Identification of New Antimicrobial Peptides from Mediterranean Medical Plant Charybdis pancration (Steinh.) Speta
Abstract
1. Introduction
2. Results
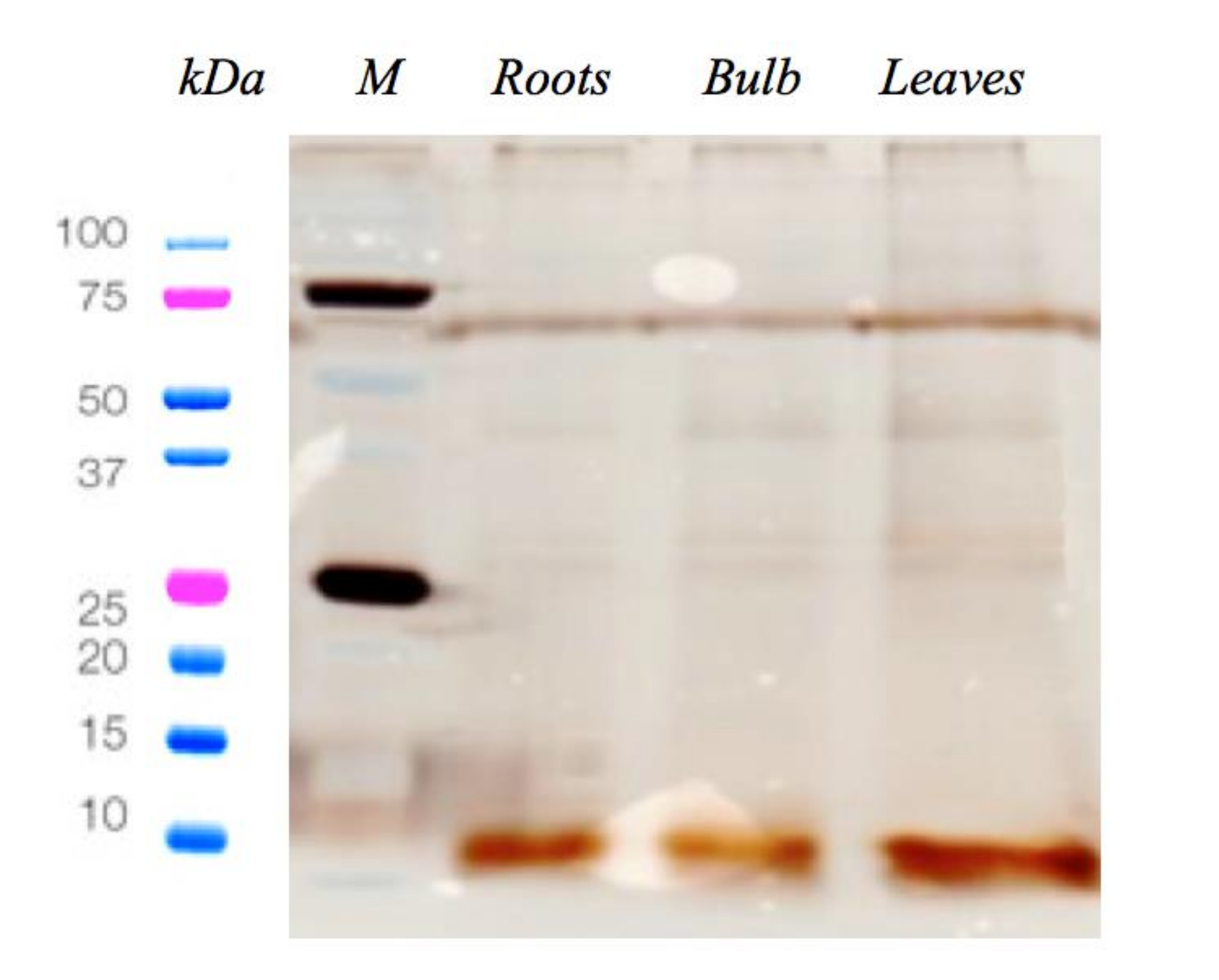
2.1. C. pancration Peptide Extraction and Antimicrobial Activity
2.2. MS Characterization of the Amino Acid Sequence of the Peptides Present in the Bulb Extract
2.3. Molecular Dynamics Simulations
3. Discussion
4. Materials and Methods
4.1. Plant Collection and Plant Extract Preparation
4.2. Protein Content Determination
4.3. Bacterial Strains
4.4. Determination of Minimal Inhibitory Concentrations (MICs)
4.5. Mass Spectrometry Analysis
4.6. Database Search
4.7. AMP Prediction and in Silico Analysis
4.8. Molecular Dynamics Simulations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Calistri, P.; Iannetti, S.; Danzetta, M.L.; Narcisi, V.; Cito, F.; Di Sabatino, D.; Bruno, R.; Sauro, F.; Atzeni, M.; Carvelli, A.; et al. The Components of ‘One World – One Health’ Approach. Transbound. Emerg. Dis. 2013, 60, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Schillaci, D.; Spanò, V.; Parrino, B.; Carbone, A.; Montalbano, A.; Barraja, P.; Diana, P.; Cirrincione, G.; Cascioferro, S. Pharmaceutical Approaches to Target Antibiotic Resistance Mechanisms. J. Med. Chem. 2017, 60, 8268–8297. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.W.; Diamond, G. The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol. 2000, 8, 402–410. [Google Scholar] [CrossRef]
- Inguglia, L.; Chiaramonte, M.; Arizza, V.; Turiák, L.; Vékey, K.; Drahos, L.; Pitonzo, R.; Avellone, G.; Di Stefano, V. Changes in the proteome of sea urchin Paracentrotus lividus coelomocytes in response to LPS injection into the body cavity. PLoS ONE 2020, 15, e0228893. [Google Scholar] [CrossRef] [PubMed]
- Crusca, E.; Rezende, A.A.; Marchetto, R.; Mendes-Giannini, M.J.S.; Fontes, W.; Castro, M.S.; Cilli, E.M. Influence of N-terminus modifications on the biological activity, membrane interaction, and secondary structure of the antimicrobial peptide hylin-a1. Biopolymers 2011, 96, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Chiaramonte, M.; Inguglia, L.; Vazzana, M.; Deidun, A.; Arizza, V. Stress and immune response to bacterial LPS in the sea urchin Paracentrotus lividus (Lamarck, 1816). Fish. Shellfish Immunol. 2019, 92, 384–394. [Google Scholar] [CrossRef]
- Graf, M.; Wilson, D.N. Intracellular antimicrobial peptides targeting the protein synthesis machinery. In Advances in Experimental Medicine and Biology; Springer: Singapore, 2019; Volume 1117, pp. 73–89. [Google Scholar]
- Le, C.F.; Fang, C.M.; Sekaran, S.D. Intracellular targeting mechanisms by antimicrobial peptides. Antimicrob. Agents Chemother. 2017, 61, e02340-16. [Google Scholar] [CrossRef]
- Tam, J.P.; Wang, S.; Wong, K.H.; Tan, W.L. Antimicrobial peptides from plants. Pharmaceuticals 2015, 8, 711–757. [Google Scholar] [CrossRef]
- Vriens, K.; Cammue, B.P.A.; Thevissen, K. Antifungal plant defensins: Mechanisms of action and production. Molecules 2014, 19, 12280–12303. [Google Scholar] [CrossRef]
- Hammami, R.; Ben Hamida, J.; Vergoten, G.; Fliss, I. PhytAMP: A database dedicated to antimicrobial plant peptides. Nucleic Acids Res. 2009, 37, D963–D968. [Google Scholar] [CrossRef]
- Almeida, M.S.; Cabral, K.M.S.; Kurtenbach, E.; Almeida, F.C.L.; Valente, A.P. Solution structure of Pisum sativum defensin 1 by high resolution NMR: Plant defensins, identical backbone with different mechanisms of action. J. Mol. Biol. 2002, 315, 749–757. [Google Scholar] [CrossRef]
- Ojeda, P.G.; Cardoso, M.H.; Franco, O.L. Pharmaceutical applications of cyclotides. Drug Discov. Today 2019, 24, 2152–2161. [Google Scholar] [CrossRef] [PubMed]
- Schillaci, D.; Cusimano, M.G.; Cascioferro, S.M.; Di Stefano, V.; Arizza, V.; Chiaramonte, M.; Inguglia, L.; Bawadekji, A.; Davino, S.; Gargano, M.L.; et al. Antibacterial activity of desert truffles from Saudi Arabia against Staphylococcus aureus and Pseudomonas aeruginosa. Int. J. Med. Mushrooms 2017, 19. [Google Scholar] [CrossRef]
- Tam, J.P.; Lu, Y.A.; Yang, J.L.; Chiu, K.W. An unusual structural motif of antimicrobial peptides containing end-to-end macrocycle and cystine-knot disulfides. Proc. Natl. Acad. Sci. USA 1999, 96, 8913–8918. [Google Scholar] [CrossRef]
- Saket, K.; Afshari, J.T.; Saburi, E.; Yousefi, M.; Salari, R. Therapeutic aspects of Squill; an evidence-based review. Curr. Drug Discov. Technol. 2020, 17, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Bonin, E.; dos Santos, A.R.; Fiori da Silva, A.; Ribeiro, L.H.; Favero, M.E.; Campanerut-Sá, P.A.Z.; de Freitas, C.F.; Caetano, W.; Hioka, N.; Mikcha, J.M.G. Photodynamic inactivation of foodborne bacteria by eosin Y. J. Appl. Microbiol. 2018, 124, 1617–1628. [Google Scholar] [CrossRef] [PubMed]
- Nejatbakhsh, F.; Karegar-Borzi, H.; Amin, G.; Eslaminejad, A.; Hosseini, M.; Bozorgi, M.; Gharabaghi, M.A. Squill Oxymel, a traditional formulation from Drimia maritima (L.) Stearn, as an add-on treatment in patients with moderate to severe persistent asthma: A pilot, triple-blind, randomized clinical trial. J. Ethnopharmacol. 2017, 196, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Aswal, S.; Kumar, A.; Semwal, R.B.; Chauhan, A.; Kumar, A.; Lehmann, J.; Semwal, D.K. Drimia indica: A plant used in traditional medicine and its potential for clinical uses. Medicina 2019, 55, 255. [Google Scholar] [CrossRef] [PubMed]
- Knittel, D.N.; Stintzing, F.C.; Kammerer, D.R. Metabolic fate of cardiac glycosides and flavonoids upon fermentation of aqueous sea squill (Drimia maritima L.) extracts. J. Pharm. Biomed. Anal. 2015, 110, 100–109. [Google Scholar] [CrossRef]
- Knittel, D.N.; Stintzing, F.C.; Kammerer, D.R. Simultaneous determination of bufadienolides and phenolic compounds in sea squill (Drimia maritima (L.) Stearn) by HPLC-DAD-MSn as a means to differentiate individual plant parts and developmental stages. Anal. Bioanal. Chem. 2014, 406, 6035–6050. [Google Scholar] [CrossRef]
- Gupta, R.C. Non-anticoagulant rodenticides. In Veterinary Toxicology; Elsevier Ltd.: Amsterdam, The Netherlands, 2007; pp. 548–560. ISBN 9780123704672. [Google Scholar]
- Mikail, H.G.; Karvouni, H.; Kotsiou, A.; Tesseromatis, C.; Magiatis, P. New alkylresorcinols from a lipophilic extract of Urginea indica L. bulbs showing experimental trauma healing activity. Fitoterapia 2015, 101, 41–45. [Google Scholar] [CrossRef] [PubMed]
- DeLeon, S.; Clinton, A.; Fowler, H.; Everett, J.; Horswill, A.R.; Rumbaugh, K.P. Synergistic interactions of Pseudomonas aeruginosa and Staphylococcus aureus in an In vitro wound model. Infect. Immun. 2014, 82, 4718–4728. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, X.; Wang, Z. APD3: The antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2016, 44, D1087–D1093. [Google Scholar] [CrossRef]
- Wu, J.; Liu, H.; Yang, H.; Yu, H.; You, D.; Ma, Y.; Ye, H.; Lai, R. Proteomic analysis of skin defensive factors of tree frog Hyla simplex. J. Proteome Res. 2011, 10, 4230–4240. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.K.; Nam, B.H.; Go, H.J.; Jeong, M.; Lee, K.Y.; Cho, S.M.; Lee, I.A.; Park, N.G. Hemerythrin-related antimicrobial peptide, msHemerycin, purified from the body of the Lugworm, Marphysa sanguinea. Fish. Shellfish Immunol. 2016, 57, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Kastin, A. Handbook of Biologically Active Peptides; Elsevier Inc.: Amsterdam, The Netherlands, 2013; ISBN 9780123850959. [Google Scholar]
- Imai, Y.; Meyer, K.J.; Iinishi, A.; Favre-Godal, Q.; Green, R.; Manuse, S.; Caboni, M.; Mori, M.; Niles, S.; Ghiglieri, M.; et al. A new antibiotic selectively kills Gram-negative pathogens. Nature 2019, 576, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Singla, D.; Rashid, M.; Raghava, G.P.S. Designing of peptides with desired half-life in intestine-like environment. Bmc Bioinform. 2014, 15, 282. [Google Scholar] [CrossRef]
- Allcock, S.; Young, E.H.; Holmes, M.; Gurdasani, D.; Dougan, G.; Sandhu, M.S.; Solomon, L.; Török, M.E. Antimicrobial resistance in human populations: Challenges and opportunities. Glob. Heal. Epidemiol. Genom. 2017, 2, 1–7. [Google Scholar]
- WHO. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics; World Health Organization: Geneva, Switzerland, 2017; pp. 348–365. [Google Scholar]
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef]
- Romero, S.M.; Cardillo, A.B.; Martínez Ceron, M.C.; Camperi, S.A.; Giudicessi, S.L. Temporins: An Approach of Potential Pharmaceutic Candidates. Surg. Infect. (Larchmt) 2019, 21, 309–322. [Google Scholar] [CrossRef]
- Bhattacharjya, S.; Straus, S.K. Design, Engineering and Discovery of Novel α-Helical and β-Boomerang Antimicrobial Peptides against Drug Resistant Bacteria. Int. J. Mol. Sci. 2020, 21, 5773. [Google Scholar] [CrossRef] [PubMed]
- Schillaci, D.; Arizza, V.; Parrinello, N.; Di Stefano, V.; Fanara, S.; Muccilli, V.; Cunsolo, V.; Haagensen, J.J.A.; Molin, S. Antimicrobial and antistaphylococcal biofilm activity from the sea urchin Paracentrotus lividus. J. Appl. Microbiol. 2010, 108, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Mauro, N.; Schillaci, D.; Varvarà, P.; Cusimano, M.G.; Geraci, D.M.; Giuffrè, M.; Cavallaro, G.; Maida, C.M.; Giammona, G. Branched High Molecular Weight Glycopolypeptide with Broad-Spectrum Antimicrobial Activity for the Treatment of Biofilm Related Infections. Acs Appl. Mater. Interfaces 2018, 10, 318–331. [Google Scholar] [CrossRef] [PubMed]
- Hess, B.; Kutzner, C.; Van Der Spoel, D.; Lindahl, E. GRGMACS 4: Algorithms for highly efficient, load-balanced, and scalable molecular simulation. J. Chem. Theory Comput. 2008, 4, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Cunsolo, V.; Fasoli, E.; Saletti, R.; Muccilli, V.; Gallina, S.; Righetti, P.G.; Foti, S. Zeus, Aesculapius, Amalthea and the proteome of goat milk. J. Proteom. 2015, 128, 69–82. [Google Scholar] [CrossRef]
- Schillaci, D.; Cusimano, M.; Cunsolo, V.; Saletti, R.; Russo, D.; Vazzana, M.; Vitale, M.; Arizza, V. Immune mediators of sea-cucumber Holothuria tubulosa (Echinodermata) as source of novel antimicrobial and anti-staphylococcal biofilm agents. Amb Express 2013, 3, 35. [Google Scholar] [CrossRef] [PubMed]
- Vazzana, M.; Celi, M.; Chiaramonte, M.; Inguglia, L.; Russo, D.; Ferrantelli, V.; Battaglia, D.; Arizza, V. Cytotoxic activity of Holothuria tubulosa (Echinodermata) coelomocytes. Fish. Shellfish Immunol. 2018, 72, 334–341. [Google Scholar] [CrossRef]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J.C. GROMACS: Fast, flexible, and free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef]
- Lindorff-Larsen, K.; Piana, S.; Palmo, K.; Maragakis, P.; Klepeis, J.L.; Dror, R.O.; Shaw, D.E. Improved side-chain torsion potentials for the Amber ff99SB protein force field. Proteins Struct. Funct. Bioinforma. 2010, 78, 1950–1958. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Bussi, G.; Donadio, D.; Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 2007, 126, 014101. [Google Scholar] [CrossRef] [PubMed]
- Parrinello, M.; Rahman, A. Polymorphic transitions in single crystals: A new molecular dynamics method. J. Appl. Phys. 1981, 52, 7182–7190. [Google Scholar] [CrossRef]
- Darden, T.; York, D.; Pedersen, L. Particle mesh Ewald: An N·log(N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]
- Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. A smooth particle mesh Ewald method. J. Chem. Phys. 1995, 103, 8577–8593. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera?A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Pérez, F.; Granger, B.E. IPython: A System for Interactive Scientific Computing. Comput. Sci. Eng. 2007, 9, 21–29. [Google Scholar] [CrossRef]
- Hunter, J.D. Matplotlib: A 2D graphics environment. Comput. Sci. Eng. 2007, 9, 99–104. [Google Scholar] [CrossRef]
- Lovell, S.C.; Davis, I.W.; Arendall, W.B.; De Bakker, P.I.W.; Word, J.M.; Prisant, M.G.; Richardson, J.S.; Richardson, D.C. Structure validation by Cα geometry: φ,ψ and Cβ deviation. Proteins Struct. Funct. Genet. 2003, 50, 437–450. [Google Scholar] [CrossRef]
- Dolinsky, T.J.; Nielsen, J.E.; McCammon, J.A.; Baker, N.A. PDB2PQR: An automated pipeline for the setup of Poisson-Boltzmann electrostatics calculations. Nucleic Acids Res. 2004, 32, W665-7. [Google Scholar] [CrossRef]
- Baker, N.A.; Sept, D.; Joseph, S.; Holst, M.J.; McCammon, J.A. Electrostatics of nanosystems: Application to microtubules and the ribosome. Proc. Natl. Acad. Sci. USA 2001, 98, 10037–10041. [Google Scholar] [CrossRef] [PubMed]

| Strains | MIC | ||
|---|---|---|---|
| Roots | Leaves | Bulb | |
| S.aureus ATCC 25923 | >50% v/v (>20 µg/mL) | >50% v/v (>45 µg/mL) | 12.5% v/v (30.5 µg/mL) |
| P.aeruginosa ATCC 15442 | >50% v/v (>20 µg/mL) | >50% v/v (>45 µg/mL) | 12.5% v/v (30.5µg/mL) |
| Protein (Acc. No; Taxa) | #No. | Identified Peptides | Predicted Ability to Interact with MembranesBetter Chance to Be an AMP | Similarity with already Described AMPs |
|---|---|---|---|---|
| Ribosome-inactivating protein charybdin (P84786; C.pancration) | ||||
| #1 | ILDISYNKNALQD | - | 46.7% frog AMP (hylain1) | |
| #2 | SEPVKLPQWMQND | - | 44.4% nematode AMP | |
| #3 | VDIANHFAFN | yes | 37.5% frog AMP (temporin) | |
| #4 | ILDISYNKNALQDAVSK | yes | 45.4% frog AMP (cruzioseptin) | |
| #5 | LPQWMQNDLEKN | - | 35.7% AMP frog (temporin) | |
| #6 | LEKNWVRFSF | yes | 40% AMP prokaryotes | |
| #7 | VDIANHFAFNLE | yes | 41.7% AMP frog (hylain 1) | |
| #8 | DILDISYNKNALQD | yes | 46.6% AMP frog (hylain 1) | |
| Elongation factor 1-alpha (multispecies identification) | ||||
| #9 | VVTFGPTGLTTEVK | - | 41.2% bacteriocin (Pediococcus pentosaceus) | |
| #10 | IERSTNLDWYKGPTLL | yes | 38.9% frog AMP (temporin) | |
| Superoxide dismutase [Cu-Zn] (multispecies identification) | ||||
| #11 | QIPLTGAHSIIGRA | yes | 40% AMP frog (temporin Rb) | |
| Superoxide dismutase [Cu-Zn], chloroplastic (multispecies identification) | ||||
| #12 | IPLSGPNAVIGRA | yes | 40% AMP frog (temporin Rb) | |
| Allene oxide synthase (multispecies identification) | ||||
| #13 | LHTFRLPPFL | - | 40% AMP frog (temporin-1Gc) | |
| #14 | LEELLLHT | - | 50% gageotetrin (Bacillus subtilis) | |
| RTM3-like protein (multispecies identification) | ||||
| #15 | LSRSMKEAGFKLDW | - | 41.2% AMP frog (temporin 1La) | |
| Photosystem P700 chlorophyll (multispecies identification) | ||||
| #16 | VSLPINELLD | yes | 50% AMP frog (temporin E) | |
| Unknown | ||||
| #17 | FVCPLNLLAE | yes | 42.8% AMP frog (Temporin-Ra) |
| Chemical-Physical Properties | Peptide #6 | Peptide #11 | Peptide #12 |
| Peptide sequence | LEKNWVRFSF | QIPLTGAHSIIGRA | IPLSGPNAVIGRA |
| Theoretical mass (Da) | 1325.67 | 1433.89 | 1264.68 |
| Net Charge | +1 | +1 | +1 |
| Isoelectric point | 6.360 | 6.401 | 6.336 |
| Wimley-White whole-residue hydrophobicity (kcal/mol) | −0.23 | 1.15 | 1.51 |
| Protein-binding potential Boman index (kcal/mol) | 2 | 0.42 | 0.05 |
| Half-life (s) | 0.0001 | 1.754 | 1.531 |
| Stability | Low | High | High |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cunsolo, V.; Schicchi, R.; Chiaramonte, M.; Inguglia, L.; Arizza, V.; Cusimano, M.G.; Schillaci, D.; Di Francesco, A.; Saletti, R.; Lo Celso, F.; et al. Identification of New Antimicrobial Peptides from Mediterranean Medical Plant Charybdis pancration (Steinh.) Speta. Antibiotics 2020, 9, 747. https://doi.org/10.3390/antibiotics9110747
Cunsolo V, Schicchi R, Chiaramonte M, Inguglia L, Arizza V, Cusimano MG, Schillaci D, Di Francesco A, Saletti R, Lo Celso F, et al. Identification of New Antimicrobial Peptides from Mediterranean Medical Plant Charybdis pancration (Steinh.) Speta. Antibiotics. 2020; 9(11):747. https://doi.org/10.3390/antibiotics9110747
Chicago/Turabian StyleCunsolo, Vincenzo, Rosario Schicchi, Marco Chiaramonte, Luigi Inguglia, Vincenzo Arizza, Maria Grazia Cusimano, Domenico Schillaci, Antonella Di Francesco, Rosaria Saletti, Fabrizio Lo Celso, and et al. 2020. "Identification of New Antimicrobial Peptides from Mediterranean Medical Plant Charybdis pancration (Steinh.) Speta" Antibiotics 9, no. 11: 747. https://doi.org/10.3390/antibiotics9110747
APA StyleCunsolo, V., Schicchi, R., Chiaramonte, M., Inguglia, L., Arizza, V., Cusimano, M. G., Schillaci, D., Di Francesco, A., Saletti, R., Lo Celso, F., Barone, G., & Vitale, M. (2020). Identification of New Antimicrobial Peptides from Mediterranean Medical Plant Charybdis pancration (Steinh.) Speta. Antibiotics, 9(11), 747. https://doi.org/10.3390/antibiotics9110747







