Comparative Evaluation of the Antimicrobial and Mucus Induction Properties of Selected Bacillus Strains against Enterotoxigenic Escherichia coli
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
2.2. Cell Lines and Culture Conditions
2.3. Pathogen Inhibition (Agar Well Diffusion) Assay
2.4. Adhesion Assay
2.5. MUC2 and MUC3 Quantification
2.6. Statistical Analyses
3. Results and Discussion
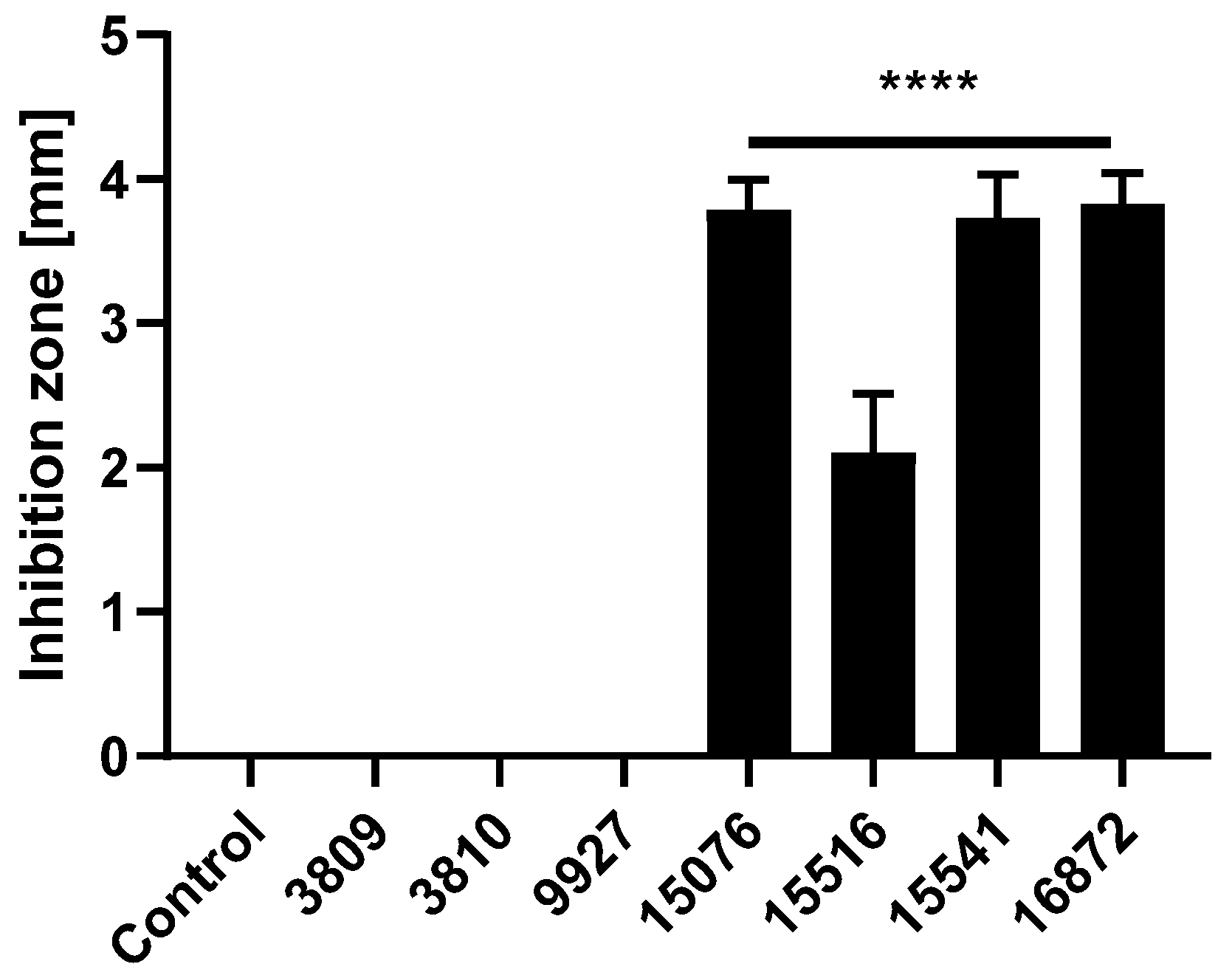
3.1. Bacillus Subtilis and Bacillus Amyloliquefaciens Strains Reduce the Growth of Pathogenic ETEC F4
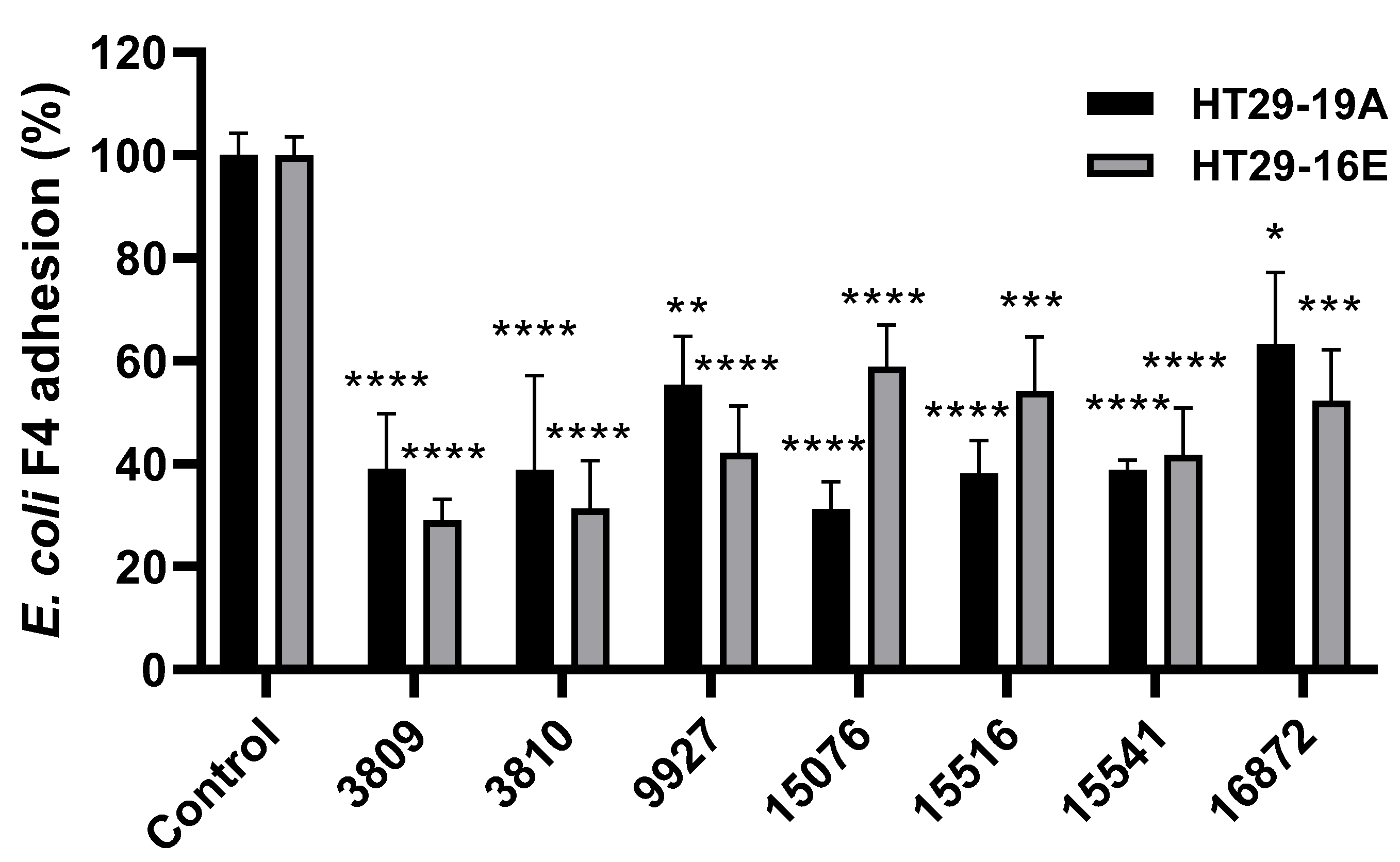
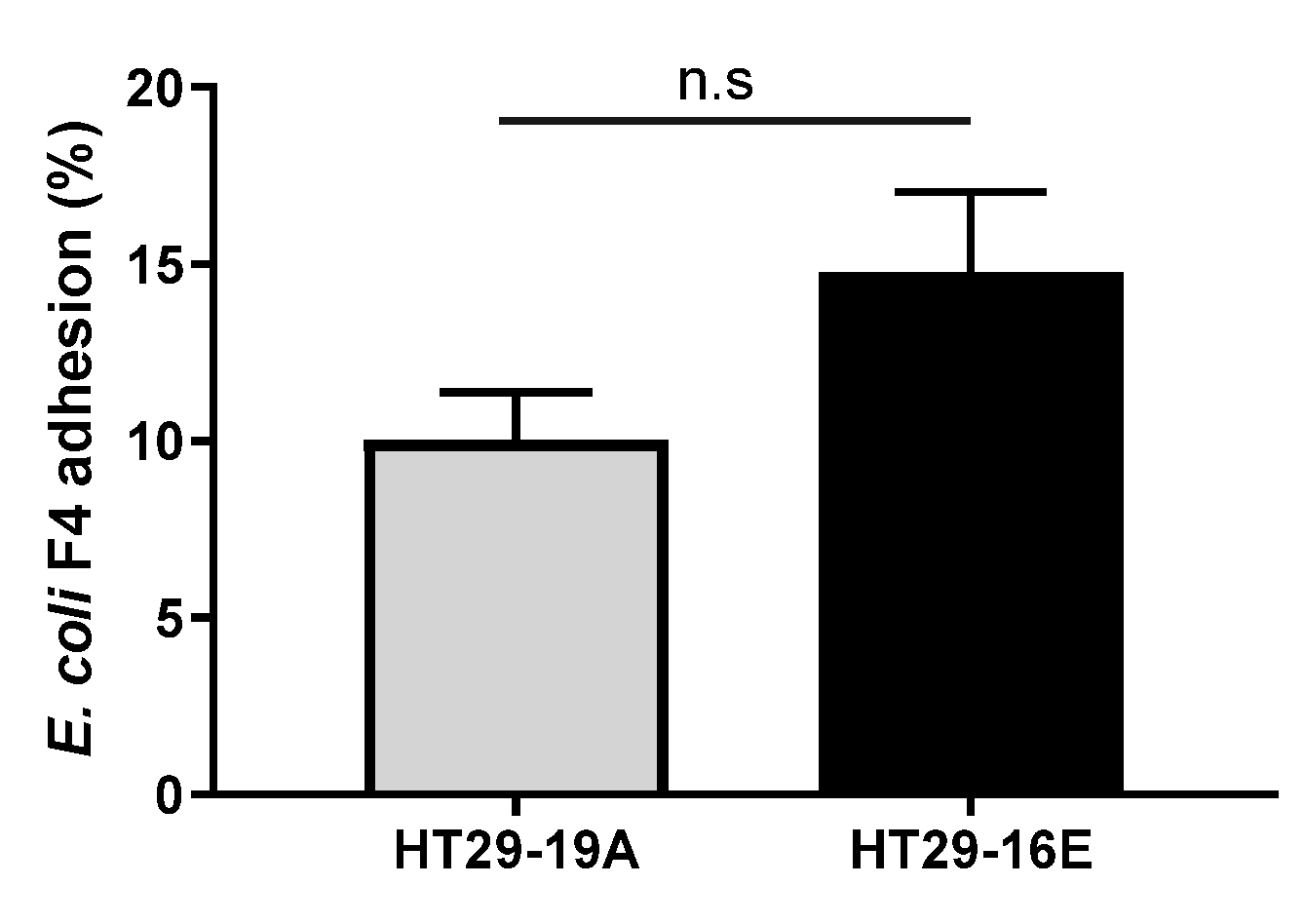
3.2. Bacillus spp. Strains Reduce Pathogenic ETEC F4 Binding to HT29 Cells
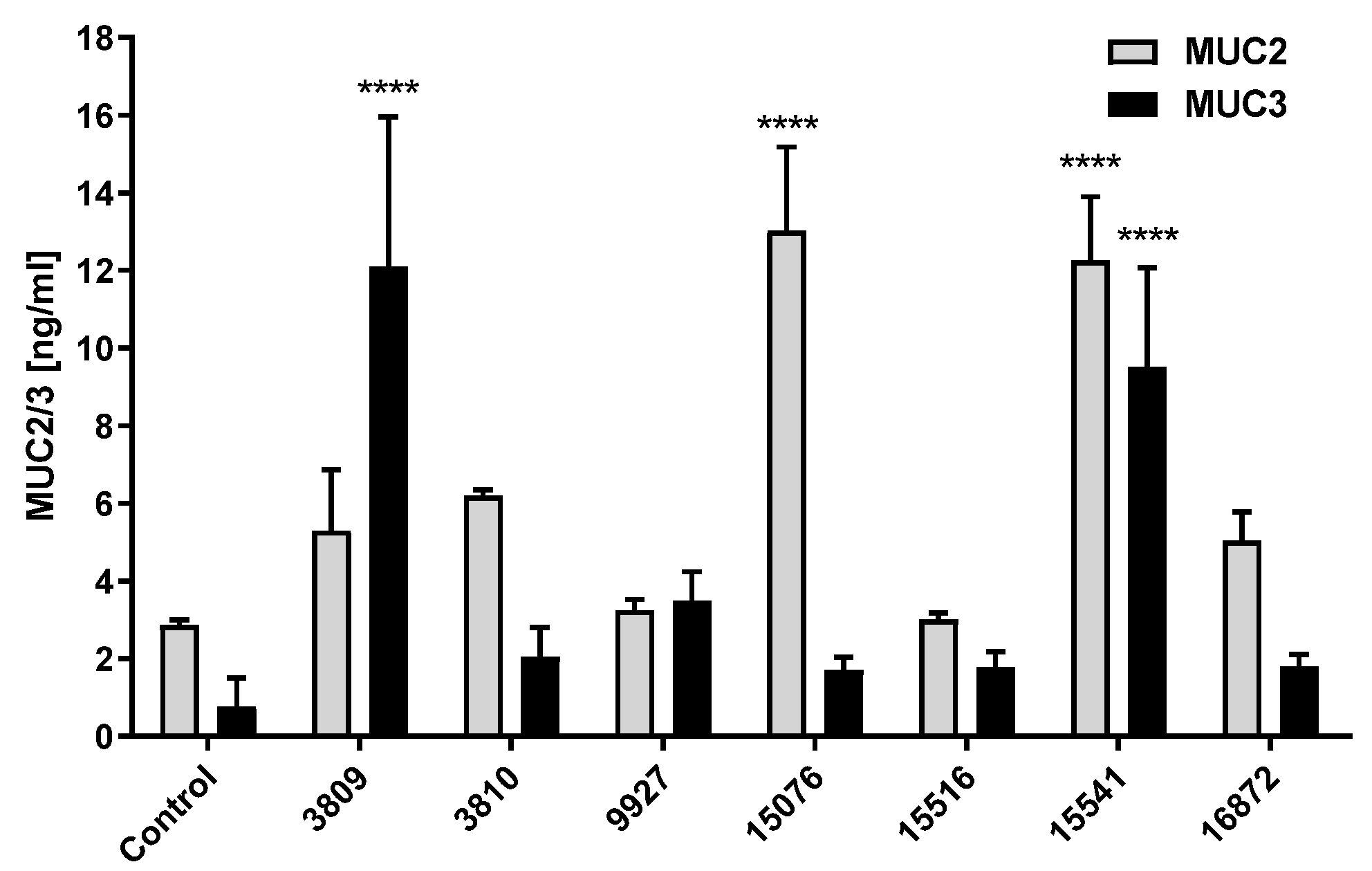
3.3. Bacillus Subtilis and Bacillus licheniformis Strains Increase Mucin Production in HT29-16E Cells at Long Periods of Incubation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sanders, M.E.; Guarner, F.; Guerrant, R.; Holt, P.R.; Quigley, E.M.M.; Sartor, R.B.; Sherman, P.M.; Mayer, E.A. An update on the use and investigation of probiotics in health and disease. Gut 2013, 62, 787–796. [Google Scholar] [CrossRef]
- Anadón, A.; Ares, I.; Martínez-Larrañaga, M.R.; Aranzazu-Martínez, M. Prebiotics and Probiotics in Feed and Animal Health. In Nutraceuticals in Veterinary Medicine; Springer International Publishing: Basel, Switzerland, 2019; pp. 261–285. ISBN 9783030046248. [Google Scholar]
- Sanz, Y.; Nadal, I.; Sánchez, E. Probiotics as Drugs Against Human Gastrointestinal Pathogens. Recent Pat. Antiinfect. Drug Discov. 2007, 2, 148–156. [Google Scholar] [CrossRef]
- Zhang, C.X.; Wang, H.Y.; Chen, T.X. Interactions between Intestinal Microflora/Probiotics and the Immune System. Biomed. Res. Int. 2019, 2019. [Google Scholar] [CrossRef]
- Monteagudo-Mera, A.; Rastall, R.A.; Gibson, G.R.; Charalampopoulos, D.; Chatzifragkou, A. Adhesion mechanisms mediated by probiotics and prebiotics and their potential impact on human health. Appl. Microbiol. Biotechnol. 2019, 103, 6463–6472. [Google Scholar] [CrossRef]
- Collado, M.C.; Meriluoto, J.; Salminen, S. Adhesion and aggregation properties of probiotic and pathogen strains. Eur. Food Res. Technol. 2008, 226, 1065–1073. [Google Scholar] [CrossRef]
- La Fata, G.; Weber, P.; Mohajeri, M.H. Probiotics and the Gut Immune System: Indirect Regulation. Probiotics Antimicrob. Proteins 2018, 10, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Mousavi Khaneghah, A.; Abhari, K.; Eş, I.; Soares, M.B.; Oliveira, R.B.A.; Hosseini, H.; Rezaei, M.; Balthazar, C.F.; Silva, R.; Cruz, A.G.; et al. Interactions between probiotics and pathogenic microorganisms in hosts and foods: A review. Trends Food Sci. Technol. 2020, 95, 205–218. [Google Scholar] [CrossRef]
- Celandroni, F.; Vecchione, A.; Cara, A.; Mazzantini, D.; Lupetti, A.; Ghelardi, E. Identification of Bacillus species: Implication on the quality of probiotic formulations. PLoS ONE 2019, 14, e0217021. [Google Scholar] [CrossRef] [PubMed]
- Elshaghabee, F.M.F.; Rokana, N.; Gulhane, R.D.; Sharma, C.; Panwar, H. Bacillus as potential probiotics: Status, concerns, and future perspectives. Front. Microbiol. 2017, 8, 1490. [Google Scholar] [CrossRef] [PubMed]
- Wehkamp, J.; Harder, J.; Wehkamp, K.; Meissner, B.W.; Schlee, M.; Enders, C.; Sonnenborn, U.; Nuding, S.; Bengmark, S.; Fellermann, K.; et al. NF-kB- and AP-1-Mediated Induction of Human Beta Defensin-2 in Intestinal Epithelial Cells by Escherichia coli Nissle 1917: A novel effect of a probiotic bacterium. Infect. Immun. 2004, 72, 5750–5758. [Google Scholar] [CrossRef]
- Preidis, G.A.; Hill, C.; Guerrant, R.L.; Ramakrishna, B.S.; Tannock, G.W.; Versalovic, J. Probiotics, Enteric and Diarrheal diseases, and global health. Gastroenterology 2011, 140, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.Y.; Ning, M.X.; Chen, D.K.; Ma, W.T. Interactions between the gut microbiota and the host innate immune response against pathogens. Front. Immunol. 2019, 10, 607. [Google Scholar] [CrossRef] [PubMed]
- Adibpour, N.; Hosseininezhad, M.; Pahlevanlo, A.; Hussain, M.A. A review on Bacillus coagulans as a Spore-Forming Probiotic. Appl. Food Biotechnol. 2019, 6, 91–100. [Google Scholar] [CrossRef]
- Lefevre, M.; Racedo, S.M.; Ripert, G.; Housez, B.; Cazaubiel, M.; Maudet, C.; Jüsten, P.; Marteau, P.; Urdaci, M.C. Probiotic strain Bacillus subtilis CU1 stimulates immune system of elderly during common infectious disease period: A randomized, double-blind placebo-controlled study. Immun. Ageing 2015, 12, 24. [Google Scholar] [CrossRef]
- Shobharani, P.; Padmaja, R.J.; Halami, P.M. Diversity in the antibacterial potential of probiotic cultures Bacillus licheniformis MCC2514 and Bacillus licheniformis MCC2512. Res. Microbiol. 2015, 166, 546–554. [Google Scholar] [CrossRef]
- Ripert, G.; Racedo, S.M.; Elie, A.M.; Jacquot, C.; Bressollier, P.; Urdaci, M.C. Secreted compounds of the probiotic Bacillus clausii strain O/C inhibit the cytotoxic effects induced by Clostridium difficile and Bacillus cereus toxins. Antimicrob. Agents Chemother. 2016, 60, 3445–3454. [Google Scholar] [CrossRef]
- Ghani, M.; Ansari, A.; Aman, A.; Zohra, R.R.; Siddiqui, N.N.; Qader, S.A.U. Isolation and characterization of different strains of Bacillus licheniformis for the production of commercially significant enzymes. Pak. J. Pharm. Sci. 2013, 26, 691–697. [Google Scholar]
- Larsen, N.; Thorsen, L.; Kpikpi, E.N.; Stuer-Lauridsen, B.; Cantor, M.D.; Nielsen, B.; Brockmann, E.; Derkx, P.M.F.; Jespersen, L. Characterization of Bacillus spp. strains for use as probiotic additives in pig feed. Appl. Microbiol. Biotechnol. 2014, 98, 1105–1118. [Google Scholar] [CrossRef]
- Jeżewska-Frąckowiak, J.; Seroczyńska, K.; Banaszczyk, J.; Jedrzejczak, G.; Żylicz-Stachula, A.; Skowron, P.M. The promises and risks of probiotic Bacillus species. Acta Biochim. Pol. 2018, 65, 509–519. [Google Scholar] [CrossRef]
- Luise, D.; Lauridsen, C.; Bosi, P.; Trevisi, P. Methodology and application of Escherichia coli F4 and F18 encoding infection models in post-weaning pigs. J. Anim. Sci. Biotechnol. 2019, 10, 53. [Google Scholar] [CrossRef]
- Jarry, A.; Merlin, D.; Hopfer, U.; Laboisse, C.L. Cyclic AMP-induced mucin exocytosis is independent of Cl- movements in human colonic epithelial cells (HT29-Cl.16E). Biochem. J. 1994, 304, 675–678. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.M. Basic Cell Culture: A Practical Approach, 2nd ed.; University Press: Oxford, UK, 2002. [Google Scholar]
- Humphries, R.M.; Ambler, J.; Mitchell, S.L.; Castanheira, M.; Dingle, T.; Hindler, J.A.; Koeth, L.; Sei, K. CLSI Methods Development and Standardization Working Group Best Practices for Evaluation of Antimicrobial Susceptibility Tests. J. Clin. Microbiol. 2018, 56, e01934-17. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Liu, X.; Dai, R.; Xiao, Y.; Wang, X.; Bi, D.; Shi, D. Enterococcus faecium HDRsEf1 Protects the Intestinal Epithelium and Attenuates ETEC-Induced IL-8 Secretion in Enterocytes. Mediators Inflamm. 2016, 2016, 7474306. [Google Scholar] [CrossRef] [PubMed]
- Lamacchia, C.; Musaico, D.; Henderson, M.E.; Bergillos-Meca, T.; Roul, M.; Landriscina, L.; Decina, I.; Corona, G.; Costabile, A. Temperature-treated gluten proteins in Gluten-FriendlyTM bread increase mucus production and gut-barrier function in human intestinal goblet cells. J. Funct. Foods 2018, 48, 507–514. [Google Scholar] [CrossRef]
- Dorsey, F.C.; Fischer, J.F.; Fleckenstein, J.M. Directed delivery of heat-labile enterotoxin by enterotoxigenic Escherichia coli. Cell. Microbiol. 2006, 8, 1516–1527. [Google Scholar] [CrossRef]
- Bergstrom, K.S.B.; Kissoon-Singh, V.; Gibson, D.L.; Ma, C.; Montero, M.; Sham, H.P.; Ryz, N.; Huang, T.; Velcich, A.; Finlay, B.B.; et al. Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS Pathog. 2010, 6, e1000902. [Google Scholar] [CrossRef]
- McGuckin, M.A.; Lindén, S.K.; Sutton, P.; Florin, T.H. Mucin dynamics and enteric pathogens. Nat. Rev. Microbiol. 2011, 9, 265–278. [Google Scholar] [CrossRef]
- Luo, Q.; Kumar, P.; Vickers, T.J.; Sheikh, A.; Lewis, W.G.; Rasko, D.A.; Sistrunk, J.; Fleckenstein, J.M. Enterotoxigenic Escherichia coli secretes a highly conserved mucin-degrading metalloprotease to effectively engage intestinal epithelial cells. Infect. Immun. 2014, 82, 509–521. [Google Scholar] [CrossRef]
- Bademler, S.; Zirtiloglu, A.; Sari, M.; Ucuncu, M.Z.; Dogru, E.B.; Karabulut, S. Clinical significance of serum membrane-bound mucin-2 levels in breast cancer. Biomolecules 2019, 9, 40. [Google Scholar] [CrossRef]
- Caulier, S.; Nannan, C.; Gillis, A.; Licciardi, F.; Bragard, C.; Mahillon, J. Overview of the Antimicrobial Compounds Produced by Members of the Bacillus subtilis Group. Front. Microbiol. 2019, 10, 302. [Google Scholar] [CrossRef]
- Hossain, M.I.; Sadekuzzaman, M.; Ha, S.-D. Probiotics as potential alternative biocontrol agents in the agriculture and food industries: A review. Food Res. Int. 2017, 100, 63–73. [Google Scholar] [CrossRef]
- Nyangale, E.P.; Farmer, S.; Keller, D.; Chernoff, D.; Gibson, G.R. Effect of prebiotics on the fecal microbiota of elderly volunteers after dietary supplementation of Bacillus coagulans GBI-30, 6086. Anaerobe 2014, 30, 75–81. [Google Scholar] [CrossRef]
- Khan, S.; Chousalkar, K.K. Salmonella Typhimurium infection disrupts but continuous feeding of Bacillus based probiotic restores gut microbiota in infected hens. J. Anim. Sci. Biotechnol. 2020, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J. How covid-19 is accelerating the threat of antimicrobial resistance. BMJ 2020, 369, 18–19. [Google Scholar] [CrossRef] [PubMed]
- Babu Rajendran, N.; Mutters, N.T.; Marasca, G.; Conti, M.; Sifakis, F.; Vuong, C.; Voss, A.; Baño, J.R.; Tacconelli, E. Mandatory surveillance and outbreaks reporting of the WHO priority pathogens for research & discovery of new antibiotics in European countries. Clin. Microbiol. Infect. 2020, 26, 943.e1–943.e6. [Google Scholar] [CrossRef]
- Sayah, R.S.; Kaneene, J.B.; Johnson, Y.; Miller, R. Patterns of antimicrobial resistance observed in Escherichia coli isolates obtained from domestic- and wild-animal fecal samples, human septage, and surface water. Appl. Environ. Microbiol. 2005, 71, 1394–1404. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, K.; Fatemeh, F.; Mehri, N.; Maryam, S. Probiotics for the treatment of pediatric Helicobacter pylori infection: A randomized double blind clinical trial. Iran. J. Pediatr. 2013, 23, 79–84. [Google Scholar]
- Lopetuso, L.R.; Scaldaferri, F.; Franceschi, F.; Gasbarrini, A. Bacillus clausii and gut homeostasis: State of the art and future perspectives. Expert Rev. Gastroenterol. Hepatol. 2016, 10, 943–948. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bravo Santano, N.; Juncker Boll, E.; Catrine Capern, L.; Cieplak, T.M.; Keleszade, E.; Letek, M.; Costabile, A. Comparative Evaluation of the Antimicrobial and Mucus Induction Properties of Selected Bacillus Strains against Enterotoxigenic Escherichia coli. Antibiotics 2020, 9, 849. https://doi.org/10.3390/antibiotics9120849
Bravo Santano N, Juncker Boll E, Catrine Capern L, Cieplak TM, Keleszade E, Letek M, Costabile A. Comparative Evaluation of the Antimicrobial and Mucus Induction Properties of Selected Bacillus Strains against Enterotoxigenic Escherichia coli. Antibiotics. 2020; 9(12):849. https://doi.org/10.3390/antibiotics9120849
Chicago/Turabian StyleBravo Santano, Natalia, Erik Juncker Boll, Lena Catrine Capern, Tomasz Maciej Cieplak, Enver Keleszade, Michal Letek, and Adele Costabile. 2020. "Comparative Evaluation of the Antimicrobial and Mucus Induction Properties of Selected Bacillus Strains against Enterotoxigenic Escherichia coli" Antibiotics 9, no. 12: 849. https://doi.org/10.3390/antibiotics9120849
APA StyleBravo Santano, N., Juncker Boll, E., Catrine Capern, L., Cieplak, T. M., Keleszade, E., Letek, M., & Costabile, A. (2020). Comparative Evaluation of the Antimicrobial and Mucus Induction Properties of Selected Bacillus Strains against Enterotoxigenic Escherichia coli. Antibiotics, 9(12), 849. https://doi.org/10.3390/antibiotics9120849





