Development and Application of a Pragmatic Algorithm to Guide Definitive Carbapenemase Testing to Identify Carbapenemase-Producing Pseudomonas aeruginosa
Abstract
1. Introduction
2. Results
2.1. Algorithm Development
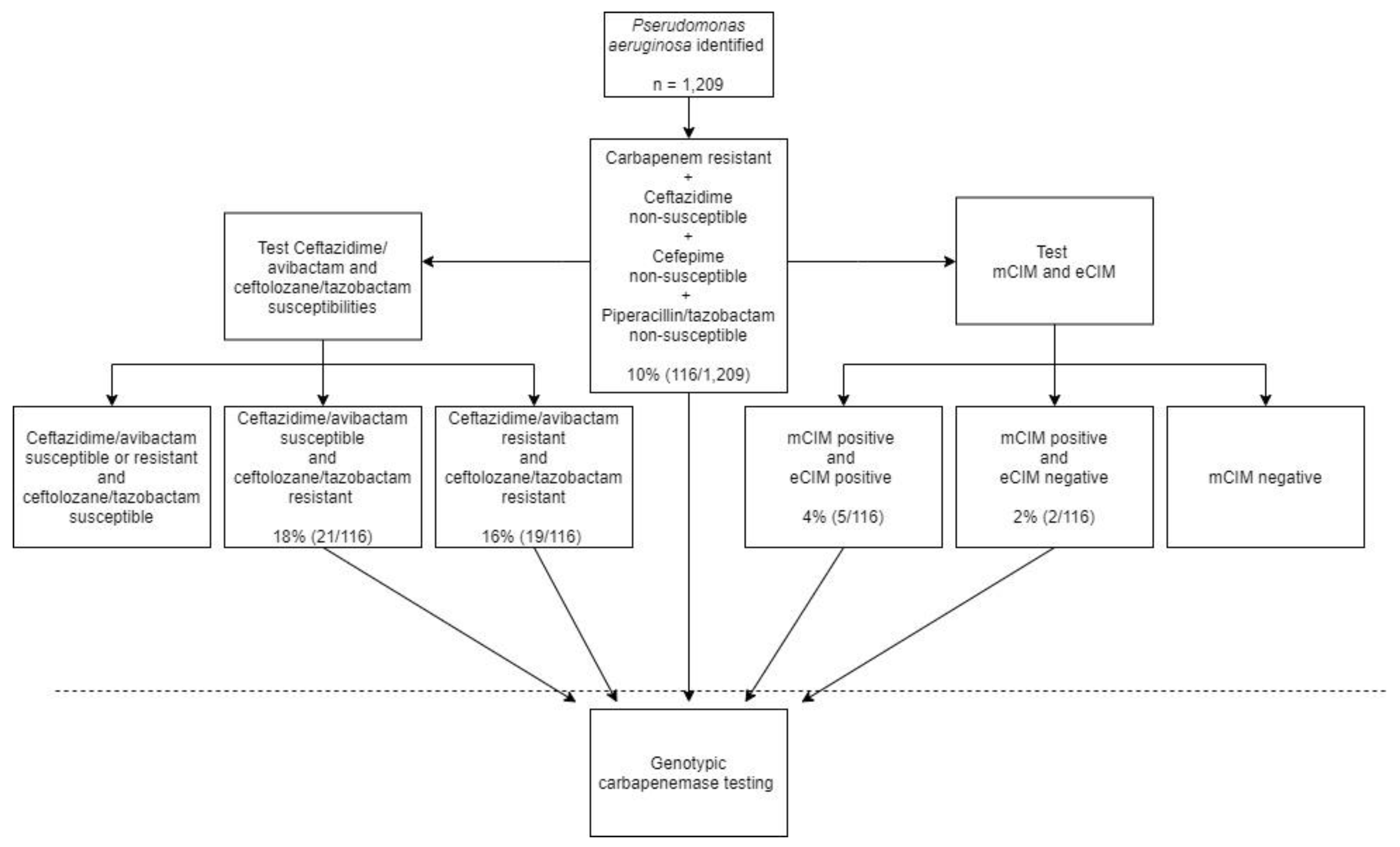
2.2. Application of Algorithm against Clinical P. aeruginosa Isolates
3. Discussion
4. Materials and Methods
4.1. Algorithm Development
4.1.1. Bacterial Isolates
4.1.2. Antimicrobial Susceptibility Testing
4.2. US Surveillance Study
4.2.1. Organism Collection
4.2.2. Phenotypic and Genotypic Carbapenemase Testing
4.3. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hong, D.J.; Bae, I.K.; Jang, I.H.; Jeong, S.H.; Kang, H.K.; Lee, K. Epidemiology and characteristics of metallo-ß-lactamase-producing Pseudomonas aeruginosa. Infect. Chemother. 2015, 47, 81–97. [Google Scholar] [CrossRef]
- Asempa, T.E.; Nicolau, D.P.; Kuti, J.L. Carbapenem-Nonsusceptible Pseudomonas aeruginosa Isolates from Intensive Care Units in the United States: A Potential Role for New β-Lactam Combination Agents. J. Clin. Microbiol. 2019, 57. [Google Scholar] [CrossRef]
- Woodworth, K.R.; Walters, M.S.; Weiner, L.M.; Edwards, J.; Brown, A.C.; Huang, J.Y.; Malik, S.; Slayton, R.B.; Paul, P.; Capers, C.; et al. Vital signs: Containment of novel multidrug-resistant organisms and resistance mechanisms—United States, 2006–2017. Morb. Mortal. Wkly. Rep. 2018, 67, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Lister, P.D.; Wolter, D.J.; Hanson, N.D. Antibacterial-Resistant Pseudomonas aeruginosa: Clinical Impact and Complex Regulation of Chromosomally Encoded Resistance Mechanisms. Clin. Microbiol. Rev. 2009, 22, 582–610. [Google Scholar] [CrossRef] [PubMed]
- Pogue, J.M.; Kaye, K.S.; Veve, M.P.; Patel, T.S.; Gerlach, A.T.; Davis, S.L.; Puzniak, L.A.; File, T.M.; Olson, S.; Dhar, S.; et al. Ceftolozane/Tazobactam vs Polymyxin or Aminoglycoside-based Regimens for the Treatment of Drug-resistant Pseudomonas aeruginosa. Clin. Infect. Dis. 2019. [Google Scholar] [CrossRef] [PubMed]
- van Duin, D.; Bonomo, R.A. Ceftazidime/Avibactam and Ceftolozane/Tazobactam: Second-generation β-Lactam/β-Lactamase Inhibitor Combinations. Clin. Infect. Dis. 2016, 63, 234–241. [Google Scholar] [CrossRef]
- Castanheira, M.; Deshpande, L.M.; Costello, A.; Davies, T.A.; Jones, R.N. Epidemiology and carbapenem resistance mechanisms of carbapenem-non-susceptible Pseudomonas aeruginosa collected during 2009-11 in 14 European and Mediterranean countries. J. Antimicrob. Chemother. 2014, 69, 1804–1814. [Google Scholar] [CrossRef]
- Labarca, J.A.; Salles, M.J.C.; Seas, C.; Guzmán-Blanco, M. Carbapenem resistance in Pseudomonas aeruginosa and Acinetobacter baumannii in the nosocomial setting in Latin America. Crit. Rev. Microbiol. 2014, 7828, 1–17. [Google Scholar] [CrossRef]
- McCracken, M.G.; Adam, H.J.; Blondeau, J.M.; Walkty, A.J.; Karlowsky, J.A.; Hoban, D.J.; Zhanel, G.G.; Mulvey, M.R.; Zhanel, G.G.; Hoban, D.J.; et al. Characterization of carbapenem-resistant and XDR Pseudomonas aeruginosa in Canada: Results of the CANWARD 2007–16 study. J. Antimicrob. Chemother. 2019, 74, iv32–iv38. [Google Scholar] [CrossRef]
- Botelho, J.; Grosso, F.; Peixe, L. Antibiotic resistance in Pseudomonas aeruginosa—Mechanisms, epidemiology and evolution. Drug Resist. Updat. 2019, 44, 100640. [Google Scholar] [CrossRef]
- Partridge, S.R.; Kwong, S.M.; Firth, N.; Jensen, S.O. Mobile genetic elements associated with antimicrobial resistance. Clin. Microbiol. Rev. 2018, 31, 1–61. [Google Scholar] [CrossRef]
- Laupland, K.B.; Parkins, M.D.; Church, D.L.; Gregson, D.B.; Louie, T.J.; Conly, J.M.; Elsayed, S.; Pitout, J.D.D. Population-Based Epidemiological Study of Infections Caused by Carbapenem-Resistant Pseudomonas aeruginosa in the Calgary Health Region: Importance of Metallo-β-Lactamase (MBL)–Producing Strains. J. Infect. Dis. 2005, 192, 1606–1612. [Google Scholar] [CrossRef]
- Lucena, A.; Dalla Costa, L.M.; Nogueira, K.S.; Matos, A.P.; Gales, A.C.; Paganini, M.C.; Castro, M.E.S.; Raboni, S.M. Nosocomial infections with metallo-beta-lactamase-producing Pseudomonas aeruginosa: Molecular epidemiology, risk factors, clinical features and outcomes. J. Hosp. Infect. 2014, 87, 234–240. [Google Scholar] [CrossRef]
- Willmann, M.; Kuebart, I.; Marschal, M.; Schröppel, K.; Vogel, W.; Flesch, I.; Markert, U.; Autenrieth, I.B.; Hölzl, F.; Peter, S. Effect of metallo-β-lactamase production and multidrug resistance on clinical outcomes in patients with Pseudomonas aeruginosa bloodstream infection: A retrospective cohort study. BMC Infect. Dis. 2013, 13, 1–9. [Google Scholar] [CrossRef]
- Tamma, P.D.; Simner, P.J. Phenotypic detection of carbapenemase-producing organisms from clinical isolates. J. Clin. Microbiol. 2018, 56, 1–13. [Google Scholar] [CrossRef]
- Dortet, L.; Cuzon, G.; Plésiat, P.; Naas, T. Prospective evaluation of an algorithm for the phenotypic screening of carbapenemase-producing Enterobacteriaceae. J. Antimicrob. Chemother. 2016, 71, 135–140. [Google Scholar] [CrossRef]
- Choquet, M.; Guiheneuf, R.; Castelain, S.; Pluquet, E.; Decroix, V. Prospective evaluation of a screening algorithm for carbapenemase-producing Enterobacteriaceae. J. Clin. Lab. Anal. 2019, 33, e22706. [Google Scholar] [CrossRef]
- Robert, J.; Pantel, A.; Merens, A.; Meiller, E.; Lavigne, J.; Nicolas-Chanoine, M.-H. Development of an algorithm for phenotypic screening of carbapenemase-producing Enterobacteriaceae in the routine laboratory. BMC Infect. Dis. 2017, 17, 78. [Google Scholar] [CrossRef]
- Samuelsen, Ø.; Buarø, L.; Giske, C.G.; Simonsen, G.S.; Aasnæs, B.; Sundsfjord, A. Evaluation of phenotypic tests for the detection of metallo-β-lactamase-producing Pseudomonas aeruginosa in a low prevalence country. J. Antimicrob. Chemother. 2008, 61, 827–830. [Google Scholar] [CrossRef][Green Version]
- Naas, T.; Dortet, L.; Iorga, B.I. Structural and Functional Aspects of Class A Carbapenemases. Curr. Drug Targets 2016, 17, 1006–1028. [Google Scholar] [CrossRef]
- Bonomo, R.A.; Burd, E.M.; Conly, J.; Limbago, B.M.; Poirel, L.; Segre, J.A.; Westblade, L.F. Carbapenemase-Producing Organisms: A Global Scourge. Clin. Infect. Dis. 2018, 66, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- Walters, M.S.; Grass, J.E.; Bulens, S.N.; Hancock, E.B.; Phipps, E.C.; Muleta, D.; Mounsey, J.; Kainer, M.A.; Concannon, C.; Dumyati, G.; et al. Carbapenem-Resistant Pseudomonas aeruginosa at US Emerging Infections Program Sites, 2015. Emerg. Infect. Dis. 2019, 25, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
- Kazmierczak, K.M.; Rabine, S.; Hackel, M.; McLaughlin, R.E.; Biedenbach, D.J.; Bouchillon, S.K.; Sahm, D.F.; Bradford, P.A. Multiyear, multinational survey of the incidence and global distribution of metallo-β-lactamase-producing enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2016, 60, 1067–1078. [Google Scholar] [CrossRef]
- Simner, P.J.; Kristie Johnson, J.; Brasso, W.B.; Anderson, K.; Lonsway, D.R.; Pierce, V.M.; Bobenchik, A.M.; Lockett, Z.C.; Charnot-Katsikas, A.; Westblade, L.F.; et al. Multicenter evaluation of the modified carbapenem inactivation method and the carba NP for detection of carbapenemase-producing pseudomonas aeruginosa and acinetobacter baumannii. J. Clin. Microbiol. 2018, 56, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Tran, T.T.; Rios, R.; Hanson, B.; Shropshire, W.C.; Sun, Z.; Diaz, L.; Dinh, A.Q.; Wanger, A.; Ostrosky-Zeichner, L.; et al. Extensively Drug-Resistant Pseudomonas aeruginosa ST309 Harboring Tandem Guiana Extended Spectrum β-Lactamase Enzymes: A Newly Emerging Threat in the United States. Open Forum Infect. Dis. 2019, 6, ofz273. [Google Scholar] [CrossRef] [PubMed]
- Garza-Ramos, U.; Barrios, H.; Reyna-Flores, F.; Tamayo-Legorreta, E.; Catalan-Najera, J.C.; Morfin-Otero, R.; Rodríguez-Noriega, E.; Volkow, P.; Cornejo-Juarez, P.; González, A.; et al. Widespread of ESBL- and carbapenemase GES-type genes on carbapenem-resistant Pseudomonas aeruginosa clinical isolates: A multicenter study in Mexican hospitals. Diagn. Microbiol. Infect. Dis. 2015, 81, 135–137. [Google Scholar] [CrossRef] [PubMed]
- Nordmann, P.; Poirel, L. Epidemiology and Diagnostics of Carbapenem Resistance in Gram-negative Bacteria. Clin. Infect. Dis. 2019, 69, S521–S528. [Google Scholar] [CrossRef]
- Decousser, J.; Poirel, L.; Nordmann, P. Recent advances in biochemical and molecular diagnostics for the rapid detection of antibiotic-resistant Enterobacteriaceae: A focus on ß-lactam resistance. Expert Rev. Mol. Diagn. 2017, 17, 327–350. [Google Scholar] [CrossRef]
- Banerjee, R.; Humphries, R. Clinical and laboratory considerations for the rapid detection of carbapenem-resistant Enterobacteriaceae. Virulence 2017, 8, 427–439. [Google Scholar] [CrossRef]
- Shields, R.K.; Doi, Y. Aztreonam Combination Therapy: An Answer to Metallo-β-Lactamase–Producing Gram-Negative Bacteria? Clin. Infect. Dis. 2019, 15261, 1–3. [Google Scholar] [CrossRef]
- Karlowsky, J.A.; Kazmierczak, K.M.; de Jonge, B.L.M.; Hackel, M.A.; Sahm, D.F.; Bradford, P.A. In Vitro Activity of Aztreonam-Avibactam against Enterobacteriaceae and Pseudomonas aeruginosa Isolated by Clinical Laboratories in 40 Countries from 2012 to 2015. Antimicrob. Agents Chemother. 2017, 61, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kresken, M.; Körber-Irrgang, B.; Korte-Berwanger, M.; Pfennigwerth, N.; Gatermann, S.G.; Seifert, H. Dissemination of carbapenem-resistant Pseudomonas aeruginosa isolates and their susceptibilities to ceftolozane-tazobactam in Germany. Int. J. Antimicrob. Agents 2020, 105959. [Google Scholar] [CrossRef]
- Sader, H.S.; Flamm, R.K.; Carvalhaes, C.G.; Castanheira, M. Comparison of ceftazidime-avibactam and ceftolozane-tazobactam in vitro activities when tested against gram-negative bacteria isolated from patients hospitalized with pneumonia in United States medical centers (2017–2018). Diagn. Microbiol. Infect. Dis. 2020, 96, 114833. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 29th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2019; ISBN 9781684400324. [Google Scholar]
- Lapuebla, A.; Abdallah, M.; Olafisoye, O.; Cortes, C.; Urban, C.; Quale, J.; Landman, D. Activity of meropenem combined with RPX7009, a novel β-lactamase inhibitor, against gram-negative clinical isolates in New York City. Antimicrob. Agents Chemother. 2015, 59, 4856–4860. [Google Scholar] [CrossRef] [PubMed]
- Vega, A.D.; Jimenez, A.; Rosello, G.; Claeys, K.C.; Martinez, O.V.; De Pascale, B.; Perez-Cardona, A.; Abbo, L. Implementing Carbapenem-resistance testing algorithms for Enterobacteriales and Pseudomonas aeruginosa: Diagnostic and antimicrobial stewardship with timely infection prevention. Diagn. Microbiol. Infect. Dis. 2020, 115069. [Google Scholar] [CrossRef] [PubMed]
- Almarzoky Abuhussain, S.S.; Sutherland, C.A.; Nicolau, D.P. In vitro potency of antipseudomonal β-lactams against blood and respiratory isolates of P. Aeruginosa collected from US hospitals. J. Thorac. Dis. 2019, 11, 1896–1902. [Google Scholar] [CrossRef]
- Sfeir, M.M.; Hayden, J.A.; Fauntleroy, K.A.; Mazur, C.; Johnson, J.K.; Simner, P.J.; Das, S.; Satlin, M.J.; Jenkins, S.G.; Westblade, L.F. EDTA-Modified Carbapenem Inactivation Method: A Phenotypic Method for Detecting Metallo-β-Lactamase-Producing Enterobacteriaceae. J. Clin. Microbiol. 2019, 57, 1–9. [Google Scholar] [CrossRef]
- Zankari, E.; Hasman, H.; Cosentino, S.; Vestergaard, M.; Rasmussen, S.; Lund, O.; Aarestrup, F.M.; Larsen, M.V. Identification of acquired antimicrobial resistance genes. J. Antimicrob. Chemother. 2012, 67, 2640–2644. [Google Scholar] [CrossRef]
| Susceptibility | Carbapenemase Producers, n = 57 | Non-Carbapenemase Producers, | Test Performance | ||
|---|---|---|---|---|---|
| Cephalosporinase or Efflux/Porin Mutation, n = 20 | Wild Type, n = 15 | Sensitivity, % (95% CI) | Specificity, % (95% CI) | ||
| IPM + MEM- Resistant | 57 (100%) | 15 (75%) | 1 (7%) | 100% (94–100%) | 54% (37–71%) |
| IPM + MEM- Resistant AND FEP + CAZ + TZP- Non-Susceptible | 57 (100%) | 12 (60%) | 0 (0%) | 100% (94–100%) | 66% (48–81%) |
| IPM + MEM- Resistant AND FEP + CAZ + TZP- Resistant | 47 (82%) | 6 (30%) | 0 (0%) | 83% (70–91%) | 83% (66–93%) |
| IPM + MEM- Resistant AND FEP + CAZ + TZP- Non-Susceptible + CZA- Resistant | 49 (86%) | 8 (40%) | 0 (0%) | 86% (74–94%) | 77% (60–90%) |
| IPM + MEM- Resistant AND FEP + CAZ + TZP- Non-Susceptible + C/T- Resistant | 57 (100%) | 4 (20%) | 0 (0%) | 100% (94–100%) | 89% (73–97%) |
| IPM + MEM- Resistant AND FEP + CAZ + TZP- Non-Susceptible + C/T- Resistant + CZA- Resistant | 49 (86%) | 3 (15%) | 0 (0%) | 86% (74–94%) | 91% (77–98%) |
| Algorithm-Derived Screening Criteria | Number Meeting Criteria | Carbapenemase Producers Detected | Carbapenemase Producers Missed by Criteria |
|---|---|---|---|
| IPM + MEM- Resistant AND FEP + CAZ + TZP- Non-Susceptible | 116 | 7/116 | 0 |
| IPM + MEM- Resistant AND FEP + CAZ + TZP- Non-Susceptible + CZA- Resistant | 43 | 7/43 | 0 |
| IPM + MEM- Resistant AND FEP + CAZ + TZP- Non-Susceptible + C/T- Resistant | 21 | 6/21 | 1 * |
| IPM + MEM- Resistant AND FEP + CAZ + TZP- Non-Susceptible + C/T- Resistant + CZA-Resistant | 19 | 6/19 | 1 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gill, C.M.; Asempa, T.E.; Nicolau, D.P. Development and Application of a Pragmatic Algorithm to Guide Definitive Carbapenemase Testing to Identify Carbapenemase-Producing Pseudomonas aeruginosa. Antibiotics 2020, 9, 738. https://doi.org/10.3390/antibiotics9110738
Gill CM, Asempa TE, Nicolau DP. Development and Application of a Pragmatic Algorithm to Guide Definitive Carbapenemase Testing to Identify Carbapenemase-Producing Pseudomonas aeruginosa. Antibiotics. 2020; 9(11):738. https://doi.org/10.3390/antibiotics9110738
Chicago/Turabian StyleGill, Christian M., Tomefa E. Asempa, and David P. Nicolau. 2020. "Development and Application of a Pragmatic Algorithm to Guide Definitive Carbapenemase Testing to Identify Carbapenemase-Producing Pseudomonas aeruginosa" Antibiotics 9, no. 11: 738. https://doi.org/10.3390/antibiotics9110738
APA StyleGill, C. M., Asempa, T. E., & Nicolau, D. P. (2020). Development and Application of a Pragmatic Algorithm to Guide Definitive Carbapenemase Testing to Identify Carbapenemase-Producing Pseudomonas aeruginosa. Antibiotics, 9(11), 738. https://doi.org/10.3390/antibiotics9110738




