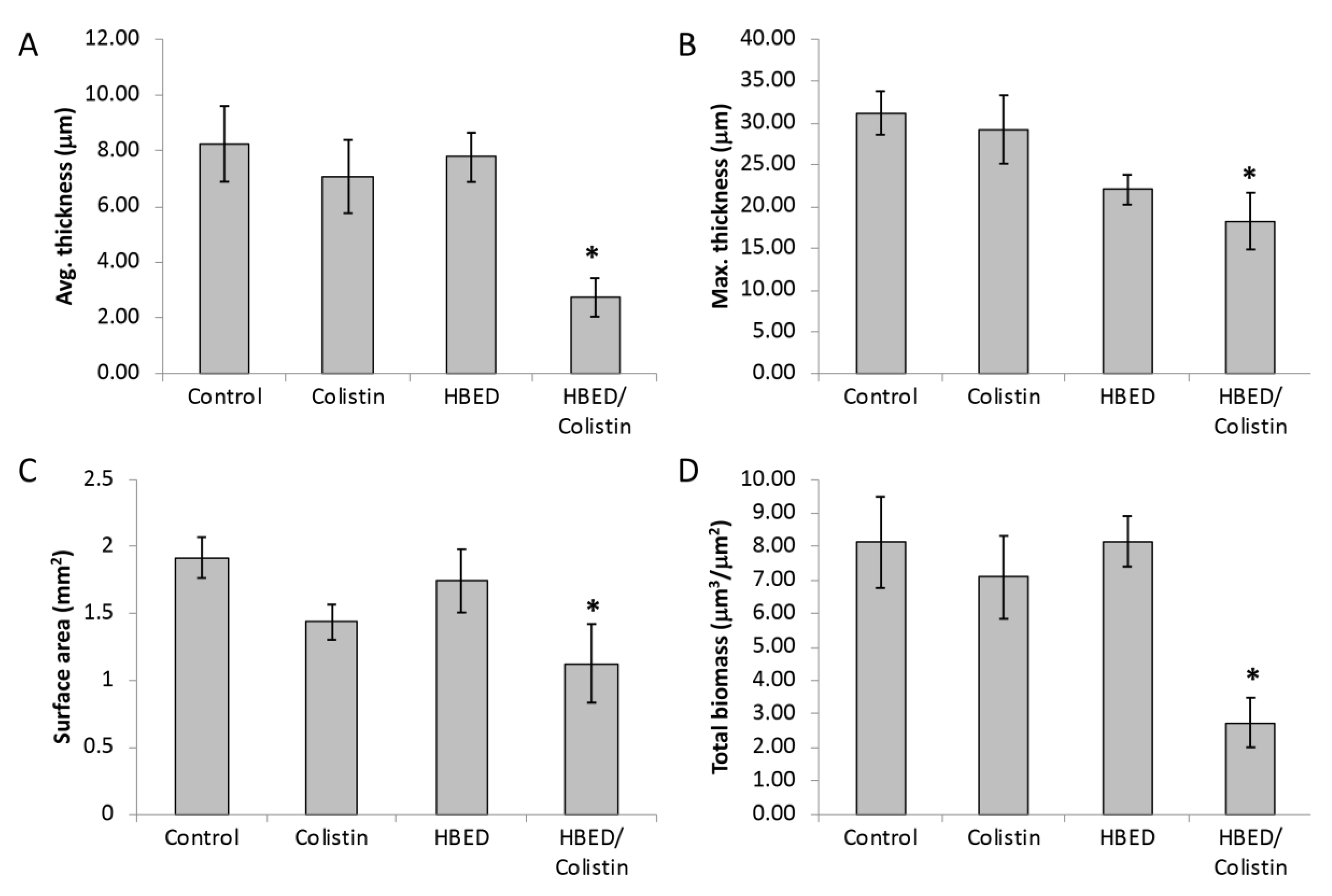
2.5. HBED is Effective against Mature Biofilms and Increases the Efficacy of Colistin
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains
4.2. Growth Media
4.3. Biofilm Assays
4.3.1. Short-Term Biofilm Assays of PAO1
4.3.2. Short-term Biofilm Assays of Clinical Isolates
4.3.3. Flow Cell Biofilm Studies
4.4. Pyoverdine Measurement
4.5. Minimal Inhibitory Concentration
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Folkesson, A.; Jelsbak, L.; Yang, L.; Johansen, H.K.; Ciofu, O.; Høiby, N.; Molin, S. Adaptation of Pseudomonas aeruginosa to the cystic fibrosis airway: An evolutionary perspective. Nat. Rev. Genet. 2012, 10, 841–851. [Google Scholar] [CrossRef]
- Reinhart, A.A.; Oglesby-Sherrouse, A.G. Regulation of Pseudomonas aeruginosa Virulence by Distinct Iron Sources. Genes 2016, 7, 126. [Google Scholar] [CrossRef] [PubMed]
- Reid, D.W.; Withers, N.J.; Francis, L.; Wilson, J.W.; Kotsimbos, T.C. Iron deficiency in cystic fibrosis: Relationship to lung disease severity and chronic Pseudomonas aeruginosa infection. Chest 2002, 121, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Reid, D.W.; Anderson, G.J.; Lamont, I.L. Role of lung iron in determining the bacterial and host struggle in cystic fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 2009, 297, L795–L802. [Google Scholar] [CrossRef] [PubMed]
- Moreau-Marquis, S.; Bomberger, J.M.; Anderson, G.G.; Swiatecka-Urban, A.; Ye, S.; O'Toole, G.A.; Stanton, B.A. The DeltaF508-CFTR mutation results in increased biofilm formation by Pseudomonas aeruginosa by increasing iron availability. Am. J. Physiol. Lung Cell Mol. Physiol. 2008, 295, L25–L37. [Google Scholar] [CrossRef] [PubMed]
- O'May, C.Y.; Sanderson, K.; Roddam, L.F.; Kirov, S.M.; Reid, D.W. Iron-binding compounds impair Pseudomonas aeruginosa biofilm formation, especially under anaerobic conditions. J. Med. Microbiol. 2009, 58, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Reid, D.W.; O'May, C.; Roddam, L.F.; Lamont, I.L. Chelated iron as an anti-Pseudomonas aeruginosa biofilm therapeutic strategy. J. Appl. Microbiol. 2009, 106, 1058. [Google Scholar] [CrossRef] [PubMed]
- Konings, A.F.; Martin, L.W.; Sharples, K.J.; Roddam, L.F.; Latham, R.; Reid, D.W.; Lamont, I.L. Pseudomonas aeruginosa uses multiple pathways to acquire iron during chronic infection in cystic fibrosis lungs. Infect. Immun. 2013, 81, 2697–2704. [Google Scholar] [CrossRef]
- Smith, D.J.; Lamont, I.L.; Anderson, G.J.; Reid, D.W. Targeting iron uptake to control Pseudomonas aeruginosa infections in cystic fibrosis. Eur. Respir. J. 2013, 42, 1723–1736. [Google Scholar] [CrossRef]
- Marvig, R.L.; Damkiær, S.; Khademi, S.M.H.; Markussen, T.M.; Molin, S.; Jelsbak, L. Within-Host Evolution of Pseudomonas aeruginosa Reveals Adaptation toward Iron Acquisition from Hemoglobin. MBio 2014, 5, e00966-14. [Google Scholar] [CrossRef]
- L'Eplattenier, F.; Murase, I.; Martell, A.E. New multidentate ligands. VI. Chelating tendencies of N,N'-di(2-hydroxybenzyl)ethylenediamine-N,N'-diacetic acid. J. Am. Chem. Soc. 1967, 89, 837–843. [Google Scholar] [CrossRef]
- Grady, R.W.; Hershko, C. HBED: A potential oral iron chelator. Ann. N. Y. Acad. Sci. 1990, 612, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Grady, R.W.; Salbe, A.D.; Hilgartner, M.W.; Giardina, P.J. Results from a phase I clinical trial of HBED. Adv. Exp. Med. Biol. 1994, 356, 351–359. [Google Scholar] [PubMed]
- Musk, D.J.; Banko, D.A.; Hergenrother, P.J. Iron salts perturb biofilm formation and disrupt existing biofilms of Pseudomonas aeruginosa. Chem. Biol. 2005, 12, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.D.; Hider, R.C. Design of iron chelators with therapeutic application. Coord. Chem. Rev. 2002, 232, 151–171. [Google Scholar] [CrossRef]
- Meyer, J.M.; Abdallah, M.A. Fluorescent pigment of Pseudomonas fluorescens—Biosynthesis, purification and physicochemical properties. J. Gen. Microbiol. 1978, 107, 319–328. [Google Scholar] [CrossRef]
- Albrechtgary, A.M.; Blanc, S.; Rochel, N.; Ocaktan, A.Z.; Abdallah, M.A. Bacterial Iron Transport—Coordination Properties of Pyoverdin PaA, a Peptidic Siderophore of Pseudomonas aeruginosa. Inorg. Chem. 1994, 33, 6391–6402. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Girardello, R.; Bispo, P.J.; Yamanaka, T.M.; Gales, A.C. Cation concentration variability of four distinct Mueller-Hinton agar brands influences polymyxin B susceptibility results. J. Clin. Microbiol. 2012, 50, 2414–2418. [Google Scholar] [CrossRef]
- O'May, C.Y.; Reid, D.W.; Kirov, S.M. Anaerobic culture conditions favor biofilm-like phenotypes in Pseudomonas aeruginosa isolates from patients with cystic fibrosis. FEMS Immunol. Med. Microbiol. 2006, 48, 373–380. [Google Scholar] [CrossRef]
- Hare, N.J.; Solis, N.; Harmer, C.; Marzook, N.B.; Rose, B.; Harbour, C.; Crossett, B.; Manos, J.; Cordwell, S.J. Proteomic profiling of Pseudomonas aeruginosa AES-1R, PAO1 and PA14 reveals potential virulence determinants associated with a transmissible cystic fibrosis-associated strain. BMC Microbiol. 2012, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- Kukavica-Ibrulj, I.; Bragonzi, A.; Paroni, M.; Winstanley, C.; Sanschagrin, F.; O'Toole, G.A.; Levesque, R.C. In vivo growth of Pseudomonas aeruginosa strains PAO1 and PA14 and the hypervirulent strain LESB58 in a rat model of chronic lung infection. J. Bacteriol. 2008, 190, 2804–2813. [Google Scholar] [CrossRef] [PubMed]
- Haussler, S.; Tummler, B.; Weissbrodt, H.; Rohde, M.; Steinmetz, I. Small-colony variants of Pseudomonas aeruginosa in cystic fibrosis. Clin. Infect. Dis. 1999, 29, 621–625. [Google Scholar] [PubMed]
- Mathee, K.; Ciofu, O.; Sternberg, C.; Lindum, P.W.; Campbell, J.I.; Jensen, P.; Johnsen, A.H.; Givskov, M.; Ohman, D.E.; Molin, S.; et al. Mucoid conversion of Pseudomonas aeruginosa by hydrogen peroxide: A mechanism for virulence activation in the cystic fibrosis lung. Microbiology 1999, 145, 1349–1357. [Google Scholar] [CrossRef]
- Eberl, L.; Tummler, B. Pseudomonas aeruginosa and Burkholderia cepacia in cystic fibrosis: Genome evolution, interactions and adaptation. Int. J. Med. Microbiol. 2004, 294, 123–131. [Google Scholar] [CrossRef]
- Smith, E.E.; Buckley, D.G.; Wu, Z.; Saenphimmachak, C.; Hoffman, L.R.; D'Argenio, D.A.; Miller, S.I.; Ramsey, B.W.; Speert, D.P.; Moskowitz, S.M.; et al. Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc. Natl. Acad. Sci. USA 2006, 103, 8487–8492. [Google Scholar] [CrossRef]
- Kidd, T.J.; Ramsay, K.A.; Hu, H.; Bye, P.T.; Elkins, M.R.; Grimwood, K.; Harbour, C.; Marks, G.B.; Nissen, M.D.; Robinson, P.J.; et al. Low rates of Pseudomonas aeruginosa misidentification in isolates from cystic fibrosis patients. J. Clin. Microbiol. 2009, 47, 1503–1509. [Google Scholar] [CrossRef]
- Bradbury, R.; Champion, A.; Reid, D.W. Poor clinical outcomes associated with a multi-drug resistant clonal strain of Pseudomonas aeruginosa in the Tasmanian cystic fibrosis population. Respirology 2008, 13, 886–892. [Google Scholar] [CrossRef]
- Hoiby, N.; Ciofu, O.; Bjarnsholt, T. Pseudomonas aeruginosa biofilms in cystic fibrosis. Future Microbiol. 2010, 5, 1663–1674. [Google Scholar] [CrossRef]
- Sauer, K.; Camper, A.K.; Ehrlich, G.D.; Costerton, J.W.; Davies, D.G. Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. J. Bacteriol. 2002, 184, 1140–1154. [Google Scholar] [CrossRef]
- VanDevanter, D.R.; Van Dalfsen, J.M. How much do Pseudomonas biofilms contribute to symptoms of pulmonary exacerbation in cystic fibrosis? Pediatr. Pulmonol. 2005, 39, 504–506. [Google Scholar] [CrossRef] [PubMed]
- Kirov, S.M.; Webb, J.S.; O'May, C.Y.; Reid, D.W.; Woo, J.K.; Rice, S.A.; Kjelleberg, S. Biofilm differentiation and dispersal in mucoid Pseudomonas aeruginosa isolates from patients with cystic fibrosis. Microbiology 2007, 153, 3264–3274. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, C.; Tolker-Nielsen, T. Growing and analyzing biofilms in flow cells. Curr. Protoc. Microbiol. 2006, 00, 1B.2.1–1B.2.15. [Google Scholar] [CrossRef]
- Kolpen, M.; Appeldorff, C.F.; Brandt, S.; Mousavi, N.; Kragh, K.N.; Aydogan, S.; Uppal, H.A.; Bjarnsholt, T.; Ciofu, O.; Hoiby, N.; et al. Increased bactericidal activity of colistin on Pseudomonas aeruginosa biofilms in anaerobic conditions. Pathog. Dis. 2016, 74, ftv086. [Google Scholar] [CrossRef]
- Haagensen, J.A.; Klausen, M.; Ernst, R.K.; Miller, S.I.; Folkesson, A.; Tolker-Nielsen, T.; Molin, S. Differentiation and distribution of colistin- and sodium dodecyl sulfate-tolerant cells in Pseudomonas aeruginosa biofilms. J. Bacteriol. 2007, 189, 28–37. [Google Scholar] [CrossRef]
- Li, J.; Turnidge, J.; Milne, R.; Nation, R.L.; Coulthard, K. In vitro pharmacodynamic properties of colistin and colistin methanesulfonate against Pseudomonas aeruginosa isolates from patients with cystic fibrosis. Antimicrob. Agents Chemother. 2001, 45, 781–785. [Google Scholar] [CrossRef]
- Ratjen, F.; Rietschel, E.; Kasel, D.; Schwiertz, R.; Starke, K.; Beier, H.; van Koningsbruggen, S.; Grasemann, H. Pharmacokinetics of inhaled colistin in patients with cystic fibrosis. J. Antimicrob. Chemother. 2006, 57, 306–311. [Google Scholar] [CrossRef]
- Banin, E.; Lozinski, A.; Brady, K.M.; Berenshtein, E.; Butterfield, P.W.; Moshe, M.; Chevion, M.; Greenberg, E.P. The potential of desferrioxamine-gallium as an anti-Pseudomonas therapeutic agent. Proc. Natl. Acad. Sci. USA 2008, 105, 16761–16766. [Google Scholar] [CrossRef]
- Moreau-Marquis, S.; O'Toole, G.A.; Stanton, B.A. Tobramycin and FDA-approved iron chelators eliminate Pseudomonas aeruginosa biofilms on cystic fibrosis cells. Am. J. Respir. Cell Mol. Biol. 2009, 41, 305–313. [Google Scholar] [CrossRef]
- Oglesby-Sherrouse, A.G.; Djapgne, L.; Nguyen, A.T.; Vasil, A.I.; Vasil, M.L. The complex interplay of iron, biofilm formation, and mucoidy affecting antimicrobial resistance of Pseudomonas aeruginosa. Pathog. Dis. 2014, 70, 307–320. [Google Scholar] [CrossRef]
- Hunter, R.C.; Asfour, F.; Dingemans, J.; Osuna, B.L.; Samad, T.; Malfroot, A.; Cornelis, P.; Newman, D.K. Ferrous iron is a significant component of bioavailable iron in cystic fibrosis airways. MBio 2013, 4, e00557-13. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.S.; Ham, S.Y.; Park, B.B.; Byun, Y.; Park, H.D. Lauroyl Arginate Ethyl Blocks the Iron Signals Necessary for Pseudomonas aeruginosa Biofilm Development. Front. Microbiol. 2017, 8, 970. [Google Scholar] [CrossRef] [PubMed]
- Samuni, A.M.; Afeworki, M.; Stein, W.; Yordanov, A.T.; DeGraff, W.; Krishna, M.C.; Mitchell, J.B.; Brechbiel, M.W. Multifunctional antioxidant activity of HBED iron chelator. Free Radic. Biol. Med. 2001, 30, 170–177. [Google Scholar] [CrossRef]
- Worlitzsch, D.; Tarran, R.; Ulrich, M.; Schwab, U.; Cekici, A.; Meyer, K.C.; Birrer, P.; Bellon, G.; Berger, J.; Weiss, T.; et al. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J. Clin. Investig. 2002, 109, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Schelstraete, P.; Haerynck, F.; Van daele, S.; Deseyne, S.; De Baets, F. Eradication therapy for Pseudomonas aeruginosa colonization episodes in cystic fibrosis patients not chronically colonized by P. aeruginosa. J Cyst. Fibros. 2013, 12, 1–8. [Google Scholar] [CrossRef]
- King, P.; Citron, D.M.; Griffith, D.C.; Lomovskaya, O.; Dudley, M.N. Effect of oxygen limitation on the in vitro activity of levofloxacin and other antibiotics administered by the aerosol route against Pseudomonas aeruginosa from cystic fibrosis patients. Diagn. Microbiol. Infect. Dis. 2010, 66, 181–186. [Google Scholar] [CrossRef]
- Xiong, Y.Q.; Vasil, M.L.; Johnson, Z.; Ochsner, U.A.; Bayer, A.S. The oxygen- and iron-dependent sigma factor pvdS of Pseudomonas aeruginosa is an important virulence factor in experimental infective endocarditis. J. Infect. Dis. 2000, 181, 1020–1026. [Google Scholar] [CrossRef]
- Lamont, I.L.; Beare, P.A.; Ochsner, U.; Vasil, A.I.; Vasil, M.L. Siderophore-mediated signaling regulates virulence factor production in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 2002, 99, 7072–7077. [Google Scholar] [CrossRef]
- Holloway, B.W. Genetic recombination in Pseudomonas aeruginosa. J. Gen. Microbiol. 1955, 13, 572–581. [Google Scholar] [CrossRef]
- Lopez-Medina, E.; Fan, D.; Coughlin, L.A.; Ho, E.X.; Lamont, I.L.; Reimmann, C.; Hooper, L.V.; Koh, A.Y. Candida albicans Inhibits Pseudomonas aeruginosa Virulence through Suppression of Pyochelin and Pyoverdine Biosynthesis. PLoS Pathog. 2015, 11, e1005129. [Google Scholar] [CrossRef]
- Miller, J.H. Experiments in Molecular Genetics; Cold Spring Harbor Laboratory: New York, NY, USA, 1972. [Google Scholar]
- Reid, D.W.; Carroll, V.; O'May, C.; Champion, A.; Kirov, S.M. Increased airway iron as a potential factor in the persistence of Pseudomonas aeruginosa infection in cystic fibrosis. Eur. Respir. J. 2007, 30, 286–292. [Google Scholar] [CrossRef] [PubMed]
- O'Toole, G.A. Microtiter dish biofilm formation assay. J. Vis. Exp. JoVE 2011, 47, 2437. [Google Scholar] [CrossRef] [PubMed]
- Webb, J.S.; Thompson, L.S.; James, S.; Charlton, T.; Tolker-Nielsen, T.; Koch, B.; Givskov, M.; Kjelleberg, S. Cell death in Pseudomonas aeruginosa biofilm development. J. Bacteriol. 2003, 185, 4585–4592. [Google Scholar] [CrossRef]
- Heydorn, A.; Nielsen, A.T.; Hentzer, M.; Sternberg, C.; Givskov, M.; Ersboll, B.K.; Molin, S. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology 2000, 146, 2395–2407. [Google Scholar] [CrossRef]
- Martin, L.W.; Reid, D.W.; Sharples, K.J.; Lamont, I.L. Pseudomonas siderophores in the sputum of patients with cystic fibrosis. Biometals 2011, 24, 1059–1067. [Google Scholar] [CrossRef] [PubMed]

| Antibiotic | Aerobic − HBED | Aerobic + 25 μM HBED | Anaerobic − HBED | Anaerobic + 10 μM HBED |
|---|---|---|---|---|
| Colistin (mg/L) | 0.5 | 0.5 | 2 | 1 |
| Ciprofloxacin (mg/L) | 0.25 | 0.25 | 0.5 | 0.5 |
| Gentamicin (mg/L) | 2 | 2 | 8 | 4 |
| Strain | Isolate Type1 | Patient Location | Patient Age at Time of Isolation | Reference |
|---|---|---|---|---|
| PA605 | First Isolate | Hobart, TAS | 7 | This study |
| AUST01 | Early Isolate | Melbourne, VIC | 10 | [27] |
| AUST01 | Late Isolate | Perth, WA | 39 | [27] |
| AUST02 | Early Isolate | Brisbane, QLD | 2 | [27] |
| AUST02 | Late Isolate | Perth, WA | 28 | [27] |
| AUST03 | Late Isolate | Hobart, TAS | 26 | [28] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mettrick, K.; Hassan, K.; Lamont, I.; Reid, D. The Iron-chelator, N,N’-bis (2-hydroxybenzyl) Ethylenediamine-N,N’-diacetic acid is an Effective Colistin Adjunct against Clinical Strains of Biofilm-Dwelling Pseudomonas aeruginosa. Antibiotics 2020, 9, 144. https://doi.org/10.3390/antibiotics9040144
Mettrick K, Hassan K, Lamont I, Reid D. The Iron-chelator, N,N’-bis (2-hydroxybenzyl) Ethylenediamine-N,N’-diacetic acid is an Effective Colistin Adjunct against Clinical Strains of Biofilm-Dwelling Pseudomonas aeruginosa. Antibiotics. 2020; 9(4):144. https://doi.org/10.3390/antibiotics9040144
Chicago/Turabian StyleMettrick, Karla, Karl Hassan, Iain Lamont, and David Reid. 2020. "The Iron-chelator, N,N’-bis (2-hydroxybenzyl) Ethylenediamine-N,N’-diacetic acid is an Effective Colistin Adjunct against Clinical Strains of Biofilm-Dwelling Pseudomonas aeruginosa" Antibiotics 9, no. 4: 144. https://doi.org/10.3390/antibiotics9040144
APA StyleMettrick, K., Hassan, K., Lamont, I., & Reid, D. (2020). The Iron-chelator, N,N’-bis (2-hydroxybenzyl) Ethylenediamine-N,N’-diacetic acid is an Effective Colistin Adjunct against Clinical Strains of Biofilm-Dwelling Pseudomonas aeruginosa. Antibiotics, 9(4), 144. https://doi.org/10.3390/antibiotics9040144






