The Usefulness of Microalgae Compounds for Preventing Biofilm Infections
Abstract
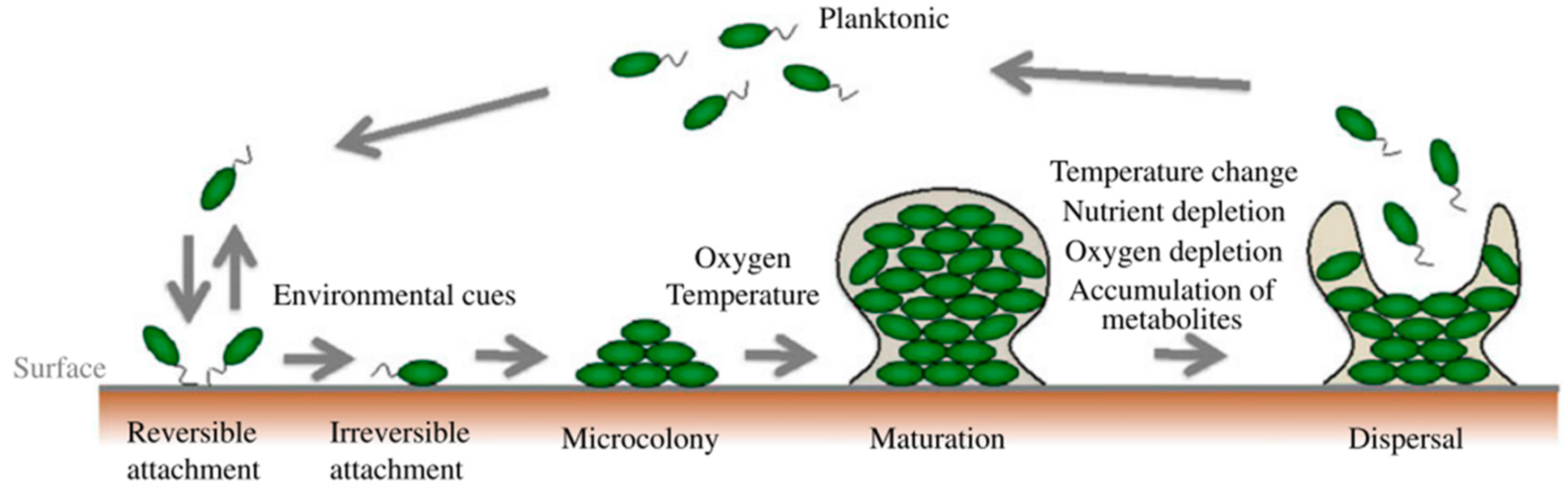
1. Biofilms and Their Role in Infectious Diseases
2. What is Needed to Fight Biofilms
3. Prevention or Treatment of Biofilm Infections
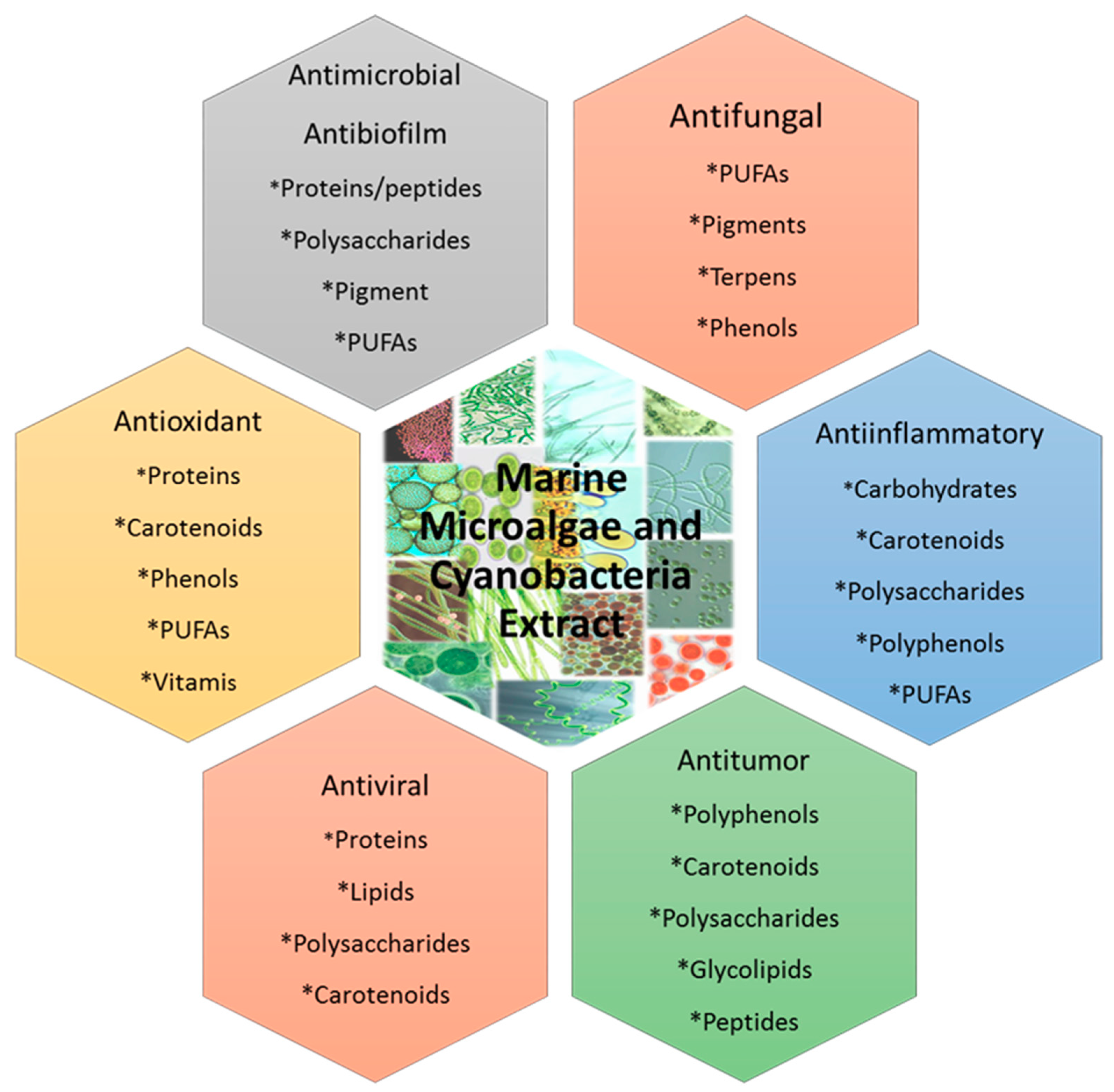
4. Microalgae as a Source of Bioactive Compounds
5. Antibiofilm Activity of Compounds Isolated from Microalgae.
6. Conclusions
7. Future Aspects
Author Contributions
Funding
Conflicts of Interest
References
- Stoodley, P.; Sauer, K.; Davies, D.G.; Costerton, J.W. Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 2002, 56, 187–209. [Google Scholar] [CrossRef]
- Soto, S.M. Role of efflux pumps in the antibiotic resistance of bacteria embedded in a biofilm. Virulence 2013, 4, 223–229. [Google Scholar] [CrossRef]
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence 2018, 9, 522–554. [Google Scholar] [CrossRef]
- Wu, H.; Moser, C.; Wang, H.Z.; Høiby, N.; Song, Z.J. Strategies for combating bacterial biofilm infections. Int. J. Oral Sci. 2015, 7, 1–7. [Google Scholar] [CrossRef]
- Macià, M.D.; Luis, J.; Díez-aguilar, M.; Guinea, J. Microbiological diagnosis of biofilm-related infections. Enferm. Infecc. Microbiol. Clin. 2018, 36, 375–381. [Google Scholar] [CrossRef]
- Costerton, J.W. Introduction to biofilm. Int. J. Antimicrob. Agents 1999, 11, 217–221. [Google Scholar] [CrossRef]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef]
- Lasa, I.; Del Pozo, J.L.; Leiva, J.; Penadés, J.R. Bacterial biofilms and infection. An. Sist. Sanit. Navar. 2005, 28, 163–175. [Google Scholar] [CrossRef]
- Goossens, G.A. Flushing and Locking of Venous Catheters: Available Evidence and Evidence Deficit. Nurs. Res. Pract. 2015, 2015, 985686. [Google Scholar] [CrossRef]
- Yasuda, H.; Ajiki, Y.; Koga, T.; Kawada, H.; Yokota, T. Interaction between biofilms formed by Pseudomonas aeruginosa and clarithromycin. Antimicrob. Agents Chemother. 1993, 37, 1749–1755. [Google Scholar] [CrossRef]
- Kumar, A.; Alam, A.; Rani, M.; Ehtesham, N.Z.; Hasnain, S.E. Biofilms: Survival and defense strategy for pathogens. Int. J. Med. Microbiol. 2017, 307, 481–489. [Google Scholar] [CrossRef]
- Ichimiya, T.; Takeoka, K.; Hiramatsu, K.; Hirai, K.; Yamasaki, T.; Nasu, M. The influence of azithromycin on the biofilm formation of pseudomonas aeruginosa in vitro. Chemotherapy 1996, 42, 186–191. [Google Scholar] [CrossRef]
- Parra-Ruiz, J.; Vidaillac, C.; Rybak, M.J. Macrolides and staphylococcal biofilms. Rev. Esp. Quimioter. 2012, 25, 10–16. [Google Scholar]
- Sano, M.; Hirose, T.; Nishimura, M.; Takahashi, S.; Matsukawa, M.; Tsukamoto, T. Inhibitory action of clarithromycin on glycocalyx produced by MRSA. J. Infect. Chemother. 1999, 5, 10–15. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, F.J.; Liu, Y.; Xiong, L.R.; Xie, L.L.; Xia, P.Y. Enhancement of biofilm formation by subinhibitory concentrations of macrolides in icaADBC-positive and -negative clinical isolates of Staphylococcus epidermidis. Antimicrob. Agents Chemother. 2010, 54, 2707–2711. [Google Scholar] [CrossRef]
- Chen, M.; Yu, Q.; Sun, H. Novel strategies for the prevention and treatment of biofilm related infections. Int. J. Mol. Sci. 2013, 14, 18488–18501. [Google Scholar] [CrossRef]
- Sambanthamoorthy, K.; Gokhale, A.A.; Lao, W.; Parashar, V.; Neiditch, M.B.; Semmelhack, M.F.; Lee, I.; Waters, C.M. Identification of a novel benzimidazole that inhibits bacterial biofilm formation in a broad-spectrum manner. Antimicrob. Agents Chemother. 2011, 55, 4369–4378. [Google Scholar] [CrossRef]
- Davies, D.G.; Marques, C.N.H. A fatty acid messenger is responsible for inducing dispersion in microbial biofilms. J. Bacteriol. 2009, 191, 1393–1403. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, Y.; Yestrepsky, B.D.; Sorenson, R.J.; Chen, M.; Larsen, S.D.; Sun, H. Novel Inhibitors of Staphylococcus aureus Virulence gene expression and biofilm formation. PLoS ONE 2012, 7, e47255. [Google Scholar] [CrossRef]
- Panmanee, W.; Taylor, D.; Shea, C.J.A.; Tang, H.; Nelson, S.; Seibel, W.; Papoian, R.; Kramer, R.; Hassett, D.J.; Lamkin, T.J. High-throughput screening for small-molecule inhibitors of Staphylococcus epidermidis RP62a biofilms. J. Biomol. Screen. 2012, 18, 820–829. [Google Scholar] [CrossRef]
- MacLehose, H.G.; Gilbert, P.; Allison, D.G. Biofilms, homoserine lactones and biocide susceptibility. J. Antimicrob. Chemother. 2004, 53, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Balaban, N.; Cirioni, O.; Giacometti, A.; Ghiselli, R.; Braunstein, J.B.; Silvestri, C.; Mocchegiani, F.; Saba, V.; Scalise, G. Treatment of Staphylococcus aureus biofilm infection by the quorum-sensing inhibitor RIP. Antimicrob. Agents Chemother. 2007, 51, 2226–2229. [Google Scholar] [CrossRef] [PubMed]
- Wood, T.K. Insights on Escherichia coli biofilm formation and inhibition from whole-transcriptome profiling. Environ. Microbiol. 2009, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Rutz, K.; Kunze, B.; Tomasch, J.; Surapaneni, S.K.; Schulz, S.; Wagner-Döbler, I. The biofilm inhibitor carolacton disturbs membrane integrity and cell division of Streptococcus mutans through the serine/threonine protein kinase PknB. J. Bacteriol. 2011, 193, 5692–5706. [Google Scholar] [CrossRef][Green Version]
- Abraham, N.M.; Lamlertthon, S.; Fowler, V.G.; Jefferson, K.K. Chelating agents exert distinct effects on biofilm formation in Staphylococcus aureus depending on strain background: Role for clumping factor B. J. Med. Microbiol. 2012, 61, 1062–1070. [Google Scholar] [CrossRef]
- Opperman, T.J.; Kwasny, S.M.; Williams, J.D.; Khan, A.R.; Peet, N.P.; Moir, D.T.; Bowlin, T.L. Aryl rhodanines specifically inhibit staphylococcal and enterococcal biofilm formation. Antimicrob. Agents Chemother. 2009, 53, 4357–4367. [Google Scholar] [CrossRef]
- Kolodkin-Gal, I.; Romero, D.; Cao, S.; Clardy, J.; Kolter, R.; Losick, R. D-Amino acids trigger biofilm disassembly. Science 2010, 328, 627–629. [Google Scholar] [CrossRef]
- Blasi, F.; Page, C.; Rossolini, G.M.; Pallecchi, L.; Matera, M.G.; Rogliani, P.; Cazzola, M. The effect of N-acetylcysteine on biofilms: Implications for the treatment of respiratory tract infections. Respir. Med. 2016, 117, 190–197. [Google Scholar] [CrossRef]
- Balain, M.; Oddie, S.J.; Mcguire, W. Antimicrobial-impregnated central venous catheters for prevention of catheter-related bloodstream infection in newborn infants. Cochrane Database Syst. Rev. 2015, 27, CD011078. [Google Scholar] [CrossRef]
- Hoffman, L.R.; D’Argenio, D.A.; MacCoss, M.J.; Zhang, Z.; Jones, R.A.; Miller, S.I. Aminoglycoside antibiotics induce bacterial biofilm formation. Nature 2005, 436, 1171–1175. [Google Scholar] [CrossRef]
- Ahearn, D.G.; Grace, D.T.; Jennings, M.J.; Borazjani, R.N.; Boles, K.J.; Rose, L.J.; Simmons, R.B.; Ahanotu, E.N. Effects of hydrogel/silver coatings on in vitro adhesion to catheters of bacteria associated with urinary tract infections. Curr. Microbiol. 2000, 41, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Drews Junior, P.; Colares, R.G.; Machado, P.; de Faria, M.; Detoni, A.; Tavano, V. Microalgae classification using semi-supervised and active learning based on Gaussian mixture models. J. Braz. Comput. Soc. 2013, 19, 411–422. [Google Scholar] [CrossRef]
- Heimann, K.; Huerlimann, R. Microalgal Classification. In Handbook of Marine Microalgae, 1st ed.; Elsevier: San Diego, CA, USA, 2015; pp. 25–41. [Google Scholar]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2010, 27, 165–237. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, K.H.M.; Guaratini, T.; Barros, M.P.; Falcão, V.R.; Tonon, A.P.; Lopes, N.P.; Campos, S.; Torres, M.A.; Souza, A.O.; Colepicolo, P.; et al. Metabolites from algae with economical impact. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2007, 146, 60–78. [Google Scholar] [CrossRef]
- Venkatesan, J.; Manivasagan, P.; Kim, S.K. Marine Microalgae Biotechnology, In Handbook of Marine Microalgae, 1st ed.; Elsevier: San Diego, CA, USA, 2015; pp. 1–9. [Google Scholar]
- Mourelle, M.L.; Gómez, C.P.; Legido, J.L. The potential use of marine microalgae and cyanobacteria in cosmetics and thalassotherapy. Cosmetics 2017, 4, 46. [Google Scholar] [CrossRef]
- Jha, D.; Jain, V.; Sharma, B.; Kant, A.; Garlapati, V.K. Microalgae-based pharmaceuticals and nutraceuticals: An emerging field with immense market potential. ChemBioEng Rev. 2017, 4, 257–272. [Google Scholar] [CrossRef]
- Singh, R.; Parihar, P.; Singh, M.; Bajguz, A.; Kumar, J.; Singh, S.; Singh, V.P.; Prasad, S.M. Uncovering potential applications of cyanobacteria and algal metabolites in biology, agriculture and medicine: Current status and future prospects. Front. Microbiol. 2017, 8, 515. [Google Scholar] [CrossRef]
- De Jesus Raposo, M.F.; Bernardo De Morais, A.M.M.; Costa De Morais, R.M.S. Bioactivity and applications of polysaccharides from Marine microalgae. In Polysaccharides: Bioactivity and Biotechnology; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Gani, P.; Sunar, N.M.; Matias-Peralta, H.; Mohamed, R.M.S.R.; Latiff, A.A.A.; Parjo, U.K. Extraction of hydrocarbons from freshwater green microalgae (Botryococcus sp.) biomass after phycoremediation of domestic wastewater. Int. J. Phytoremediation 2017, 19, 679–685. [Google Scholar] [CrossRef]
- Ibañez, E.; Cifuentes, A. Benefits of using algae as natural sources of functional ingredients. J. Sci. Food Agric. 2013, 93, 703–709. [Google Scholar] [CrossRef]
- Tang, G.; Suter, P.M. Vitamin A, nutrition, and health values of Algae: Spirulina, chlorella, and dunaliella. J. Pharm. Nutr. Sci. 2011, 1, 111–118. [Google Scholar] [CrossRef]
- Jain, R.; Raghukumar, S.; Tharanathan, R.; Bhosle, N.B. Extracellular polysaccharide production by thraustochytrid protists. Mar. Biotechnol. 2005, 7, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Sathasivam, R.; Ki, J.S. A review of the biological activities of microalgal carotenoids and their potential use in healthcare and cosmetic industries. Mar. Drugs 2018, 16, 26. [Google Scholar] [CrossRef] [PubMed]
- Neumann, U.; Derwenskus, F.; Gille, A.; Louis, S.; Schmid-Staiger, U.; Briviba, K.; Bischoff, S.C. Bioavailability and safety of nutrients from the microalgae chlorella vulgaris, nannochloropsis oceanica and phaeodactylum tricornutum in C57BL/6 mice. Nutrients 2018, 10, 965. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Li, T.; Zhou, Z.; Jiang, Y. Biological Processes for Hydrogen Production. Adv. Biochem. Eng. Biotechnol. 2016, 153, 37–58. [Google Scholar]
- De Jesus Raposo, M.F.; De Morais, A.M.B.; De Morais, R.M.S.C. Marine polysaccharides from algae with potential biomedical applications. Mar. Drugs 2015, 13, 2967–3028. [Google Scholar] [CrossRef]
- Plaza, M.; Herrero, M.; Alejandro Cifuentes, A.; Ibáñez, E. Innovative natural functional ingredients from microalgae. J. Agric. Food Chem. 2009, 57, 7159–7170. [Google Scholar] [CrossRef]
- Gayatri, K.; Soundhari, C.; Pavithra, B.P. Biofilm inhibitory effect of Chlorella extracts on pseudomonas aeruginosa. Int. J. Pharm. Sci. Res. 2019, 10, 1966–1971. [Google Scholar]
- Xin, Y.; Lu, Y.; Lee, Y.Y.; Wei, L.; Jia, J.; Wang, Q.; Wang, D.; Bai, F.; Hu, H.; Hu, Q.; et al. Producing Designer Oils in Industrial Microalgae by Rational Modulation of Co-evolving Type-2 Diacylglycerol Acyltransferases. Mol. Plant 2017, 10, 1523–1539. [Google Scholar] [CrossRef]
- Bhattacharjee, M. Pharmaceutically valuable bioactive compounds of algae. Asian J. Pharm. Clin. Res. 2016, 9, 43–47. [Google Scholar] [CrossRef]
- Liu, J.; Sun, Z.; Gerken, H.; Liu, Z.; Jiang, Y.; Chen, F. Chlorella zofingiensis as an alternative microalgal producer of astaxanthin: Biology and industrial potential. Mar. Drugs 2014, 12, 3487–3515. [Google Scholar] [CrossRef]
- Molino, A.; Iovine, A.; Casella, P.; Mehariya, S.; Chianese, S.; Cerbone, A.; Rimauro, J.; Musmarra, D. Microalgae characterization for consolidated and new application in human food, animal feed and nutraceuticals. Int. J. Environ. Res. Public Health 2018, 15, 2436. [Google Scholar] [CrossRef] [PubMed]
- LewisOscar, F.; Nithya, C.; Bakkiyaraj, D.; Arunkumar, M.; Alharbi, N.S.; Thajuddin, N. Biofilm Inhibitory Effect of Spirulina platensis Extracts on Bacteria of Clinical Significance. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2017, 87, 537–544. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Shukla, S.; Kang, S.M.; Hwang, S.K.; Song, X.; Huh, Y.S.; Han, Y.K. Developments of cyanobacteria for nano-marine drugs: Relevance of nanoformulations in cancer therapies. Mar. Drugs 2018, 16, 179. [Google Scholar] [CrossRef] [PubMed]
- De Morais, M.G.; Vaz, B.D.S.; De Morais, E.G.; Costa, J.A.V. Biologically Active Metabolites Synthesized by Microalgae. BioMed Res. Int. 2015, 2015, 835761. [Google Scholar] [CrossRef]
- Michalak, I.; Chojnacka, K. Algae as production systems of bioactive compounds. Eng. Life Sci. 2015, 15, 160–176. [Google Scholar] [CrossRef]
- Liyanage, I.N.S.G. Unravelling Complex Interactions between Microalgae and Bacteria in Biofilms. Master’s Thesis, Universiteit Gent, Gent, Belgium, 2018. [Google Scholar]
- Adnan, M.; Alshammari, E.; Patel, M.; Ashraf, S.A.; Khan, S.; Hadi, S. Significance and potential of marine microbial natural bioactive compounds against biofilms/biofouling: Necessity for green chemistry. PeerJ 2018, 27, e5049. [Google Scholar] [CrossRef]
- Jeganathan, P.; Rajasekaran, K.M.; Devi, N.K.A.; Karuppusamy, S. Antimicrobial activity and Characterization of Marine bacteria. Indian J. Pharm. Biol. Res. 2013, 1, 38–44. [Google Scholar] [CrossRef]
- El-Gendy, M.M.A.; Shaaban, M.; El-Bondkly, A.M.; Shaaban, K.A. Bioactive benzopyrone derivatives from new recombinant fusant of marine Streptomyces. Appl. Biochem. Biotechnol. 2008, 150, 85–96. [Google Scholar] [CrossRef]
- Kumar Jha, R.; Zi-Rong, X. Biomedical Compounds from Marine organisms. Mar. Drugs 2004, 2, 123–146. [Google Scholar]
- Ratha, S.K.; Babu, S.; Renuka, N.; Prasanna, R.; Prasad, R.B.N.; Saxena, A.K. Exploring nutritional modes of cultivation for enhancing lipid accumulation in microalgae. J. Basic Microbiol. 2013, 53, 440–450. [Google Scholar] [CrossRef]
- Tsuda, Y. Isolation of Natural Products. In H.E.J. Research Institute of Chemistry; Printed by Japan Analytical Industry, Co.; Ltd.; Humana Press: Totowa, NJ, USA, 2004. [Google Scholar]
- Lauritano, C.; Andersen, J.H.; Hansen, E.; Albrigtsen, M.; Escalera, L.; Esposito, F.; Helland, K.; Hanssen, K.; Romano, G.; Ianora, A. Bioactivity screening of microalgae for antioxidant, anti-inflammatory, anticancer, anti-diabetes, and antibacterial activities. Front. Mar. Sci. 2016, 3, 1–2. [Google Scholar] [CrossRef]
- Cepas, V.; López, Y.; Gabasa, Y.; Martins, C.B.; Ferreira, J.D.; Correia, M.J.; Santos, L.M.A.; Oliveira, F.; Ramos, V.; Reis, M.; et al. Inhibition of Bacterial and Fungal Biofilm Formation by 675 Extracts from Microalgae and Cyanobacteria. Antibiotics 2019, 8, 77. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Dong, J.; Xia, Y.; Shu, R. Antibacterial activities of docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) against planktonic and biofilm growing Streptococcus mutans. Microb. Pathog. 2017, 107, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Zea-Obando, C.; Tunin-Ley, A.; Turquet, J.; Culioli, G.; Briand, J.F.; Bazire, A.; Réhel, K.; Faÿ, F.; Linossier, I. Anti-bacterial adhesion activity of tropical microalgae extracts. Molecules 2018, 23, 2180. [Google Scholar] [CrossRef]
- Krishnan, T.; Yin, W.F.; Chan, K.G. Inhibition of quorum sensing-controlled virulence factor production in Pseudomonas aeruginosa PAO1 by ayurveda spice clove (Syzygium aromaticum) bud extract. Sensors 2012, 12, 4016–4030. [Google Scholar] [CrossRef]
- Asfour, H. Anti-quorum sensing natural compounds. J. Microsc. Ultrastruct. 2018, 6, 1–10. [Google Scholar] [CrossRef]
- Papenfort, K.; Bassler, B.L. Quorum sensing signal-response systems in Gram-negative bacteria. Nat. Rev. Microbiol. 2016, 14, 576–588. [Google Scholar] [CrossRef]
- Zheng, L.; Oh, S.T.; Jeon, J.Y.; Moon, B.H.; Kwon, H.S.; Lim, S.U.; An, B.K.; Kang, C.W. The dietary effects of fermented Chlorella vulgaris CBT®on production performance, iver lipids and intestinal microflora in laying hens. Asian-Australas. J. Anim. Sci. 2012, 25, 261–266. [Google Scholar] [CrossRef]
- Sridevi, N.; Dhanusha, V.; Rajeswari, M.; Santhi, N. An in-vitro antibiofilm activity of Chlorella vulgaris. Asian J. Pharm. Clin. Res. 2019, 12, 239–242. [Google Scholar] [CrossRef]
- Caufield, P.W.; Li, Y.; Dasanayake, A. Dental caries: An infectious and transmissible disease. Compend. Contin. Educ. Dent. 2005, 26 (Suppl. S1), 10–16. [Google Scholar]
- Yoshida, A.; Kuramitsu, H.K. Multiple Streptococcus mutans genes are involved in biofilm formation. Appl. Environ. Microbiol. 2002, 26, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Jafari, S.; Mobasher, M.A.; Najafipour, S.; Ghasemi, Y.; Mohkam, M.; Ebrahimi, M.A.; Mobasher, N. Antibacterial potential of Chlorella vulgaris and Dunaliella salina extracts against Streptococcus mutans. Jundishapur J. Nat. Pharm. Prod. 2018, 13, e13226. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M.; Snader, K.M. Natural products as sources of new drugs over the period 1981–2002. J. Nat. Prod. 2003, 66, 1022–1037. [Google Scholar] [CrossRef] [PubMed]
- Boisseau, P.; Loubaton, B. Nanomedicine, nanotechnology in medicine. Comptes Rendus Phys. 2011, 12, 620–636. [Google Scholar] [CrossRef]
- Ramos, A.P.; Cruz, M.A.E.; Tovani, C.B.; Ciancaglini, P. Biomedical applications of nanotechnology. Biophys. Rev. 2017, 9, 79–89. [Google Scholar] [CrossRef]
- Massironi, A.; Morelli, A.; Grassi, L.; Puppi, D.; Braccini, S.; Maisetta, G.; Esin, S.; Batoni, G.; Della Pina, C.; Chiellini, F. Ulvan as novel reducing and stabilizing agent from renewable algal biomass: Application to green synthesis of silver nanoparticles. Carbohydr. Polym. 2019, 203, 310–321. [Google Scholar] [CrossRef]
- Adebayo-Tayo, B.; Salaam, A.; Ajibade, A. Green synthesis of silver nanoparticle using Oscillatoria sp. extract, its antibacterial, antibiofilm potential and cytotoxicity activity. Heliyon 2019, 5, e02502. [Google Scholar] [CrossRef]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef]
- Qing, Y.; Cheng, L.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int. J. Nanomed. 2018, 13, 3311–3327. [Google Scholar] [CrossRef]
- Vijayan, S.R.; Santhiyagu, P.; Singamuthu, M.; Kumari Ahila, N.; Jayaraman, R.; Ethiraj, K. Synthesis and characterization of silver and gold nanoparticles using aqueous extract of seaweed, turbinaria conoides, and their antimicrofouling activity. Sci. World J. 2014, 2014, 10. [Google Scholar] [CrossRef]
- Martinez-Gutierrez, F.; Boegli, L.; Agostinho, A.; Sánchez, E.M.; Bach, H.; Ruiz, F.; James, G. Anti-biofilm activity of silver nanoparticles against different microorganisms. Biofouling 2013, 29, 651–660. [Google Scholar] [CrossRef] [PubMed]



| Molecule | Mechanism | Effect | Ref. |
|---|---|---|---|
| Anti-virulence compounds | Inhibition of gene expression of virulence factors | Inhibition of biofilm formation by S. aureus | [19] |
| Anti-biofilm compounds | Unknown | Inhibition of biofilm formation by S. epidermidis | [20] |
| Acyl Homoserine Lactones (AHLs) | Autoinducers-QS | Inhibition of biofilm formation by P. aeruginosa | [21] |
| Autoinducing peptides (AIPs) | Signaling molecules | Inhibition of biofilm formation by S. aureus | [22] |
| ABC-1 | Inhibition of c-di-GMP-inducible transcription | Inhibition of biofilm formation by multiple Gram-negative and Gram-positive bacterial pathogens | [17] |
| Indole and derivatives | Oxidized indole metabolites | Inhibition of biofilm formation by E. coli, P. aeruginosa, Staphylococcal species | [23] |
| Carolacton | Affect the expression of two component signal transduction systems | Inhibition of biofilm formation by S. mutans | [24] |
| Chelators | Interference with metal ion’s function in biofilm formation | Inhibition of biofilm formation by S. aureus | [25] |
| Aryl rhodanines | Unknown | Inhibition of biofilm formation by S. aureus and S. epidermidis | [26] |
| Cis-2-decenoic acid | Unknown | Dispersion of biofilms by E. coli, K. pneumoniae, P. mirabilis, S. pyogenes, B. subtilis, S. aureus, and C. albicans | [18] |
| D-amino acids | Unknown | Inhibition of biofilm formation by S. aureus and P. aeruginosa | [27] |
| N-acetylcysteine | Interference with exopolysaccharide formation in biofilms | Inhibition of biofilm formation by S. epidermidis | [28] |
| Microalgae | Bioactive Compounds | Use | Ref. |
|---|---|---|---|
| Arthrospira platensis | Methanolic extracts of exopolysaccharides | Antioxidant | [40] |
| Botryococcus braunii | Linear alkadienes (C25, C27, C29, and C31), triene (C29) | Phycoremediation | [41] |
| Chlorella sp. | Carotenoids, sulfated polysaccharides, sterols, PUFAs (n-3) (a) fatty acids, chlorophyll | Moisturizing and thickener agent, dentifrices and deodorants, antimicrobial, antibiofilm | [42,43,44] |
| Chlorella ellipsoidea | Zeaxanthin (b), violaxanthin | Health and cosmetic as UV protection, antioxidant and antibiofilm | [45] |
| Chlorella minutissima | Eicosapentaenoic acid (EPA) | Food supplements | [46] |
| Chlorella protothecoides | Lutein, zeaxanthin, canthaxanthin | Health and cosmetic as UV protection, antioxidant | [47,48] |
| Chlorella pyrenoidosa | Lutein, sulfated polysaccharide | Health and cosmetic as UV protection, antioxidant | [47,49] |
| Chlorella vulgaris | Canthaxanthin, astaxanthin, peptide, oleic acid, Diethyl phthalate (c), trimethyl (4-tertbutyl phenoxy) silane (d), chlorella vulgaris extracts | Antioxidant, antimicrobial, antibiofilm, anti-ageing | [49,50,51] |
| Chlorella zofingiensis | Astaxanthin | Health and cosmetic as UV protection, antioxidant | [51] |
| Dunaliella salina | Trans-betacarotene, cis-betacarotene, β-carotene, oleic acid, linolenic (e) acid, palmitic acid (f), β-Cryptoxanthin and glucosyltransferases (GTF) (g) | Health and cosmetic as UV protection. Anti-inflammator, antibacterial and antibiofilm. | [43,52] |
| Dunaliella sp | Diacylglycerols | Acylation stimulating protein | [53] |
| Haematococcus pluvialis | Astaxanthin, lutein, zeaxanthin, canthaxanthin, lutein, β-carotene, oleic acid | Health and cosmetic as UV protection, antioxidant | [54] |
| Oscillatoria sp | Oscillatoria sp. extract | Antioxidant, antimicrobial, antibiofilm | [51] |
| Spirulina sp | Polysaccharides | Food and in cosmetics | [37] |
| Spirulina platensis | Phycocyanin, C-phycocyanin, phenolic acids, tocopherols (vitamin E), neophytadiene, phytol, PUFAs (n-3) fatty acids, oleic acid, linolenic acid, palmitoleic acid | Food, health and cosmetics, antimicrobial, antibiofilm | [55] |
| Spirulina fusiformis | Diacylglycerols | Acylation stimulating protein | [51] |
| Nostoclinckia/Nostocspongiaeforme | Borophycin, cryptophycin | Anti-tumor compounds | [56] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López, Y.; Soto, S.M. The Usefulness of Microalgae Compounds for Preventing Biofilm Infections. Antibiotics 2020, 9, 9. https://doi.org/10.3390/antibiotics9010009
López Y, Soto SM. The Usefulness of Microalgae Compounds for Preventing Biofilm Infections. Antibiotics. 2020; 9(1):9. https://doi.org/10.3390/antibiotics9010009
Chicago/Turabian StyleLópez, Yuly, and Sara M. Soto. 2020. "The Usefulness of Microalgae Compounds for Preventing Biofilm Infections" Antibiotics 9, no. 1: 9. https://doi.org/10.3390/antibiotics9010009
APA StyleLópez, Y., & Soto, S. M. (2020). The Usefulness of Microalgae Compounds for Preventing Biofilm Infections. Antibiotics, 9(1), 9. https://doi.org/10.3390/antibiotics9010009





