Rifaximin Alleviates Endotoxemia with Decreased Serum Levels of Soluble CD163 and Mannose Receptor and Partial Modification of Gut Microbiota in Cirrhotic Patients
Abstract
1. Introduction
2. Results
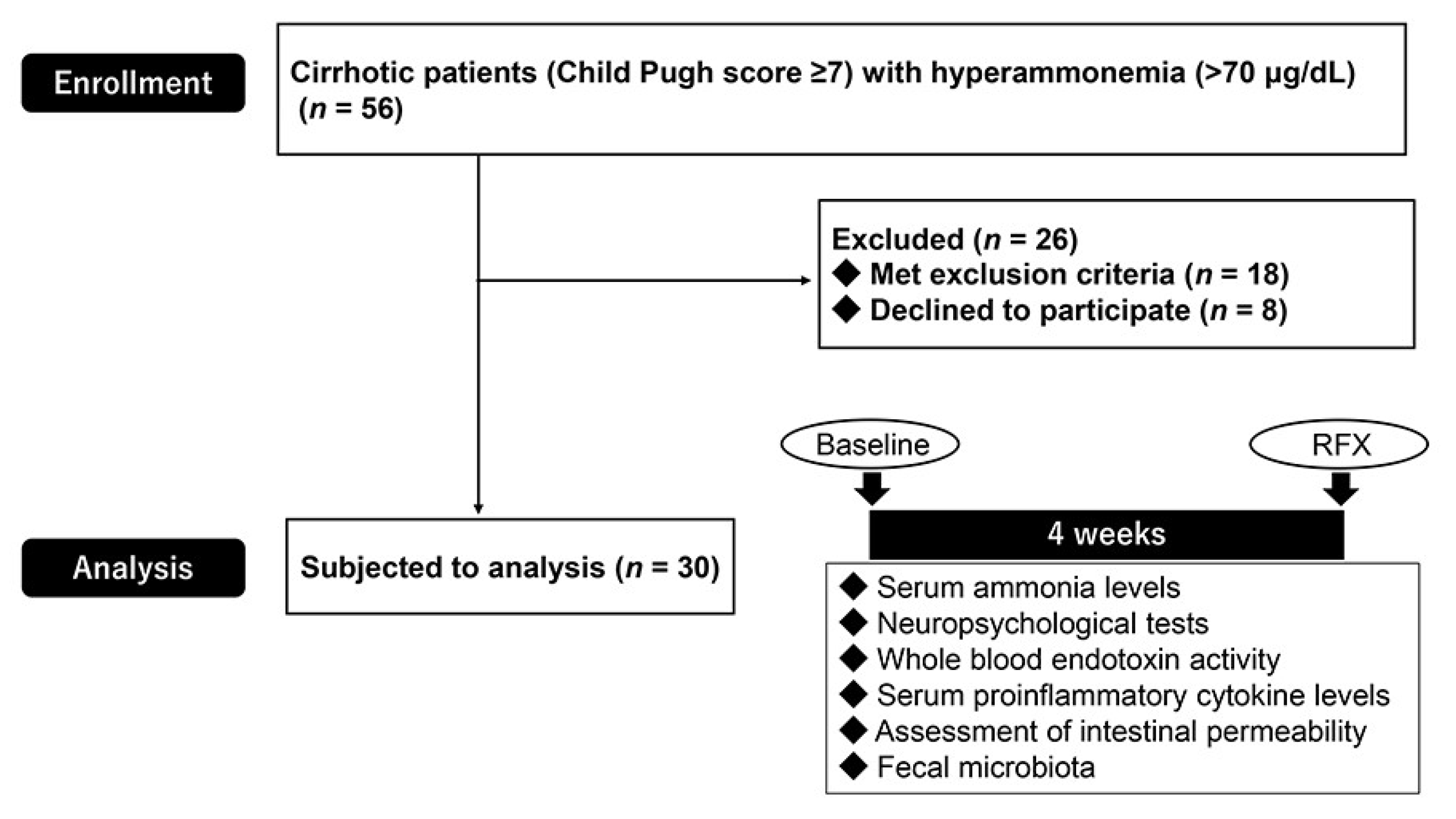
2.1. Patient Characteristics
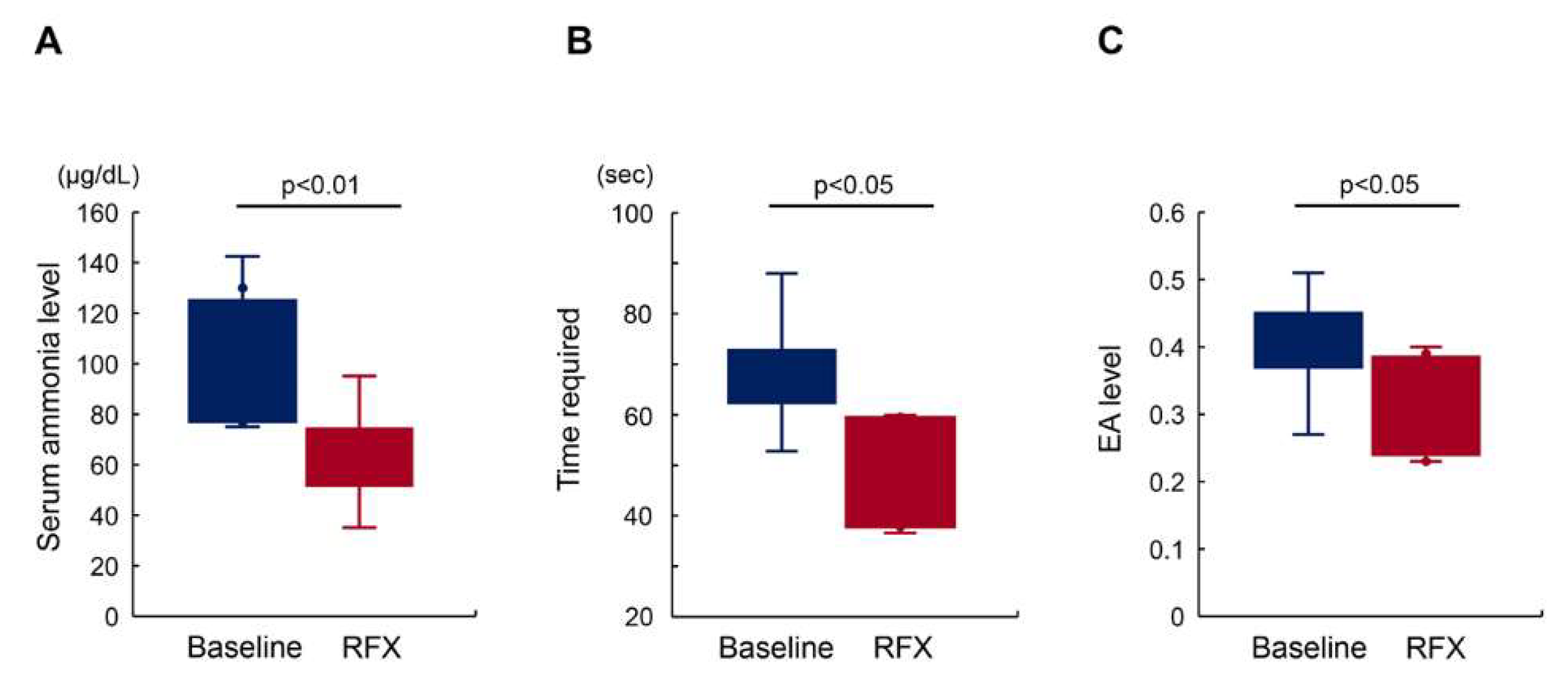
2.2. Rifaximin Improves Minimal Hepatic Encephalopathy with Reduced Endotoxin Activity
2.3. Rifaximin Lowers Serum sCD163 and sMR Levels
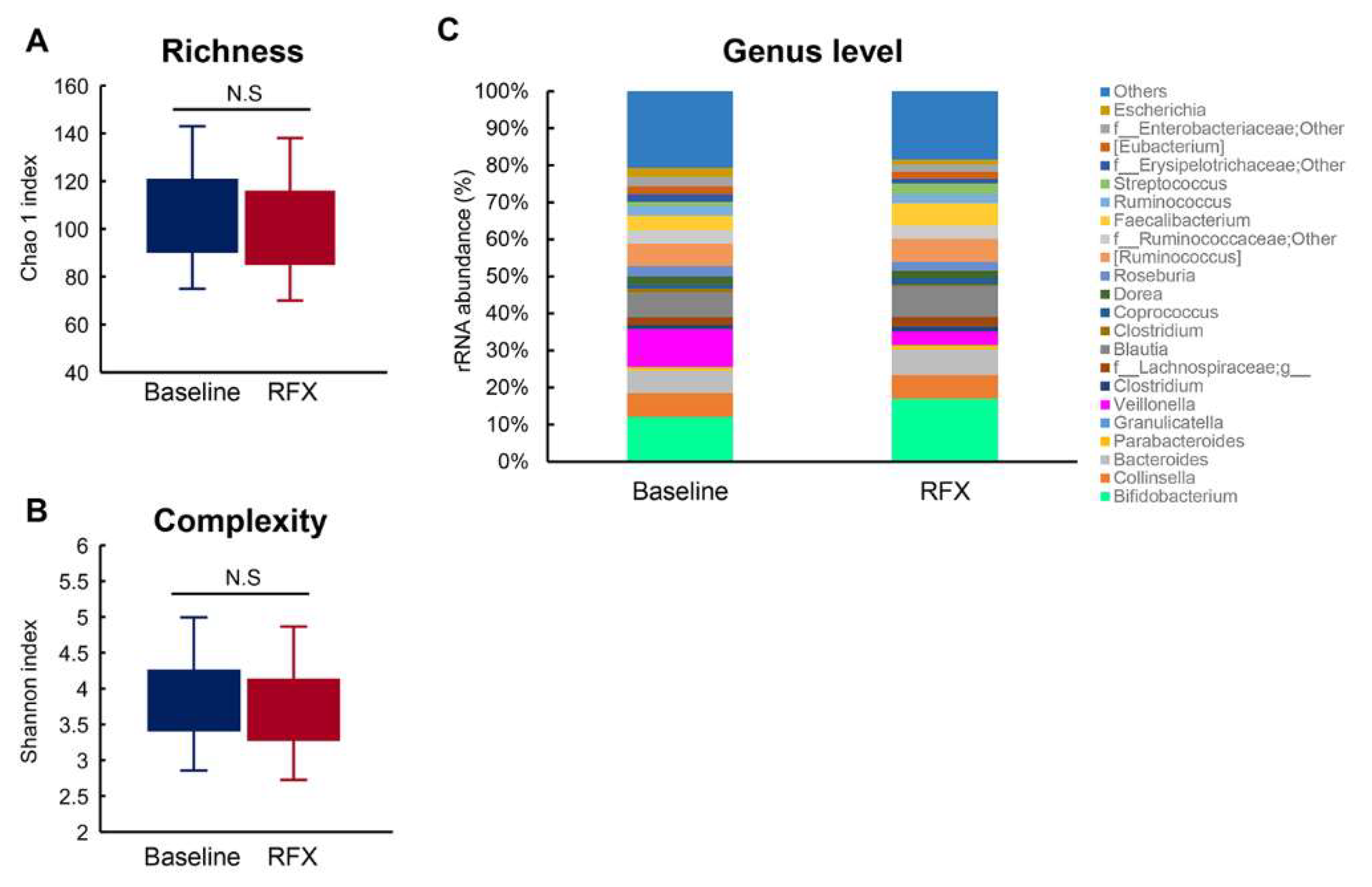
2.4. Alterations to Fecal Microbiota Composition
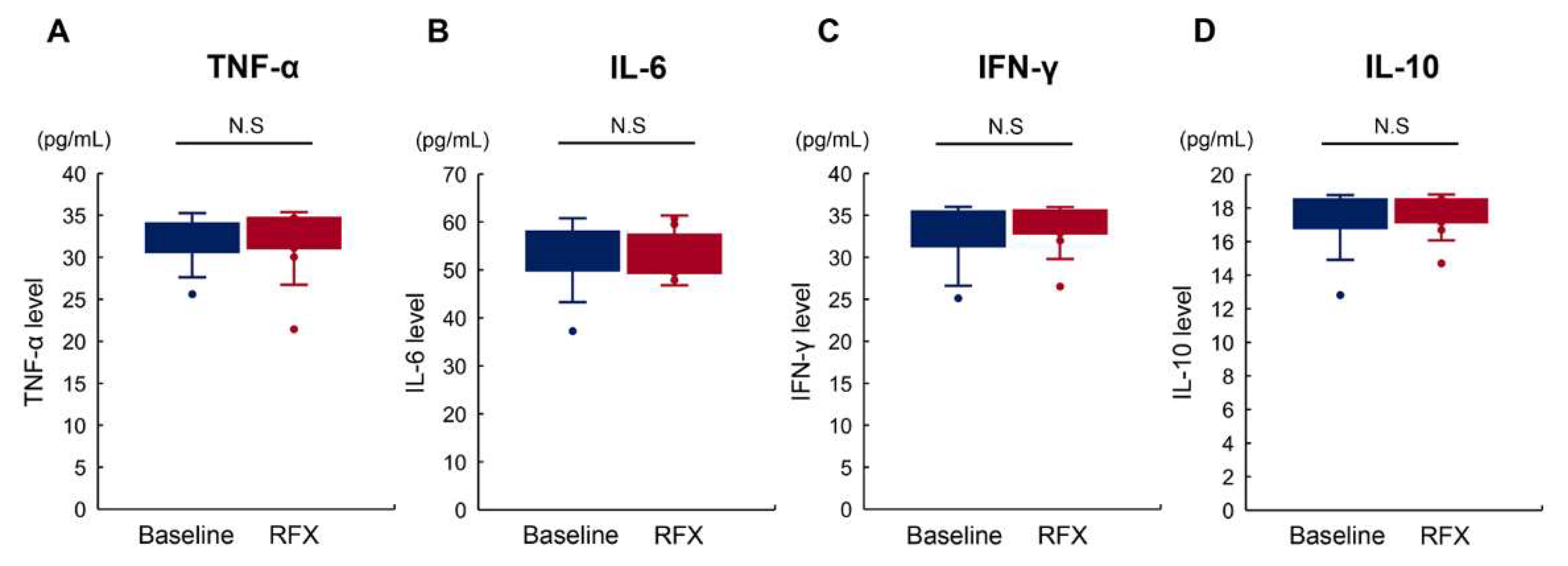
2.5. Rifaximin Does Not Affect Serum Proinflammatory Cytokine Levels
3. Discussion
4. Methods
4.1. Subjects
4.2. Study Design and Ethical Approval
4.3. Neuropsychological Testing
4.4. Whole Blood Endotoxin Activity
4.5. Proinflammatory Cytokines and Intestinal Permeability
4.6. Fecal Microbiome Analysis
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| HE | hepatic encephalopathy |
| EA | endotoxin activity |
| sCD163 | soluble CD163 |
| sMR | mannose receptor |
| rRNA | ribosomal RNA |
| mHE | minimal hepatic encephalopathy |
| TJPs | tight junction proteins |
| SIBO | small-intestinal bacterial overgrowth |
| LPS | lipopolysaccharide |
| LMR | lactulose/mannitol ratio |
| NCT | number connection test |
| EAA | endotoxin activity assay |
| TNF-α | tumor necrosis factor-alpha |
| IL-6 | interleukin-6 |
| IFN-γ | interferon-gamma |
| IL-10 | interleukin-10 |
| ELISA | enzyme-linked immunosorbent assay |
| OTUs | operational taxonomic units |
| SD | standard deviation |
| MELD | median model of end-stage liver disease |
| CRP | C-reactive protein |
| WBC | white blood cells |
| BTR | branched chain amino acid and tyrosine ratio |
| PAMPs | pathogen-associated molecular patterns |
| TLR4 | toll-like receptor 4 |
| PXR | pregnane X receptor |
| HBV | hepatitis B virus |
| HCV | hepatitis C virus |
| NASH | non-alcoholic steatohepatitis; |
| AIH | autoimmune hepatitis |
| PBC | primary biliary cholangitis |
References
- Prakash, R.; Mullen, K.D. Mechanisms, diagnosis and management of hepatic encephalopathy. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Rikkers, L.; Jenko, P.; Rudman, D.; Freides, D. Subclinical hepatic encephalopathy: Detection, prevalence, and relationship to nitrogen metabolism. Gastroenterology 1978, 75, 462–469. [Google Scholar] [CrossRef]
- American Association for the Study of Liver Diseases; European Association for the Study of the Liver. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases. J. Hepatol. 2014, 61, 642–659. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.A.; Smith, G.E. Minimal hepatic encephalopathy. Nat. Clin. Pract. Gastroenterol. Hepatol. 2007, 4, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, M.; Jacas, C.; Cordoba, J. Minimal hepatic encephalopathy: Diagnosis, clinical significance and recommendations. J. Hepatol. 2005, 42, S45–S53. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.S.; Lee, F.Y.; Lee, S.D.; Tsai, Y.T.; Lin, H.C.; Lu, R.H.; Hsu, W.C.; Huang, C.C.; Wang, S.S.; Lo, K.J.; et al. Endotoxemia in patients with chronic liver diseases: Relationship to severity of liver diseases, presence of esophageal varices, and hyperdynamic circulation. J. Hepatol. 1995, 22, 165–172. [Google Scholar] [CrossRef]
- Jain, L.; Sharma, B.C.; Sharma, P.; Srivastava, S.; Agrawal, A.; Sarin, S.K. Serum endotoxin and inflammatory mediators in patients with cirrhosis and hepatic encephalopathy. Dig. Liver Dis. 2012, 44, 1027–1031. [Google Scholar] [CrossRef]
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef]
- Parlesak, A.; Schafer, C.; Schutz, T.; Bode, J.C.; Bode, C. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J. Hepatol. 2000, 32, 742–747. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Heuman, D.M.; Sanyal, A.J.; Bajaj, A.S.; Heuman, D.M.; Sanyal, A.J.; Hylemon, P.B.; Sterling, R.K.; ToddStravitz, R.; Fuchs, M.; et al. Modulation of the metabiome by rifaximin in patients with cirrhosis and minimal hepatic encephalopathy. PLoS ONE 2013, 8, e60042. [Google Scholar] [CrossRef]
- Kimer, N.; Pedersen, J.S.; Tavenier, J.; Christensen, J.E.; Busk, T.M.; Hobolth, L.; Krag, A.; Al-Soud, W.A.; Mortensen, M.S.; Sørensen, S.J.; et al. Rifaximin has minor effects on bacterial composition, inflammation, and bacterial translocation in cirrhosis: A randomized trial. J. Gastroenterol. Hepatol. 2018, 33, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Kaji, K.; Takaya, H.; Saikawa, S.; Furukawa, M.; Sato, S.; Kawaratani, H.; Kitade, M.; Moriya, K.; Namisaki, T.; Akahane, T.; et al. Rifaximin ameliorates hepatic encephalopathy and endotoxemia without affecting the gut microbiome diversity. World J. Gastroenterol. 2017, 23, 8355–8366. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Feng, Y.; Cao, B.; Tian, Q. Effects of SIBO and rifaximin therapy on MHE caused by hepatic cirrhosis. Int. J. Clin. Exp. Med. 2015, 8, 2954–2957. [Google Scholar] [PubMed]
- Andersen, E.S.; Rodgaard-Hansen, S.; Moessner, B.; Christensen, P.B.; Moller, H.J.; Weis, N. Macrophage-related serum biomarkers soluble CD163 (sCD163) and soluble mannose receptor (sMR) to differentiate mild liver fibrosis from cirrhosis in patients with chronic hepatitis C: A pilot study. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Rode, A.; Nicoll, A.; Moller, H.J.; Lim, L.; Angus, P.W.; Kronborg, I.; Arachchi, N.; Gorelik, A.; Liew, D.; Kazankov, K.; et al. Hepatic macrophage activation predicts clinical decompensation in chronic liver disease. Gut 2013, 62, 1231–1232. [Google Scholar] [CrossRef] [PubMed]
- Kristiansen, M.; Graversen, J.H.; Jacobsen, C.; Sonne, O.; Hoffman, H.J.; Law, S.K.; Moestrup, S.K. Identification of the haemoglobin scavenger receptor. Nature 2001, 409, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Sandahl, T.D.; Gronbaek, H.; Moller, H.J.; Stoy, S.; Thomsen, K.L.; Dige, A.K.; Agnholt, J.; Hamilton-Dutoit, S.; Thiel, S.; Vilstrup, H. Hepatic macrophage activation and the LPS pathway in patients with alcoholic hepatitis: A prospective cohort study. Am. J. Gastroenterol. 2014, 109, 1749–1756. [Google Scholar] [CrossRef]
- Rodgaard-Hansen, S.; Rafique, A.; Christensen, P.A.; Maniecki, M.B.; Sandahl, T.D.; Nexo, E.; Moller, H.J. A soluble form of the macrophage-related mannose receptor (MR/CD206) is present in human serum and elevated in critical illness. Clin. Chem. Lab. Med. 2014, 52, 453–461. [Google Scholar] [CrossRef]
- Rainer, F.; Horvath, A.; Sandahl, T.D.; Leber, B.; Schmerboeck, B.; Blesl, A.; Groselj-Strele, A.; Stauber, R.E.; Fickert, P.; Stiegler, P.; et al. Soluble CD163 and soluble mannose receptor predict survival and decompensation in patients with liver cirrhosis, and correlate with gut permeability and bacterial translocation. Aliment. Pharmacol. Ther. 2018, 47, 657–664. [Google Scholar] [CrossRef]
- Moreno-Navarrete, J.M.; Sabater, M.; Ortega, F.; Ricart, W.; Fernandez-Real, J.M. Circulating zonulin, a marker of intestinal permeability, is increased in association with obesity-associated insulin resistance. PLoS ONE 2012, 7, e37160. [Google Scholar] [CrossRef]
- Raparelli, V.; Basili, S.; Carnevale, R.; Napoleone, L.; Del Ben, M.; Nocella, C.; Bartimoccia, S.; Lucidi, C.; Talerico, G.; Riggio, O.; et al. Low-grade endotoxemia and platelet activation in cirrhosis. Hepatology 2017, 65, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Sturgeon, C.; Fasano, A. Zonulin, a regulator of epithelial and endothelial barrier functions, and its involvement in chronic inflammatory diseases. Tissue Barriers 2016, 4, e1251384. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.R.; Turner, J.R. Inflammatory bowel disease: Is it really just another break in the wall? Gut 2007, 56, 6–8. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.H.; Wu, X.J.; Li, J.S. Influence of portal pressure change on intestinal permeability in patients with portal hypertension. Hepatobiliary Pancreat. Dis. Int. 2002, 1, 510–514. [Google Scholar]
- Fukui, H. Gut-liver axis in liver cirrhosis: How to manage leaky gut and endotoxemia. World J. Hepatol. 2015, 7, 425–442. [Google Scholar] [CrossRef]
- Pijls, K.E.; Jonkers, D.M.; Elamin, E.E.; Masclee, A.A.; Koek, G.H. Intestinal epithelial barrier function in liver cirrhosis: An extensive review of the literature. Liver Int. 2013, 33, 1457–1469. [Google Scholar] [CrossRef]
- Assimakopoulos, S.F.; Tsamandas, A.C.; Tsiaoussis, G.I.; Karatza, E.; Triantos, C.; Vagianos, C.E.; Spiliopoulou, I.; Kaltezioti, V.; Charonis, A.; Nikolopoulou, V.N.; et al. Altered intestinal tight junctions’ expression in patients with liver cirrhosis: A pathogenetic mechanism of intestinal hyperpermeability. Eur. J. Clin. Investig. 2012, 42, 439–446. [Google Scholar] [CrossRef]
- Scarpellini, E.; Valenza, V.; Gabrielli, M.; Lauritano, E.C.; Perotti, G.; Merra, G.; Dal Lago, A.; Ojetti, V.; Ainora, M.E.; Santoro, M.; et al. Intestinal permeability in cirrhotic patients with and without spontaneous bacterial peritonitis: Is the ring closed? Am. J. Gastroenterol. 2010, 105, 323–327. [Google Scholar] [CrossRef]
- Pascual, S.; Such, J.; Esteban, A.; Zapater, P.; Casellas, J.A.; Aparicio, J.R.; Girona, E.; Gutierrez, A.; Cainices, F.; Palazon, J.M.; et al. Intestinal permeability is increased in patients with advanced cirrhosis. Hepato Gastroenterol. 2003, 50, 1482–1486. [Google Scholar]
- Douhara, A.; Moriya, K.; Yoshiji, H.; Noguchi, R.; Namisaki, T.; Kitade, M.; Kaji, K.; Aihara, Y.; Nishimura, N.; Takeda, K.; et al. Reduction of endotoxin attenuates liver fibrosis through suppression of hepatic stellate cell activation and remission of intestinal permeability in a rat non-alcoholic steatohepatitis model. Mol. Med. Rep. 2015, 11, 1693–1700. [Google Scholar] [CrossRef]
- Jin, Y.; Ren, X.; Li, G.; Li, Y.; Zhang, L.; Wang, H.; Qian, W.; Hou, X. Beneficial effects of Rifaximin in post-infectious irritable bowel syndrome mouse model beyond gut microbiota. J. Gastroenterol. Hepatol. 2018, 33, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Finlin, B.S.; Zhu, B.; Boyechko, T.; Westgate, P.M.; Chia, C.W.; Egan, J.M.; Kern, P.A. Effect of Rifaximin Treatment on Endotoxemia and Insulin Sensitivity in Humans. J. Endocr. Soc. 2019, 3, 1641–1651. [Google Scholar] [CrossRef] [PubMed]
- Yaroustovsky, M.; Plyushch, M.; Popov, D.; Samsonova, N.; Abramyan, M.; Popok, Z.; Krotenko, N. Prognostic value of endotoxin activity assay in patients with severe sepsis after cardiac surgery. J. Inflamm. 2013, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Keshavarzian, A.; Holmes, E.W.; Patel, M.; Iber, F.; Fields, J.Z.; Pethkar, S. Leaky gut in alcoholic cirrhosis: A possible mechanism for alcohol-induced liver damage. Am. J. Gastroenterol. 1999, 94, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Delwiche, E.A.; Pestka, J.J.; Tortorello, M.L. The veillonellae: Gram-negative cocci with a unique physiology. Annu. Rev. Microbiol. 1985, 39, 175–193. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Hylemon, P.B.; Ridlon, J.M.; Heuman, D.M.; Daita, K.; White, M.B.; Monteith, P.; Noble, N.A.; Sikaroodi, M.; Gillevet, P.M. Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G675–G685. [Google Scholar] [CrossRef]
- Gao, J.; Gillilland, M.G., 3rd; Owyang, C. Rifaximin, gut microbes and mucosal inflammation: Unraveling a complex relationship. Gut Microbes 2014, 5, 571–575. [Google Scholar] [CrossRef]
- Guo, S.; Al-Sadi, R.; Said, H.M.; Ma, T.Y. Lipopolysaccharide causes an increase in intestinal tight junction permeability in vitro and in vivo by inducing enterocyte membrane expression and localization of TLR-4 and CD14. Am. J. Pathol. 2013, 182, 375–387. [Google Scholar] [CrossRef]
- Ma, X.; Shah, Y.M.; Guo, G.L.; Wang, T.; Krausz, K.W.; Idle, J.R.; Gonzalez, F.J. Rifaximin is a gut-specific human pregnane X receptor activator. J. Pharmacol. Exp. Ther. 2007, 322, 391–398. [Google Scholar] [CrossRef]
- Esposito, G.; Nobile, N.; Gigli, S.; Seguella, L.; Pesce, M.; d’Alessandro, A.; Bruzzese, E.; Capoccia, E.; Steardo, L.; Cuomo, R.; et al. Rifaximin Improves Clostridium difficile Toxin A-Induced Toxicity in Caco-2 Cells by the PXR-Dependent TLR4/MyD88/NF-kappaB Pathway. Front. Pharmacol. 2016, 7, 120. [Google Scholar] [CrossRef]
- Kimer, N.; Gudmann, N.S.; Pedersen, J.S.; Moller, S.; Nielsen, M.J.; Leeming, D.J.; Karsdal, M.A.; Moller, H.J.; Bendtsen, F.; Gronbek, H.; et al. No effect of rifaximin on soluble CD163, mannose receptor or type III and IV neoepitope collagen markers in decompensated cirrhosis: Results from a randomized, placebo controlled trial. PLoS ONE 2018, 13, e0203200. [Google Scholar] [CrossRef] [PubMed]
- Cariello, R.; Federico, A.; Sapone, A.; Tuccillo, C.; Scialdone, V.R.; Tiso, A.; Miranda, A.; Portincasa, P.; Carbonara, V.; Palasciano, G.; et al. Intestinal permeability in patients with chronic liver diseases: Its relationship with the aetiology and the entity of liver damage. Dig. Liver Dis. 2010, 42, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, T.; Konishi, M.; Kato, A.; Kato, M.; Kooka, Y.; Sawara, K.; Endo, R.; Torimura, T.; Suzuki, K.; Takikawa, Y.; et al. Updating the neuropsychological test system in Japan for the elderly and in a modern touch screen tablet society by resetting the cut-off values. Hepatol. Res. 2017, 47, 1335–1339. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Fu, L.; Niu, B.; Wu, S.; Wooley, J. Ultrafast clustering algorithms for metagenomic sequence analysis. Brief. Bioinform. 2012, 13, 656–668. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]

| Parameters | Baseline | RFX | p Value |
|---|---|---|---|
| Age | 67.3 (23–89) | ||
| Sex (male/female) | 18/12 | ||
| Etiology | |||
| Alcohol | 5 (16.7%) | ||
| Hepatitis B virus (HBV) | 4 (13.3%) | ||
| Hepatitis C virus (HCV) | 11 (36.7%) | ||
| Non-alcoholic steatohepatitis | 4 (13.3%) | ||
| Autoimmune hepatitis | 2 (6.7%) | ||
| Primary biliary cholangitis | 2 (6.7%) | ||
| Alcohol + HBV or HCV | 2 (6.7%) | ||
| Child class (A/B/C) | 0/28/2 | ||
| MELD score | 8.5 (1.3–17.4) | 7.9 (1.7–18.5) | 0.546 |
| Child-pugh score | 7 (7–13) | 8 (7–12) | 0.539 |
| Aspartate aminotransferase (U/L) | 47 ± 21 | 52 ± 30 | 0.507 |
| Alanine aminotransferase (U/L) | 31 ± 15 | 30 ± 15 | 0.928 |
| Albumin (g/dL) | 3.3 ± 0.6 | 3.4 ± 0.6 | 0.761 |
| Total bilirubin (mg/dL) | 1.7 ± 0.8 | 1.5 ± 0.7 | 0.412 |
| Prothrombin time (INR) | 1.30 ± 0.12 | 1.29 ± 0.13 | 0.819 |
| C-reactive protein (mg/dL) | 0.3 ± 0.5 | 0.3 ± 0.4 | 0.916 |
| Leukocyte (103/μL) | 3.8 ± 1.9 | 3.8 ± 1.9 | 0.95 |
| Platelet (104/μL) | 8.4 ± 3.8 | 8.2 ± 3.9 | 0.9 |
| Branched chain amino acid and tyrosine ratio | 3.6 ± 1.4 | 4.3 ± 3.5 | 0.33 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaji, K.; Saikawa, S.; Takaya, H.; Fujinaga, Y.; Furukawa, M.; Kitagawa, K.; Ozutsumi, T.; Kaya, D.; Tsuji, Y.; Sawada, Y.; et al. Rifaximin Alleviates Endotoxemia with Decreased Serum Levels of Soluble CD163 and Mannose Receptor and Partial Modification of Gut Microbiota in Cirrhotic Patients. Antibiotics 2020, 9, 145. https://doi.org/10.3390/antibiotics9040145
Kaji K, Saikawa S, Takaya H, Fujinaga Y, Furukawa M, Kitagawa K, Ozutsumi T, Kaya D, Tsuji Y, Sawada Y, et al. Rifaximin Alleviates Endotoxemia with Decreased Serum Levels of Soluble CD163 and Mannose Receptor and Partial Modification of Gut Microbiota in Cirrhotic Patients. Antibiotics. 2020; 9(4):145. https://doi.org/10.3390/antibiotics9040145
Chicago/Turabian StyleKaji, Kosuke, Soichiro Saikawa, Hiroaki Takaya, Yukihisa Fujinaga, Masanori Furukawa, Koh Kitagawa, Takahiro Ozutsumi, Daisuke Kaya, Yuki Tsuji, Yasuhiko Sawada, and et al. 2020. "Rifaximin Alleviates Endotoxemia with Decreased Serum Levels of Soluble CD163 and Mannose Receptor and Partial Modification of Gut Microbiota in Cirrhotic Patients" Antibiotics 9, no. 4: 145. https://doi.org/10.3390/antibiotics9040145
APA StyleKaji, K., Saikawa, S., Takaya, H., Fujinaga, Y., Furukawa, M., Kitagawa, K., Ozutsumi, T., Kaya, D., Tsuji, Y., Sawada, Y., Kawaratani, H., Moriya, K., Namisaki, T., Akahane, T., Mitoro, A., & Yoshiji, H. (2020). Rifaximin Alleviates Endotoxemia with Decreased Serum Levels of Soluble CD163 and Mannose Receptor and Partial Modification of Gut Microbiota in Cirrhotic Patients. Antibiotics, 9(4), 145. https://doi.org/10.3390/antibiotics9040145







