Powerful Antibacterial Peptides from Egg Albumin Hydrolysates
Abstract
1. Introduction
2. Results
2.1. Chemical Characterization of HEA
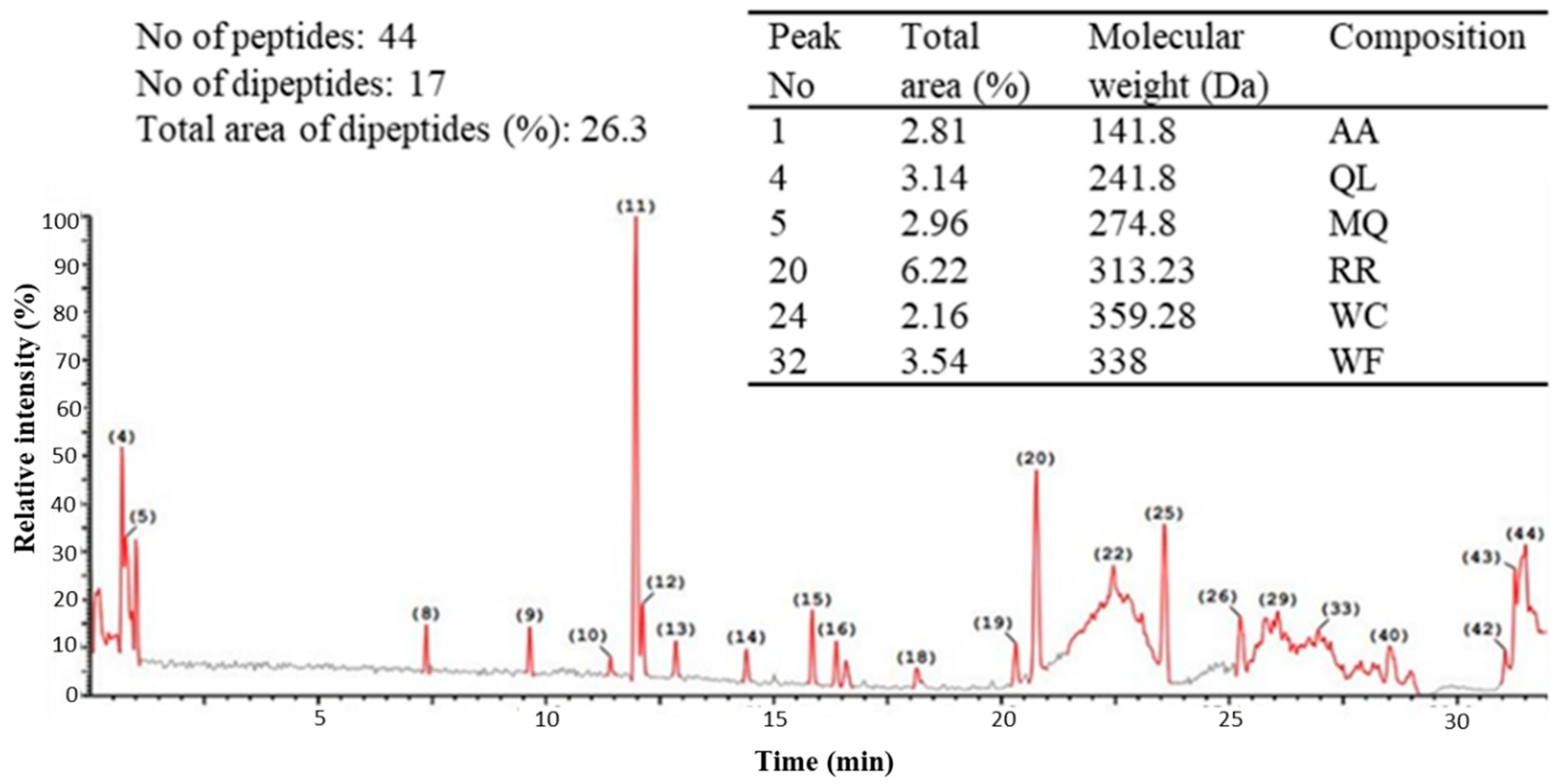
2.2. Ultra-Performance Liquid Chromatography (UPLC) of the Peptic Hydrolysate of Egg Albumin
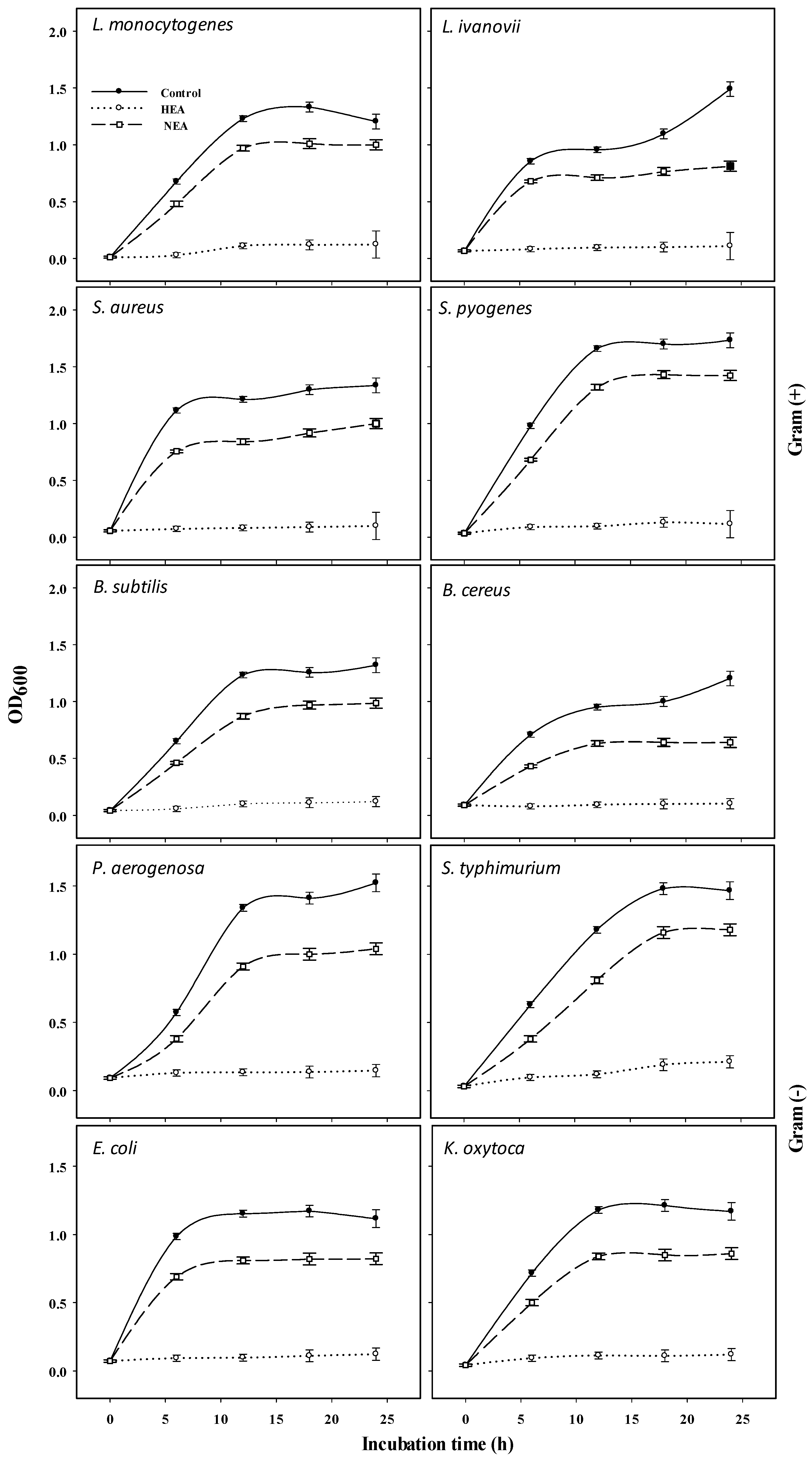
2.3. Antibacterial Activity of HEA and NEA against Gram (+) and Gram (−) Bacteria
2.4. Antibacterial Activity of Antibiotics and HEA-Antibiotic Combinations by Disc Diffusion Assay
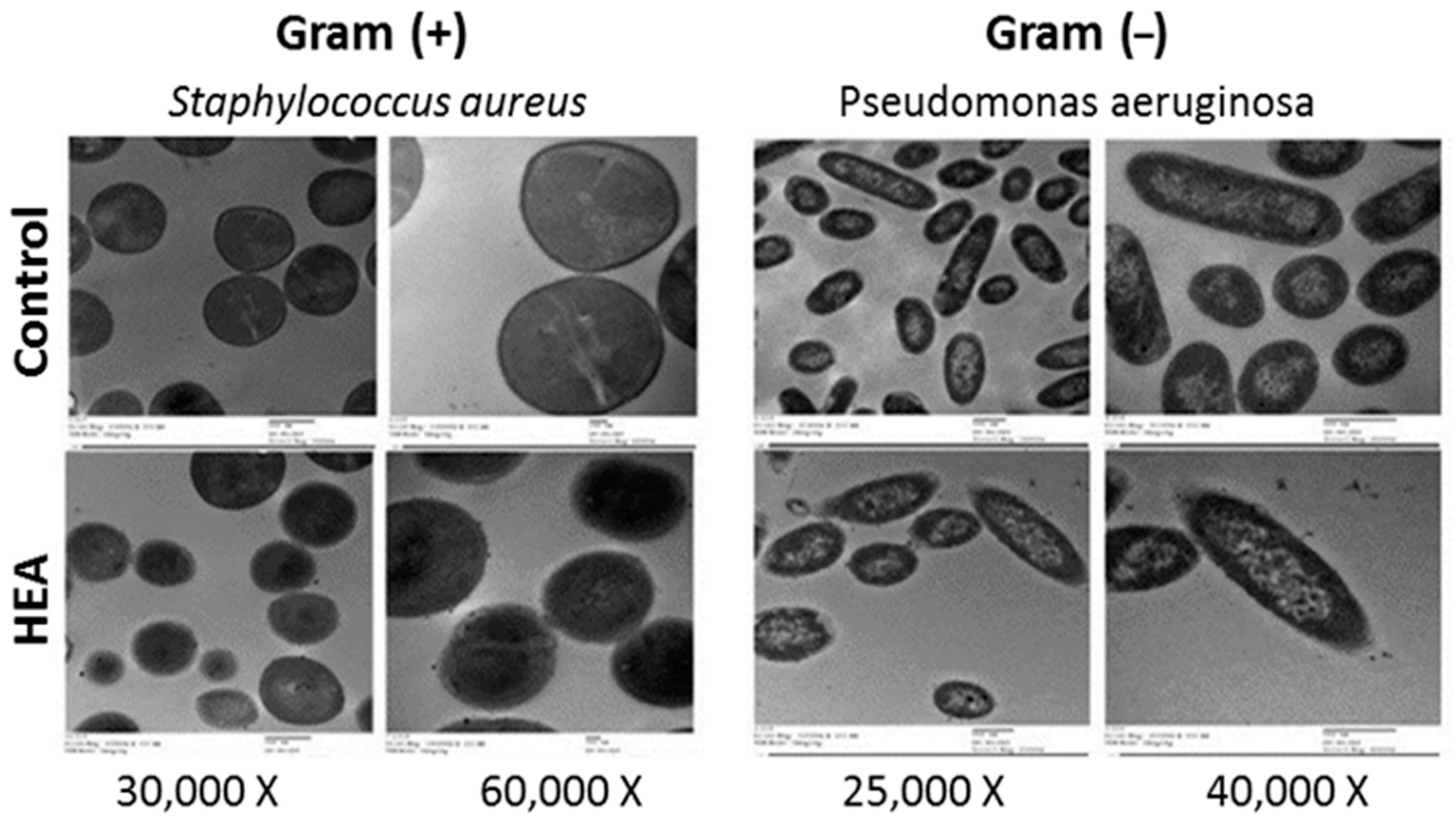
2.5. TEM Analysis
2.6. Investigation of Toxicity of HEA in Wistar Albino Rats
2.6.1. Acute Oral Toxicity
2.6.2. Subacute Toxicity
2.6.3. Hematological Parameters
2.6.4. Biochemical Indicators
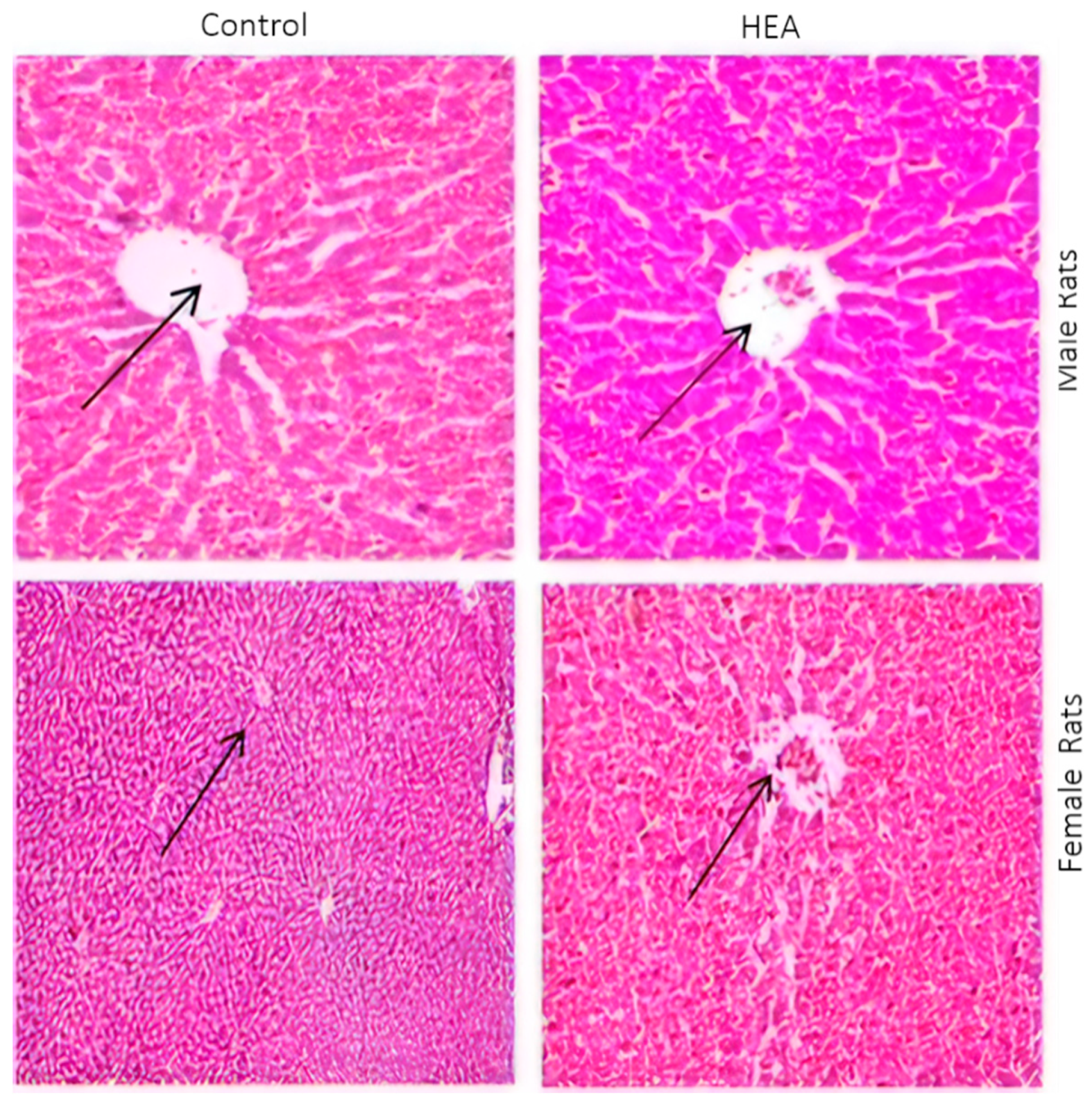
2.6.5. Histo-Pathological Examination
3. Discussion
4. Materials and Methods
4.1. Collection of Pathogenic Bacteria
4.2. Egg Albumin Preparation
4.3. Enzymatic Hydrolysis of Egg Albumin
Characterization of Egg Albumin Hydrolysates
4.4. Antibacterial Activity Estimation
4.4.1. Disc Diffusion Assay
4.4.2. Minimum Inhibitory Concentration (MIC)
4.4.3. Antibacterial Activity of Antibiotics and Combinations of Both HEA and Antibiotics
4.4.4. Quantitative Inhibition of Pathogenic Bacteria by HEA
4.5. Transmission Electron Microscopy Examination
4.6. Investigation of Toxicity for HEA in Wistar Albino Rats
4.6.1. Acute Oral Toxicity
4.6.2. Subacute Toxicity
4.6.3. Biochemical Analyses
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sitohy, M.Z.; Osman, A.O. Enhancing milk preservation with esterified legume proteins. Probiotics Antimicrob. Proteins 2011, 3, 48–56. [Google Scholar] [CrossRef]
- Enan, G.; Addallah, F.M.; Sobhy, H. Effect of acyclovir on bovine herpesvirus type 1 infection in vitro cultured cells. Int. J. Virol. 2012, 8, 307–312. [Google Scholar] [CrossRef]
- Enan, G.; Abdel-Shafi, S.; Ouda, S.; Negm, S. Novel antibacterial activity of Lactococcus lactis subspecies lactis Z11 isolated from Zabady. Int. J. Biomed. Sci. 2013, 9, 144–180. [Google Scholar]
- Enan, G.; Abdel-Shafi, S.; Abdel-Halem, M.F.; Negm, S. Characterization of probiotic lactic acid bacteria to be used as starter and protective cultures for dairy fermentations. Int. J. Probiotics Prebiotics 2013, 8, 157–163. [Google Scholar]
- Enan, G.; Osman, M.E.; Abdel-Haliem, M.E.F.; Abdel-Ghany, S.E. Advances in Microbial and Nucleic Acids Biotechnology. BioMed Res. Int. 2018, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Shafi, S.; Osman, A.; Al-Mohammadi, A.-R.; Enan, G.; Kamal, N.; Sitohy, M. Biochemical, biological characteristics and antibacterial activity of glycoprotein extracted from the epidermal mucus of African catfish (Clarias gariepinus). Int. J. Biol. Macromol. 2019, 138, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Ismaiel, A.A.; Ali, A.-E.S.; Enan, G. Incidence of Listeria in Egyptian meat and dairy samples. Food Sci. Biotechnol. 2014, 23, 179–185. [Google Scholar] [CrossRef]
- Mahgoub, S.A.M.; Osman, A.; Ramadan, M.F. Inhibitory effect of Nigella sativa oil against Listeria monocytogenes and Salmonella enteritidis inoculated in minced beef meat. J. Food Meas. Charact. 2017, 11, 2043–2051. [Google Scholar] [CrossRef]
- Osman, A.; El-Didamony, G.; Sitohy, M.; Khalifa, M.; Enan, G. Soybean glycinin basic subunit inhibits methicillin resistant-vancomycin intermediate Staphylococcus aureus (MRSA-VISA) in vitro. Int. J. Appl. Res. Nat. Prod. 2016, 9, 17–26. [Google Scholar]
- Osman, A.; Goda, H.A.; Sitohy, M. Storage stability of minced beef supplemented with chickpea legumin at 4 °C as a potential substitute for nisin. Food Sci. Technol. 2018, 93, 434–441. [Google Scholar] [CrossRef]
- Osman, A.O.; Mahgoub, S.A.; Sitohy, M.Z. Preservative action of 11S (glycinin) and 7S (β-conglycinin) soy globulin on bovine raw milk stored either at 4 or 25 °C. J. Dairy Res. 2013, 80, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Sitohy, M.; Osman, A. Bioactive Compounds in Soybean Proteins and Its Applications in Food Systems, Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2019; pp. 147–160. [Google Scholar]
- Enan, G.; Abdul Aziz, A.-A. Novel plantaricin UG1 production by Lactobacillus plantarum UG1 in enriched whey permeate in batch fermentation processes. J. Food Agric. Environ. 2006, 4, 84–88. [Google Scholar]
- Abdel-Shafi, S.; Ouda, S.M.; Elbalat, I.; Enan, G. Characterization and identification of multidrug resistant bacteria from some Egyptian patients. Biotechnology 2013, 12, 65–73. [Google Scholar] [CrossRef]
- Enan, G.; Al-Mohammadi, A.-R.; El-Didamony, G.; Abdel-Haliem, M.E.F. Antimicrobial activity of Enterococcus faecium NM2 isolated from urine: Purification, characterization and bactericidal action of enterocin NM2. Asian J. Appl. Sci. 2014, 7, 721–734. [Google Scholar] [CrossRef]
- Abdel-Shafi, S.; Al-Mohammadi, A.-R.; Negm, S.; Enan, G. Antibacterial activity of Lactobacillus delbreukii subspecies bulgaricus isolated from zabady. Life Sci. J. 2014, 11, 264–270. [Google Scholar]
- Enan, G.; El-Didamony, G.; Mohammed, M.E.F.; Zakaria, A. Novel antibacterial activity of Enterococcus faecium NM2 isolated from urine of healthy people. Asian J. Appl. Sci. 2014, 7, 66–78. [Google Scholar] [CrossRef]
- Osman, A.; Abbas, E.; Mahgoub, S.; Sitohy, M. Inhibition of Penicillium digitatum in vitro and in postharvest orange fruit by a soy protein fraction containing mainly β-conglycinin. J. Gen. Plant Pathol. 2016, 82, 293–301. [Google Scholar] [CrossRef]
- Akyil, D.; Ozkara, A.; Konuk, M. Pesticides, Environmental Pollution, and Health. In Environmental Health Risk-Hazardous Factors to Living Species; Intech Open: London, UK, 2016. [Google Scholar] [CrossRef]
- Osman, A.; Abdel-Shafi, S.; Al-Mohammadi, A.R.; Kamal, N.; Enan, G.; Sitohy, M. Catfish glycoprotein, a highly powerful safe preservative of minced beef stored at 4 °C for 15 Days. Foods 2020, 9, 1115. [Google Scholar] [CrossRef]
- Abbas, E.; Osman, A.; Sitohy, M. Biochemical control of Alternaria tenuissima infecting post-harvest fig fruit by chickpea vicilin. J. Sci. Food Agric. 2020, 100, 2889–2897. [Google Scholar] [CrossRef]
- Mahgoub, S.; Osman, A.; Sitohy, M. Inhibition of growth of pathogenic bacteria in raw milk by legume protein esters. J. Food Prot. 2011, 74, 1475–1481. [Google Scholar] [CrossRef]
- Mahgoub, S.A.; Sitohy, M.Z.; Osman, A.O. Counteracting recontamination of pasteurized milk by methylated soybean protein. Food Bioproc. Tech. 2013, 6, 101–109. [Google Scholar] [CrossRef]
- Osman, A.; Mahgoub, S.; El-Masry, R.; Al-Gaby, A.; Sitohy, M. Extending the technological validity of raw buffalo milk at room temperature by esterified legume proteins. J. Food Process. Preserv. 2014, 38, 223–231. [Google Scholar] [CrossRef]
- Osman, A.; Mahgoub, S.; Sitohy, M. Hindering milk quality storage deterioration by mild thermization combined with methylated chickpea protein. Int. Food Res. J. 2014, 21, 693–701. [Google Scholar]
- Sitohy, M.; Mahgoub, S.; Osman, A. Controlling psychrotrophic bacteria in raw buffalo milk preserved at 4 °C with esterified legume proteins. Food Sci. Technol. 2011, 44, 1697–1702. [Google Scholar] [CrossRef]
- Abdel-Hamid, M.; Romeih, E.; Saporito, P.; Osman, A.; Mateiu, R.V.; Mojsoska, B.; Jenssen, H. Camel milk whey hydrolysate inhibits growth and biofilm formation of Pseudomonas aeruginosa PAO1 and methicillin-resistant Staphylococcus aureus. Food Control. 2020, 111, 107056. [Google Scholar] [CrossRef]
- Osman, A.; El-Araby, G.M.; Taha, H. Potential use as a bio-preservative from lupin protein hydrolysate generated by alcalase in food system. J. Appl. Biol. Biotechnol. 2016, 4, 076–081. [Google Scholar]
- Saad, A.M.; Osman, A.O.M.; Mohamed, A.S.; Ramadan, M.F. Enzymatic hydrolysis of Phaseolus vulgaris protein isolate: Characterization of hydrolysates and effect on the quality of minced beef during cold storage. Int. J. Pept. Res. Ther. 2020, 26, 567–577. [Google Scholar] [CrossRef]
- Abdel-Hamid, M.; Otte, J.; De Gobba, C.; Osman, A.; Hamad, E. Angiotensin I-converting enzyme inhibitory activity and antioxidant capacity of bioactive peptides derived from enzymatic hydrolysis of buffalo milk proteins. Int. Dairy J. 2017, 66, 91–98. [Google Scholar] [CrossRef]
- Abdel-Hamid, M.; Osman, A.; El-Hadary, A.; Romeih, E.; Sitohy, M.; Li, L. Hepatoprotective action of papain-hydrolyzed buffalo milk protein on carbon tetrachloride oxidative stressed albino rats. J. Dairy Sci. 2020, 103, 1884–1893. [Google Scholar] [CrossRef]
- Abdel-Hamid, M.; Goda, H.A.; De Gobba, C.; Jenssen, H.; Osman, A. Antibacterial activity of papain hydrolysed camel whey and its fractions. Int. Dairy J. 2016, 61, 91–98. [Google Scholar] [CrossRef]
- Osman, A.; Goda, H.A.; Abdel-Hamid, M.; Badran, S.M.; Otte, J. Antibacterial peptides generated by Alcalase hydrolysis of goat whey. Food Sci. Technol. 2016, 65, 480–486. [Google Scholar] [CrossRef]
- Abdel-Shafi, S.; Al-Mohammadi, A.-R.; Osman, A.; Enan, G.; Abdel-Hameid, S.; Sitohy, M. Characterization and antibacterial activity of 7S and 11S Globulins isolated from Cowpea seed protein. Molecules 2019, 24, 1082. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Shafi, S.; Al-Mohammadi, A.R.; Sitohy, M.; Mosa, B.; Ismaiel, A.; Enan, G.; Osman, A. Antimicrobial activity and chemical constitution of the crude, phenolic-rich extracts of Hibiscus sabdariffa, Brassica oleracea and Beta vulgaris. Molecules 2019, 24, 4280. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Shafi, S.; Al-Mohammadi, A.R.; Almanaa, T.N.; Moustafa, A.H.; Saad, T.M.M.; Ghonemy, A.; Anacarso, I.; Enan, G.; El-Gazzar, N. Identification and testing antidermatophyticoxaborole-6-benzene sulphonoamide derivative (OXBS) from Streptomyces atrovirens KM192347 isolated from soil. Antibiotics 2020, 9, 176. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Shafi, S.; Osman, A.; Enan, G.; El-Nemer, M.; Sitohy, M. Antibacterial activity of methylated egg white proteins against pathogenic G+ and G− bacteria matching antibiotics. SpringerPlus 2016, 5, 983. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Yin, Y.; Zhao, W.; Chen, F.; Liu, J. Application and bioactive properties of proteins and peptides derived from hen eggs: Opportunities and challenges. J. Sci. Food Agric. 2014, 94, 2839–2845. [Google Scholar] [CrossRef]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef]
- Cruz, J.; Ortiz, C.; Guzman, F.; Fernandez-Lafuente, R.; Torres, R. Antimicrobial peptides: Promising compounds against pathogenic microorganisms. Curr. Med. Chem. 2014, 21, 2299–2321. [Google Scholar] [CrossRef]
- Fosgerau, K.; Hoffmann, T. Peptide therapeutics: Current status and future directions. Drug Discov. Today 2015, 20, 122–128. [Google Scholar] [CrossRef]
- Enan, G.; Abdel-Shafi, S.; Ouda, S.M.; Elbalat, I. Genetic linkage of the antibiotic resistance ability in the Escherichia coli UR4 strain isolated from urine. J. Med. Sci. 2013, 13, 261–268. [Google Scholar] [CrossRef][Green Version]
- Simon, C.; Foxman, B.; Nriagu, J. Prevalence of antibiotic resistance bacteria and treatment. Appl. Environ. Microbiol. 2009, 75, 5714–5718. [Google Scholar]
- Enan, G.; Sitohy, M.; Osman, A.; Khalifa, M.; El-Didamony, G. Inhibition of Methicillin Resistant-Vancomycin Intermediate S. aureus (MRSA-VISA) by Glycin Basic Subunit In Vitro and in Minced Meat; First International Congress on Food Safety and Regulatory Measures: Birmingham, UK, 2015; pp. 37–45. [Google Scholar]
- Chopra, I.; Hodgson, J.; Metcalf, B.; Poste, G. New approaches to control of infections caused by antibiotic resistant bacteria. Jama 1996, 275, 401–403. [Google Scholar] [CrossRef]
- Chopra, I.; Hodgson, J.; Metcalf, B.; Poste, G. The search for antimicrobial agents effective against bacterial resistant to multiple antibiotics. Antimicorb. Agents Chemother. 1997, 41, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, C.L.; Moyles, D.; Beveridge, T.J.; Hancock, R.E.W. Antimicrobial action of structurally diverse cationic peptides on Gram-positive bacteria. Antimicrob. Agents Chemother. 2000, 44, 2086–2092. [Google Scholar] [CrossRef] [PubMed]
- Halpin, M.I.; Richardson, T. Elected functionality changes of Beta-lacto globulin upon esterification of side chain carboxyl groups. J. Dairy Sci. 1985, 68, 3189–3198. [Google Scholar] [CrossRef]
- Sitohy, M.; Mahgoub, S.; Osman, A. Bioactive proteins against pathogenic and spoilage bacteria. Funct. Food Health Dis. 2014, 4, 451–462. [Google Scholar] [CrossRef][Green Version]
- Liu, Q.; Kong, B.; Xiong, Y.L.; Xia, X. Antioxidant activity and functional properties of porcine plasma protein hydrolysate as influenced by the degree of hydrolysis. Food Chem. 2010, 118, 403–410. [Google Scholar] [CrossRef]
- Klompong, V.; Benjakul, S.; Kantachote, D.; Shahidi, F. Antioxidative activity and functional properties of protein hydrolysate of yellow stripe trevally (Selaroides leptolepis) as influence by the degree of hydrolysis and enzyme type. Food Chem. 2007, 102, 1317–1327. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, R.; Rana, R. Bioactive Peptides: A Review. Int. J. Bioautom. 2011, 15, 223–250. [Google Scholar]
- El-Zahar, K.; Sitohy, M.; Choiset, Y.; Métro, F.; Haertle, T.; Chobert, J.-M. Peptic hydrolysis of ovine β-lactoglobulin and α-lactalbumin exceptional susceptibility of native ovine β-lactoglobulin to pepsinolysis. Int. Dairy J. 2005, 15, 17–27. [Google Scholar] [CrossRef]
- Sitohy, M.; Chobert, J.; Haertlé, T. Peptic hydrolysis of methyl-, ethyl-, and propyl-esters of beta-casein and alpha-lactalbumin. Milchwissenshaft Milk Sci. Int. 2001, 56, 303–307. [Google Scholar]
- Sitohy, M.; Chobert, J.M.; Dalgalarrondo, M.; Haertle, T. Factors influencing pepsinolysis of methyl-, ethyl-, and propyl-esters of derivatives of β-lactoglobulin. J. Food Biochem. 2001, 25, 181–198. [Google Scholar] [CrossRef]
- Lehninger, A.L.; Nelson, D.L.; Cox, M.M. Lehninger Principles of Biochemistry; Macmillan: New York, NY, USA, 2005. [Google Scholar]
- El-Zahar, K.; Chobert, J.M.; Sitohy, M.; Dalgalarrondo, M.; Haertlé, T. Proteolytic degradation of ewe milk proteins during fermentation of yoghurts and storage. Nahr. Food. 2003, 47, 199–206. [Google Scholar] [CrossRef] [PubMed]
- El-Zahar, K.; Sitohy, M.; Choiset, Y.; Metro, F.; Haertle, T.; Chobert, J. Antimicrobial activity of ovine whey protein and their peptic hydrolysates. Milchwissenshaft Milk Sci. Int. 2004, 59, 653–656. [Google Scholar]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Sitohy, M.Z.; Mahgoub, S.A.; Osman, A.O. In vitro and in situ antimicrobial action and mechanism of glycinin and its basic subunit. Int. J. Food Microbiol. 2012, 154, 19–29. [Google Scholar] [CrossRef]
- Enan, G.; El-Essawy, A.A.; Uytendaale, M.; Debevere, J. Antibacterial activity of L. plantarum UG1 isolated from dry sausage: Characterization, production and bactericidal action of plantaricinUG1. Int. J. Food Microbiol. 1996, 30, 189–215. [Google Scholar] [CrossRef]
- Utsumi, S.; Matsumura, Y.; Mori, T. Structure-function relationships of soy proteins. In Food Proteins and Their Application; Damodaran, S., Paraf, A., Eds.; Marcel Dekker: New York, NY, USA, 1997; pp. 257–291. [Google Scholar]
- Sitohy, M.; Mahgoub, S.; Osman, A.; El-Masry, R.; Al-Gaby, A. Extent and mode of action of cationic legume proteins against Listeria monocytogenes and Salmonella enteritidis. Probiotics Antimicrob. Proteins 2013, 5, 195–205. [Google Scholar] [CrossRef]
- Kuipers, B.J. Gruppen, H. Identification of strong aggregating regions in soy glycinin upon enzymatic hydrolysis. J. Agric. Food Chem. 2008, 56, 3818–3827. [Google Scholar] [CrossRef]
- Ibrahim, H.R. On the novel catalytically-independent antimicrobial function of hen egg-white lysozyme: A conformation-dependent activity. Nahrung 1998, 42, 187–193. [Google Scholar] [CrossRef]
- Hughey, V.L.; Johnson, E.A. Antimicrobial activity of lysozyme against bacteria involved in food spoilage and food-borne disease. Appl. Environ. Microbiol. 1987, 53, 2165–2170. [Google Scholar] [CrossRef] [PubMed]
- Bera, A.; Herbert, S.; Jacob, A.; Vollmer, W.; Gotz, F. Why are pathogenic Staphylococci so lysozyme resistant? The peptidoglycan O-acetyltransferaze OatA is the major determinant for lysozyme resistance of Staphylococcus aureus. Mol. Microbiol. 2005, 55, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Atrih, A.; Bacher, G.; Allmaier, G.; Williamson, M.P.; Foster, S.J. Analysis of peptidoglycan structure from vegetative cells of Bacillus subtilis 168 and role of PBP 5 in peptidoglycan maturation. J. Bacteriol. 1999, 181, 3956–3966. [Google Scholar] [CrossRef]
- Szwajkowska, M.; Wolanciuk, A.; Barłowska, J.; Król, J.; Litwińczuk, Z. Bovine milk proteins as the source of bioactive peptides influencing the consumers’ immune system—A review. Anim. Sci. Pap. Rep. 2011, 29, 269–280. [Google Scholar]
- Jeremy, J.M.; Sondra, C.F.; Anthony, C.J.L. Longer-duration uses of tetracyclines and penicillins in U.S. food-producing animals: Indications and microbiologic effects. Environ. Int. 2011, 37, 991–1004. [Google Scholar]
- Lavalette, D.; Te´treau, C.; Tourbez, M.; Blouquit, Y. Microscopic viscosity and rotational diffusion of proteins in a macromolecular environment. Biophys. J. 1999, 76, 2744–2751. [Google Scholar] [CrossRef]
- Murzyn, K.; Rog, T.; Pasenkiewicz-Gierula, M. Phosphatidylethanolamine-phosphatidyl glycerol bilayer as a model of the inner bacterial membrane. Biophys. J. 2005, 88, 1091–1103. [Google Scholar] [CrossRef]
- Enan, G.; Al-Mohammadi, A.-R.; Mahgoub, S.; Abdel-Shafi, S.; Askar, E.; Ghaly, M.F.; Taha, M.A.; El-Gazzar, N. Inhibition of Staphylococcus aureus LC 554891 by Moringa oleifera seed extract either singly or in combination with antibiotics. Molecules 2020, 25, 4583. [Google Scholar] [CrossRef]
- Hugo, W.; Russel, A. Pharmaceutical Microbiology, 5th ed.; Black Well Scientific Publications: Oxford, UK, 1990; pp. 369–380; 487–492. [Google Scholar]
- Giuliani, A.; Pirri, G.; Rinaldi, A.C. Antimicrobial peptides: The LPS connection. Methods Mol. Biol. 2010, 618, 137–154. [Google Scholar]
- Castro, A.; Silva, J.; Teixeira, P. Staphylococcus aureus, a Food Pathogen: Virulence Factors and Antibiotic Resistance. Food Borne Dis. 2018, 213–238. [Google Scholar] [CrossRef]
- Hoek, K.S.; Milne, J.M.; Grieve, P.A.; Dionysius, D.A.; Smith, R. Antibacterial activity of bovine lactoferrin–derived peptides. Antimicrob. Agents Chemother. 1997, 41, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Loomis, T.A.; Hayes, A.W. Loomis’s Essentials of Toxicology; Academic Press: San Diego, CA, USA, 1996; pp. 205–248. [Google Scholar]
- Andersen, H.; Larsen, S.; Spliid, H.; Christensen, N.D. Multivariate statistical analysis of organ weights in toxicity studies. Toxicology 1999, 136, 67–77. [Google Scholar] [CrossRef]
- Morita, C.; Nishida, T.; Ito, K. Biological toxicity of acid electrolyzed functional water: Effect of oral administration on mouse digestive tract and changes in body weight. Arch. Oral Biol. 2011, 56, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Sitohy, M.; Osman, A.; Gharib, A.; Chobert, J.-M.; Haertlé, T. Preliminary assessment of potential toxicity of methylated soybean protein and methylated β-lactoglobulin in male Wistar rats. Food Chem. Toxicol. 2013, 59, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Otte, J.; Abdel-Hamid, M.; Osman, A. Comparative assessment of peptide concentration in milk protein hydrolysates and fractions. Int. J. Dairy Sci. 2015, 10, 228–235. [Google Scholar] [CrossRef]
- Adler-Nissen, J. Enzymic Hydrolysis of Food Proteins; Elsevier Applied Science Publishers: Amsterdam, The Netherlands, 1986. [Google Scholar]
- Sitohy, M.; Osman, A. Antimicrobial activity of native and esterified legume proteins against Gram-negative and Gram-positive bacteria. Food Chem. 2010, 120, 66–73. [Google Scholar] [CrossRef]
- Evans, R.W.; Williams, J. The electrophoresis of transferrins in urea/polyacrylamide gels. Biochem. J. 1980, 189, 541. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing: Eighteenth Informational Supplement; CLSI: Wayne, PA, USA, 2008. [Google Scholar]
- National Committee for Clinical Laboratory Standards; Barry, A.L. Methods for Determining Bactericidal Activity of Antimicrobial Agents: Approved Guideline; National Committee for Clinical Laboratory Standards Wayne: Wayne, PA, USA, 1999.
- Schafer, E.W. The acute oral toxicity of 369 pesticidal, pharmaceutical and other chemicals to wild birds. Toxicol. Appl. Pharmacol. 1972, 21, 315–330. [Google Scholar] [CrossRef]
- OECD. Guidelines for The Testing of Chemicals: Acute Oral Toxicity—Fixed Dose Procedure; OECD/OCDE 420; OECD Environment Directorate, Environment, Health and Safety Division: Paris, France, 2001. [Google Scholar]
- Osman, A.; Abd-Elaziz, S.; Salama, A.; Eita, A.A.; Sitohy, M. Health protective actions of phycocyanin obtained from an Egyptian isolate of Spirulina platensis on albino rats. Eur. Asian J. Biosci. 2019, 13, 105–112. [Google Scholar]
- Guidelines for Hematoxylin and Eosin Staining; National Society for Histotechnology: Ellicott City, MD, USA, 2001.
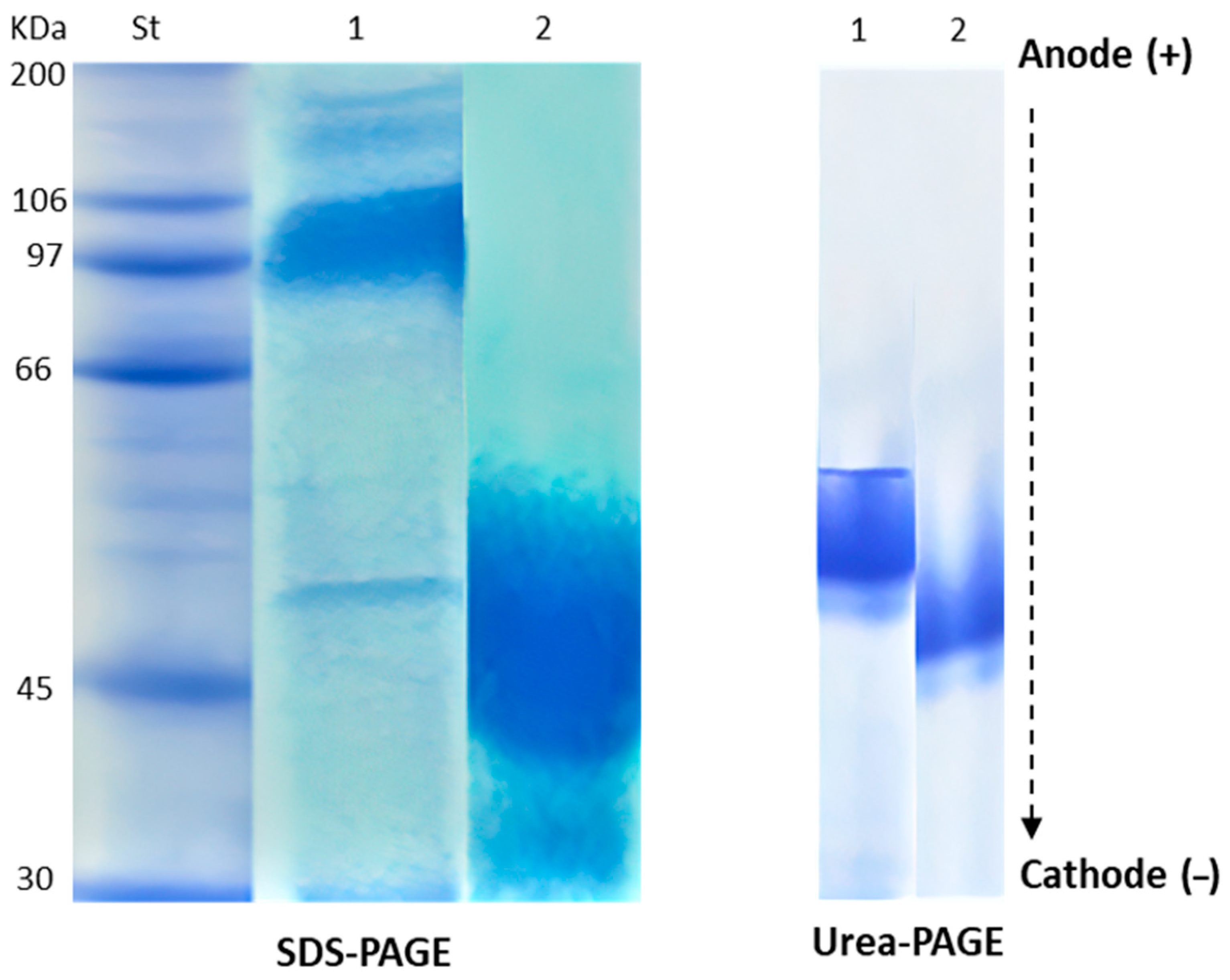


| Band Number | NEA | HEA | ||
|---|---|---|---|---|
| MW (kDa) | Band Intensity | MW (kDa) | Band Intensity | |
| 1 | 180 | 1.2 | 51 | 10 |
| 2 | 144 | 1.7 | - | - |
| 3 | 97 | 6.9 | - | - |
| 4 | 50 | 1.9 | - | - |
| 5 | 30 | 2.4 | - | - |
| Inhibition Zone Diameter (IZD) (mm/HEA µg mL−1) | IZD of NEA (mm/NEA µg mL−1) | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 50 | 100 | 150 | 200 | 250 | 250 | |
| L. monocytogens | 0 | 0 | 34 ± 1.16 a | 36 ± 2.31 a | 39 ± 1.73 a | 42 ± 2.31 a | 7 ± 0.58 c |
| K. oxytoca | 0 | 0 | 9 ± 0.58 d | 28 ± 1.73 c | 34 ± 1.73 b | 38 ± 1.73 abc | 5 ± 0.44 d |
| S. pyogenes | 0 | 0 | 24 ± 1.73 b | 32 ± 1.73 b | 35 ± 2.89 ab | 39 ± 2.89 ab | 3 ± 0.38 e |
| L. ivanovii | 0 | 0 | 0 f | 17 ± 0.58 d | 33 ± 1.73 b | 34 ± 1.16 bc | 10 ± 0.58 b |
| S. aureus | 0 | 0 | 20 ± 1.16 c | 28 ± 1.16 c | 30 ± 1.16 bc | 33 ± 1.73 c | 11 ± 1.05 a |
| B. subtilis | 0 | 0 | 0 f | 9 ± 0.58 e | 12 ± 0.58 e | 14 ± 0.58 e | 12 ± 1.28 a |
| P. aerugenosa | 0 | 0 | 0 f | 9 ± 0.58 e | 28 ± 1.73 c | 33 ± 1.73 c | 3 ± 0.11 e |
| S. typhimurium | 0 | 0 | 7 ± 0.57 de | 13 ± 1.16 e | 18 ± 1.16 d | 21 ± 1.15 d | 1 ± 0.08 ef |
| E. coli | 0 | 0 | 0 f | 0 f | 10 ± 0.58 e | 13 ± 1.16 e | 2 ± 0.09 e |
| B. cereus | 0 | 0 | 6 ± 0.58 e | 11 ± 1.16 e | 12 ± 1.16 e | 14 ± 1.16 e | 2 ± 0.22 e |
| Microorganisms | Antibiotic | Antibiotic MIC (µg/mL) | Inhibition Zone (mm) | ||
|---|---|---|---|---|---|
| Antibiotic | HEA | Antibiotic: HEA | |||
| L. monocytogenes | Ciprofloxacin | 2 | 18 ± 1.73 c | 34 ± 1.73 a | 36 ± 2.31 a |
| K. oxytoca | Gentamycin | 6 | 14 ± 1.16 b | 9 ± 0.58 c | 16 ± 0.58 a |
| S. pyogenes | Penicillin G | 0.016 | 29 ± 1.16 b | 24 ± 1.16 c | 34 ± 1.16 a |
| L. ivanovii | Chloramphenicol | 7.5 | 19 ± 1.73 b | 17 ± 1.16 b | 29 ± 1.16 a |
| S. aureus | Vancomycin | 1 | 14 ± 0.58 c | 20 ± 1.16 b | 34 ± 0.58 a |
| B. subtilis | Chloramphenicol | 2 | 18 ± 1.16 b | 9 ± 1.16 c | 20 ± 1.16 a |
| P. aeruginosa | Ciprofloxacin | 1 | 28 ± 1.73 b | 9 ± 0.58 c | 30 ± 1.16 a |
| S. typhimurium | Ciprofloxacin | 4 | 18 ± 1.16 a | 7 ± 0.58 b | 19 ± 1.16 a |
| E. coli | Gentamycin | 1 | 19 ± 1.16 a | 10 ± 1.16 c | 15 ± 1.16 b |
| B. cereus | Vancomycin | 4 | 5 ± 0.29 b | 6 ± 0.29 b | 15 ± 0.58 a |
| Treatment | Control | Tested Proteins Doses (mg/kg b. wt *./day) | |
|---|---|---|---|
| HEA | |||
| 500 | 2500 | ||
| Male | |||
| Initial body wt (g) | 165 ± 2.9 | 163 ± 2.9 | 160 ± 2.9 |
| Final body wt (g) | 245 ± 5.8 | 249 ± 3.5 | 250 ± 2.9 |
| Body wt gain (g) | 80 ± 2.9 | 86 ± 2.3 | 90 ± 5.8 |
| Female | |||
| Initial body wt (g) | 135 ± 2.9 | 140 ± 5.1 | 135 ± 2.9 |
| Final body wt (g) | 187 ± 5.8 | 196 ± 2.3 | 196 ± 5.8 |
| Body wt gain (g) | 52 ± 1.7 b | 56 ± 2.3 ab | 61 ± 2.9 a |
| Organ Weight (g) | Control | Tested Proteins Doses (mg/kg b. wt./day) | |
|---|---|---|---|
| HEA | |||
| 500 | 2500 | ||
| Male | |||
| Liver | 6.3 ± 0.35 | 6.23 ± 0.14 | 6.16 ± 0.26 |
| Kidney | 1.45 ± 0.13 | 1.37 ± 0.13 | 1.28 ± 0.06 |
| Brain | 1.37 ± 0.09 | 1.36 ± 0.14 | 1.32 ± 0.09 |
| Spleen | 0.65 ± 0.07 | 0.65 ± 0.07 | 0.63 ± 0.05 |
| Lung | 1.27 ± 0.08 | 1.27 ± 0.08 | 1.25 ± 0.06 |
| Heart | 0.98 ± 0.11 | 0.94 ± 0.08 | 0.95 ± 0.07 |
| Testis | 1.28 ± 0.13 | 1.26 ± 0.12 | 1.24 ± 0.13 |
| Female | |||
| Liver | 5.8 ± 0.23 | 5.78 ± 0.21 | 5.68 ± 0.15 |
| Kidney | 1.29 ± 0.11 | 1.26 ± 0.08 | 1.24 ± 0.09 |
| Brain | 1.27 ± 0.07 | 1.26 ± 0.08 | 1.24 ± 0.15 |
| Spleen | 0.61 ± 0.06 | 0.6 ± 0.04 | 0.58 ± 0.09 |
| Lung | 1.15 ± 0.12 | 1.16 ± 0.08 | 1.14 ± 0.06 |
| Heart | 0.85 ± 0.05 | 0.84 ± 0.13 | 0.83 ± 0.07 |
| Ovary | 0.067 ± 0.02 | 0.064 ± 0.03 | 0.062 ± 0.02 |
| Blood Biochemical Parameters | Control | Tested Proteins Doses (mg/kg b. wt./day) | |
|---|---|---|---|
| HEA | |||
| 500 | 2500 | ||
| Male | |||
| WBC a (×103/µL) | 7.12 ± 0.13 | 7.09 ± 0.07 | 7.1 ± 0.13 |
| RBC b (×106 µL) | 8.23 ± 0.11 | 8.23 ± 0.09 | 8.22 ± 0.12 |
| HGB c (g/dL) | 12.89 ± 0.23 | 12.89 ± 0.34 | 12.93 ± 0.27 |
| HCT d (%) | 42.7 ± 0.31 | 42.12 ± 0.12 | 42.9 ± 0.17 |
| MCV e (FL) | 51.76 ± 0.17 | 51.56 ± 0.19 | 51.65 ± 0.34 |
| MCH f (pg) | 16.98 ± 0.17 | 16.96 ± 0.13 | 16.94 ± 0.11 |
| MCHC g (g/dL) | 34.45 ± 0.15 | 34.44 ± 0.11 | 34.43 ± 0.09 |
| PLT h (×103/µL) | 820 ± 3.45 | 827 ± 5.91 | 822 ± 4.9 |
| LYM i (%) | 5.27 ± 0.17 | 5.65 ± 0.04 | 5.16 ± 0.06 |
| Female | |||
| WBC a (×103/µL) | 8.02 ± 0.12 | 8.01 ± 0.08 | 8.06 ± 0.12 |
| RBC b (×106 µL) | 8.25 ± 0.11 | 8.22 ± 0.12 | 8.21 ± 0.11 |
| HGB c (g/dL) | 12.99 ± 0.21 | 12.94 ± 0.31 | 12.96 ± 0.22 |
| HCT d (%) | 44.8 ± 0.31 | 43.19 ± 0.15 | 44.9 ± 0.07 |
| MCV e (FL) | 53.70 ± 0.11 | 52.96 ± 0.14 | 53.68 ± 0.14 |
| MCH f (pg) | 17.55 ± 0.12 | 17.86 ± 0.14 | 17.90 ± 0.21 |
| MCHC g (g/dL) | 36.41 ± 0.16 | 36.04 ± 0.09 | 36.33 ± 0.19 |
| PLT h (×103/µL) | 880 ± 62 | 885 ± 5.44 | 882 ± 9.51 |
| LYM i (%) | 6.34 ± 0.11 | 6.25 ± 0.08 | 6.34 ± 0.09 |
| Blood Biochemical Parameters | Control | Tested Proteins Doses (mg/kg b. wt./day) | |
|---|---|---|---|
| HEA | |||
| 500 | 2500 | ||
| Male | |||
| ALT (U/mL) | 44.82 ± 2.30 a | 37.38 ± 1.71 b | 34.27 ± 1.15 b |
| AST (U/mL) | 113.56 ± 4.05 | 103 ± 4.05 | 98.05 ± 5.84 |
| ALP (U/L) | 145.512 ± 5.8 | 140.05 ± 5.8 | 134.54 ± 5.81 |
| AChE (U/L) | 393.45 ± 5.8 | 376.02 ± 11.60 | 371.36 ± 5.80 |
| T.P. (g/dL) | 6.43 ± 0.58 | 6.18 ± 0.58 | 5.92 ± 0.17 |
| Alb. (g/dL) | 3.28 ± 0.12 | 3.13 ± 0.11 | 3.08 ± 0.91 |
| Urea (mg/dL) | 36.78 ± 3.50 | 32.08 ± 1.15 | 31.23 ± 1.15 |
| Creatinine (mg/dL) | 0.48 ± 0.045 | 0.47 ± 0.024 | 0.42 ± 0.052 |
| Female | |||
| ALT (U/mL) | 38.32 ± 2.91 | 38.02 ± 2.91 | 36.04 ±2.34 |
| AST (U/mL) | 97.19 ± 5.82 | 96.82 ± 3.51 | 93.39 ± 2.94 |
| ALP (U/L) | 114.26 ± 5.81 | 113.53 ± 4.11 | 109.25 ± 5.80 |
| AChE (U/L) | 412.65 ± 11.60 | 408.21 ± 5.80 | 405.03 ± 3.70 |
| T.P. (g/dL) | 6.38 ± 0.58 | 6.37 ± 0.09 | 6.33 ± 0.07 |
| Alb. (g/dL) | 3.31 ± 0.58 | 3.29 ± 0.29 | 3.25 ± 0.14 |
| Urea (mg/dL) | 40.32 ± 2.91 | 39.83 ± 3.51 | 36.83 ± 3.51 |
| Creatinine (mg/dL) | 0.47 ± 0.037 | 0.47 ± 0.04 | 0.45 ± 0.067 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Mohammadi, A.-R.; Osman, A.; Enan, G.; Abdel-Shafi, S.; El-Nemer, M.; Sitohy, M.; Taha, M.A. Powerful Antibacterial Peptides from Egg Albumin Hydrolysates. Antibiotics 2020, 9, 901. https://doi.org/10.3390/antibiotics9120901
Al-Mohammadi A-R, Osman A, Enan G, Abdel-Shafi S, El-Nemer M, Sitohy M, Taha MA. Powerful Antibacterial Peptides from Egg Albumin Hydrolysates. Antibiotics. 2020; 9(12):901. https://doi.org/10.3390/antibiotics9120901
Chicago/Turabian StyleAl-Mohammadi, Abdul-Raouf, Ali Osman, Gamal Enan, Seham Abdel-Shafi, Mona El-Nemer, Mahmoud Sitohy, and Mohamed A. Taha. 2020. "Powerful Antibacterial Peptides from Egg Albumin Hydrolysates" Antibiotics 9, no. 12: 901. https://doi.org/10.3390/antibiotics9120901
APA StyleAl-Mohammadi, A.-R., Osman, A., Enan, G., Abdel-Shafi, S., El-Nemer, M., Sitohy, M., & Taha, M. A. (2020). Powerful Antibacterial Peptides from Egg Albumin Hydrolysates. Antibiotics, 9(12), 901. https://doi.org/10.3390/antibiotics9120901







