The Safety and Efficacy of Phage Therapy for Bone and Joint Infections: A Systematic Review
Abstract
1. Introduction
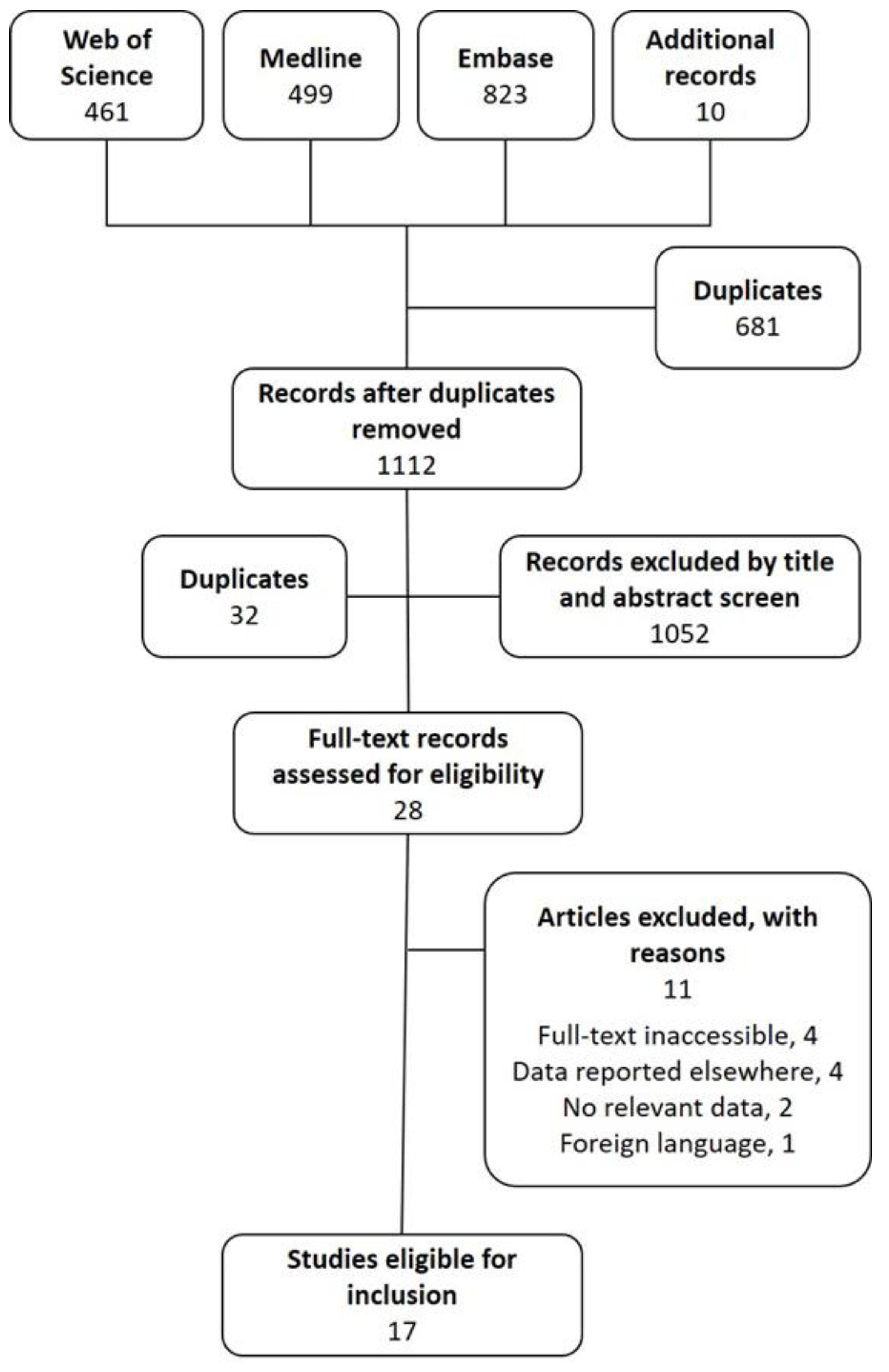
2. Methods
2.1. Search Strategy
2.2. Study Selection Criteria
2.3. Data Extraction and Critical Appraisal
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Availability of Data and Materials
Abbreviations
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase |
| CRP | C-reactive protein |
| DAIR | debridement, antibiotics and implant retention |
| IV | intravenous |
| MRSA | methicillin-resistant Staphylococcus aureus |
| PFU | plaque forming unit |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| SC | subcutaneous |
| SPL | Staphylococcus phage lysate |
| WBC | white blood cell |
References
- Fleming, A. Penicillin. 1945. Available online: https://www.nobelprize.org/uploads/2018/06/fleming-lecture.pdf (accessed on 9 November 2020).
- Wittebole, X.; De Roock, S.; Opal, S.M. A historical overview of bacteriophage therapy as an alternative to antibiotics for the treatment of bacterial pathogens. Virulence 2014, 5, 226–235. [Google Scholar] [CrossRef]
- Almeida, G.M.D.F.; Sundberg, L.-R. The forgotten tale of Brazilian phage therapy. Lancet Infect. Dis. 2020, 20, e90–e101. [Google Scholar] [CrossRef]
- Abedon, S.T. Bacteriophage Clinical Use as Antibacterial “Drugs”: Utility and Precedent. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef]
- Myelnikov, D. An Alternative Cure: The Adoption and Survival of Bacteriophage Therapy in the USSR, 1922–1955. J. Hist. Med. Allied Sci. 2018, 73, 385–411. [Google Scholar] [CrossRef] [PubMed]
- Summers, W.C. The strange history of phage therapy. Bacteriophage 2012, 2, 130–133. [Google Scholar] [CrossRef]
- Żaczek, M.; Weber-Dąbrowska, B.; Międzybrodzki, R.; Łusiak-Szelachowska, M.; Górski, A. Phage Therapy in Poland—A Centennial Journey to the First Ethically Approved Treatment Facility in Europe. Front. Microbiol. 2020, 11, 1056. [Google Scholar] [CrossRef]
- Kutateladze, M.; Adamia, R. Phage therapy experience at the Eliava Institute. Méd. Mal. Infect. 2008, 38, 426–430. [Google Scholar] [CrossRef]
- Hall, C.W.; Mah, T.-F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef]
- Davies, D. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2003, 2, 114–122. [Google Scholar] [CrossRef]
- Ciofu, O.; Rojo-Molinero, E.; Macià, M.D.; Oliver, A. Antibiotic treatment of biofilm infections. APMIS 2017, 125, 304–319. [Google Scholar] [CrossRef]
- Pozo, J.L.D. Biofilm-related disease. Expert Rev. Anti Infect. Ther. 2018, 16, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Zimmerli, W.; Sendi, P. Orthopaedic biofilm infections. APMIS 2017, 125, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Zimmerli, W.; Trampuz, A.; Ochsner, P.E. Prosthetic-Joint Infections. N. Engl. J. Med. 2004, 351, 1645–1654. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Beswick, A.D.; Whitehouse, M.R.; Wylde, V.; Blom, A.W. Debridement, antibiotics and implant retention for periprosthetic joint infections: A systematic review and meta-analysis of treatment outcomes. J. Infect. 2018, 77, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Kallala, R.F.; Vanhegan, I.S.; Ibrahim, M.S.; Sarmah, S.; Haddad, F.S. Financial analysis of revision knee surgery based on NHS tariffs and hospital costs. Bone Jt. J. 2015, 97-B, 197–201. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Lau, E.; Watson, H.; Schmier, J.K.; Parvizi, J. Economic Burden of Periprosthetic Joint Infection in the United States. J. Arthroplast. 2012, 27, 61–65. [Google Scholar] [CrossRef]
- Slopek, S.; Kucharewicz-Krukowska, A.; Weber-Dabrowska, B.; Dabrowski, M. Results of bacteriophage treatment of suppurative bacterial infections. V. Evaluation of the results obtained in children. Arch. Immunol. Ther. Exp. (Warsz.) 1985, 33, 241–259. [Google Scholar]
- Slopek, S.; Weber-Dabrowska, B.; Dabrowski, M.; Kucharewicz-Krukowska, A. Results of bacteriophage treatment of supparative bacterial infections in the years 1981–1986. Arch. Immunol. Ther. Exp. (Warsz.) 1987, 35, 569–583. [Google Scholar]
- Baker, A. Staphylococcus bacteriophage lysate topical and parenteral use in allergic patients. Pa. Med. J. 1963, 66, 25–28. [Google Scholar]
- Weber-Dabrowska, B.; Mulczyk, M.; Górski, A. Bacteriophage therapy of bacterial infections: An update of our institute’s experience. Arch. Immunol. Ther. Exp. (Warsz.) 2000, 48, 547–551. [Google Scholar]
- Slopek, S.; Kucharewicz-Krukowska, A.; Weber-Dabrowska, B.; Dabrowski, M. Results of bacteriophage treatment of suppurative bacterial infections. VI. Analysis of treatment of suppurative staphylococcal infections. Arch. Immunol. Ther. Exp. (Warsz.) 1985, 33, 261–273. [Google Scholar] [PubMed]
- Southwest Regional Wound Care Centre Expanded Phage Case Histories. Available online: https://pdfs.semanticscholar.org/b7f2/9b704ba13aafa35ff0809d67eac89807f456.pdf (accessed on 25 February 2020).
- Bernstein, V.S.; Sokobenzon, E.E.; Yakhnina, N.A. On phage therapy of the festering processes. Surg. Khirurgia 1940, 56–58. [Google Scholar]
- Matusis, E.E.; Shumilkina, E.I.; Boiarinova, L.V. Use of therapeutic bacteriophages in complex treatment of faked joints of shin bones aggravated with osteomyelitis. In Selected Articles of the Jubilee Symposium Dedicated to the 50th Anniversary of the Tbilisi Institute of Vaccine and Sera; TIVS: Tbilisi, Georgia, 1974; pp. 390–392. [Google Scholar]
- Ferry, T.; Batailler, C.; Petitjean, C.; Chateau, J.; Fevre, C.; Forestier, E.; Brosset, S.; Leboucher, G.; Kolenda, C.; Laurent, F. The Potential Innovative Use of Bacteriophages Within the DAC® Hydrogel to Treat Patients With Knee Megaprosthesis Infection Requiring “Debridement Antibiotics and Implant Retention” and Soft Tissue Coverage as Salvage Therapy. Front. Med. 2020, 7, 342. [Google Scholar] [CrossRef] [PubMed]
- Cano, E.J.; Caflisch, K.M.; Bollyky, P.L.; Van Belleghem, J.D.; Patel, R.; Fackler, J.; Brownstein, M.J.; Horne, B.; Biswas, B.; Henry, M. Phage Therapy for Limb-threatening Prosthetic Knee Klebsiella pneumoniae Infection: Case Report and In Vitro Characterization of Anti-biofilm Activity. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of Observational Studies in Epidemiology: A Proposal for Reporting. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef]
- The Joanna Briggs Institute Critical Appraisal Tools—JBI. Available online: http://joannabriggs.org/research/critical-appraisal-tools.html (accessed on 31 March 2020).
- GraphPad Analyze A 2 × 2 Contingency Table. Available online: https://www.graphpad.com/quickcalcs/contingency1.cfm (accessed on 23 September 2020).
- Chanishvili, N. A Literature Review of the Practical Applications of Bacteriophage Research; Eliava Institute of Bacteriophage, Microbiology & Virology: Tbilisi, Georgia, 2009. [Google Scholar]
- Doub, J.B.; Ng, V.Y.; Johnson, A.J.; Slomka, M.; Fackler, J.; Horne, B.; Brownstein, M.J.; Henry, M.; Malagon, F.; Biswas, B. Salvage Bacteriophage Therapy for a Chronic MRSA Prosthetic Joint Infection. Antibiotics 2020, 9, 241. [Google Scholar] [CrossRef]
- Fish, R.; Kutter, E.; Wheat, G.; Blasdel, B.; Kutateladze, M.; Kuhl, S. Bacteriophage treatment of intransigent Diabetic toe ulcers: A case series. J. Wound Care 2016, 25, S27–S33. [Google Scholar] [CrossRef]
- Fish, R.; Kutter, E.; Bryan, D.; Wheat, G.; Kuhl, S. Resolving Digital Staphylococcal Osteomyelitis Using Bacteriophage—A Case Report. Antibiotics 2018, 7, 87. [Google Scholar] [CrossRef]
- Fish, R.; Kutter, E.; Wheat, G.; Blasdel, B.; Kutateladze, M.; Kuhl, S. Compassionate use of bacteriophage therapy for foot ulcer treatment as an effective step for moving toward clinical trials. In Bacteriophage Therapy: From Lab to Clinical Practice; Azeredo, J., Sillankorva, S., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2018; ISBN 978-1-4939-7394-1. [Google Scholar]
- Nir-Paz, R.; Gelman, D.; Khouri, A.; Sisson, B.M.; Fackler, J.; Alkalay-Oren, S.; Khalifa, L.; Rimon, A.; Yerushalmy, O.; Bader, R. Successful Treatment of Antibiotic-resistant, Poly-microbial Bone Infection With Bacteriophages and Antibiotics Combination. Clin. Infect. Dis. 2019, 69, 2015–2018. [Google Scholar] [CrossRef]
- Onsea, J.; Soentjens, P.; Djebara, S.; Merabishvili, M.; Depypere, M.; Spriet, I.; De Munter, P.; Debaveye, Y.; Nijs, S.; Vanderschot, P. Bacteriophage Application for Difficult-to-treat Musculoskeletal Infections: Development of a Standardized Multidisciplinary Treatment Protocol. Viruses 2019, 11, 891. [Google Scholar] [CrossRef]
- Tkhilaishvili, T.; Winkler, T.; Müller, M.; Perka, C.; Trampuz, A. Bacteriophages as Adjuvant to Antibiotics for the Treatment of Periprosthetic Joint Infection Caused by Multidrug-Resistant Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2019, 64, e00924-19. [Google Scholar] [CrossRef]
- Ferry, T.; Boucher, F.; Fevre, C.; Perpoint, T.; Chateau, J.; Petitjean, C.; Josse, J.; Chidiac, C.; L’hostis, G.; Leboucher, G. Innovations for the treatment of a complex bone and joint infection due to XDR Pseudomonas aeruginosa including local application of a selected cocktail of bacteriophages. J. Antimicrob. Chemother. 2018, 73, 2901–2903. [Google Scholar] [CrossRef] [PubMed]
- Ferry, T.; Leboucher, G.; Fevre, C.; Herry, Y.; Conrad, A.; Josse, J.; Batailler, C.; Chidiac, C.; Medina, M.; Lustig, S. Salvage Debridement, Antibiotics and Implant Retention (“DAIR”) With Local Injection of a Selected Cocktail of Bacteriophages: Is It an Option for an Elderly Patient With Relapsing Staphylococcus aureus Prosthetic-Joint Infection? Open Forum Infect. Dis. 2018, 5, ofy269. [Google Scholar] [CrossRef]
- Albee, F.H. The treatment of osteomyelitis by bacteriophage. JBJS 1933, 15, 58–66. [Google Scholar]
- Slopek, S.; Durlakowa, I.; Weber-Dabrowska, B.; Kucharewicz-Krukowska, A.; Dabrowski, M.; Bisikiewicz, R. Results of bacteriophage treatment of suppurative bacterial infections. I. General evaluation of the results. Arch. Immunol. Ther. Exp. (Warsz.) 1983, 31, 267–291. [Google Scholar] [PubMed]
- Efremov, I.M.; Sibaev, F.Y.; Shevalaev, G.A. Two-stage reosteosynthesis of tibia in the patient with fracture non-union complicated by postoperative osteomyelitis. Traumatol. Orthop. Russ. 2018, 24, 108–114. [Google Scholar] [CrossRef]
- Green, S.I.; Kaelber, J.T.; Ma, L.; Trautner, B.W.; Ramig, R.F.; Maresso, A.W. Bacteriophages from ExPEC Reservoirs Kill Pandemic Multidrug-Resistant Strains of Clonal Group ST131 in Animal Models of Bacteremia. Sci. Rep. 2017, 7, 46151. [Google Scholar] [CrossRef] [PubMed]
- Henry, M.; Lavigne, R.; Debarbieux, L. Predicting In Vivo Efficacy of Therapeutic Bacteriophages Used To Treat Pulmonary Infections. Antimicrob. Agents Chemother. 2013, 57, 5961–5968. [Google Scholar] [CrossRef]
- Beridze, M.A. Role of Bacteriophage Therapy in Combating Purulent Skin Infections; Medgiz: Tbilisi, Georgia, 1938. [Google Scholar]
- Chan, B.K.; Abedon, S.T.; Loc-Carrillo, C. Phage cocktails and the future of phage therapy. Future Microbiol. 2013, 8, 769–783. [Google Scholar] [CrossRef]
- Segall, A.M.; Roach, D.R.; Strathdee, S.A. Stronger together? Perspectives on phage-antibiotic synergy in clinical applications of phage therapy. Curr. Opin. Microbiol. 2019, 51, 46–50. [Google Scholar] [CrossRef]
- Comeau, A.M.; Hatfull, G.F.; Krisch, H.M.; Lindell, D.; Mann, N.H.; Prangishvili, D. Exploring the prokaryotic virosphere. Res. Microbiol. 2008, 159, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Tagliaferri, T.L.; Jansen, M.; Horz, H.-P. Fighting Pathogenic Bacteria on Two Fronts: Phages and Antibiotics as Combined Strategy. Front. Cell. Infect. Microbiol. 2019, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Pires, D.; Melo, L.; Vilas Boas, D.; Sillankorva, S.; Azeredo, J. Phage therapy as an alternative or complementary strategy to prevent and control biofilm-related infections. Curr. Opin. Microbiol. 2017, 39, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Ferriol-González, C.; Domingo-Calap, P. Phages for Biofilm Removal. Antibiotics 2020, 9, 268. [Google Scholar] [CrossRef]
- Akturk, E.; Oliveira, H.; Santos, S.B.; Costa, S.; Kuyumcu, S.; Melo, L.D.R.; Azeredo, J. Synergistic Action of Phage and Antibiotics: Parameters to Enhance the Killing Efficacy Against Mono and Dual-Species Biofilms. Antibiotics 2019, 8, 103. [Google Scholar] [CrossRef]
- Tkhilaishvili, T.; Lombardi, L.; Klatt, A.-B.; Trampuz, A.; Di Luca, M. Bacteriophage Sb-1 enhances antibiotic activity against biofilm, degrades exopolysaccharide matrix and targets persisters of Staphylococcus aureus. Int. J. Antimicrob. Agents 2018, 52, 842–853. [Google Scholar] [CrossRef]
- Seth, A.K.; Geringer, M.R.; Nguyen, K.T.; Agnew, S.P.; Dumanian, Z.; Galiano, R.D.; Leung, K.P.; Mustoe, T.A.; Hong, S.J. Bacteriophage therapy for Staphylococcus aureus biofilm-infected wounds: A new approach to chronic wound care. Plast. Reconstr. Surg. 2013, 131, 225–234. [Google Scholar] [CrossRef]
- Fabijan, A.P.; Lin, R.C.Y.; Ho, J.; Maddocks, S.; Ben Zakour, N.L.; Iredell, J.R. Safety of bacteriophage therapy in severe Staphylococcus aureus infection. Nat. Microbiol. 2020, 5, 465–472. [Google Scholar] [CrossRef]
- Schooley, R.T.; Biswas, B.; Gill, J.J.; Hernandez-Morales, A.; Lancaster, J.; Lessor, L.; Barr, J.J.; Reed, S.L.; Rohwer, F.; Benler, S. Development and Use of Personalized Bacteriophage-Based Therapeutic Cocktails To Treat a Patient with a Disseminated Resistant Acinetobacter baumannii Infection. Antimicrob. Agents Chemother. 2017, 61, e00954-17. [Google Scholar] [CrossRef]
- Suffredini, A.F.; Noveck, R.J. Human Endotoxin Administration as an Experimental Model in Drug Development. Clin. Pharmacol. Ther. 2014, 96, 418–422. [Google Scholar] [CrossRef]
- Pirnay, J.-P.; Blasdel, B.G.; Bretaudeau, L.; Buckling, A.; Chanishvili, N.; Clark, J.R.; Corte-Real, S.; Debarbieux, L.; Dublanchet, A.; De Vos, D. Quality and Safety Requirements for Sustainable Phage Therapy Products. Pharm. Res. 2015, 32, 2173–2179. [Google Scholar] [CrossRef]
- Gibson, S.B.; Green, S.I.; Liu, C.G.; Salazar, K.C.; Clark, J.R.; Terwilliger, A.L.; Kaplan, H.B.; Maresso, A.W.; Trautner, B.W.; Ramig, R.F. Constructing and Characterizing Bacteriophage Libraries for Phage Therapy of Human Infections. Front. Microbiol. 2019, 10, 2537. [Google Scholar] [CrossRef] [PubMed]
- Philipson, C.; Voegtly, L.; Lueder, M.; Long, K.; Rice, G.; Frey, K.; Biswas, B.; Cer, R.; Hamilton, T.; Bishop-Lilly, K. Characterizing Phage Genomes for Therapeutic Applications. Viruses 2018, 10, 188. [Google Scholar] [CrossRef] [PubMed]
- Shusharin, A.G.; Polovinka, M.P. Effective treatment of opportunistic infections in patients with rheumatoid arthritis and gout using bacteriophages. Osteoporos. Int. 2014, 25, S403. [Google Scholar]
- Negut, A.C.; Sandulescu, O.; Streinu-Cercel, A.; Magdalena Motoi, M.; Loan Popa, M.; Streinu-Cercel, A. A novel apporach for managing hard-to-treat infections. BMC Infect. Dis. 2016, 16, A19. [Google Scholar]
- Dublanchet, A.; Patey, O. Phage therapy for bone and joint infections: Report of french cases. Orthop. Proc. 2017, 99-B, 35. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clarke, A.L.; De Soir, S.; Jones, J.D. The Safety and Efficacy of Phage Therapy for Bone and Joint Infections: A Systematic Review. Antibiotics 2020, 9, 795. https://doi.org/10.3390/antibiotics9110795
Clarke AL, De Soir S, Jones JD. The Safety and Efficacy of Phage Therapy for Bone and Joint Infections: A Systematic Review. Antibiotics. 2020; 9(11):795. https://doi.org/10.3390/antibiotics9110795
Chicago/Turabian StyleClarke, Alex L., Steven De Soir, and Joshua D. Jones. 2020. "The Safety and Efficacy of Phage Therapy for Bone and Joint Infections: A Systematic Review" Antibiotics 9, no. 11: 795. https://doi.org/10.3390/antibiotics9110795
APA StyleClarke, A. L., De Soir, S., & Jones, J. D. (2020). The Safety and Efficacy of Phage Therapy for Bone and Joint Infections: A Systematic Review. Antibiotics, 9(11), 795. https://doi.org/10.3390/antibiotics9110795



