Development and Challenges of Antimicrobial Peptides for Therapeutic Applications
Abstract
1. Introduction
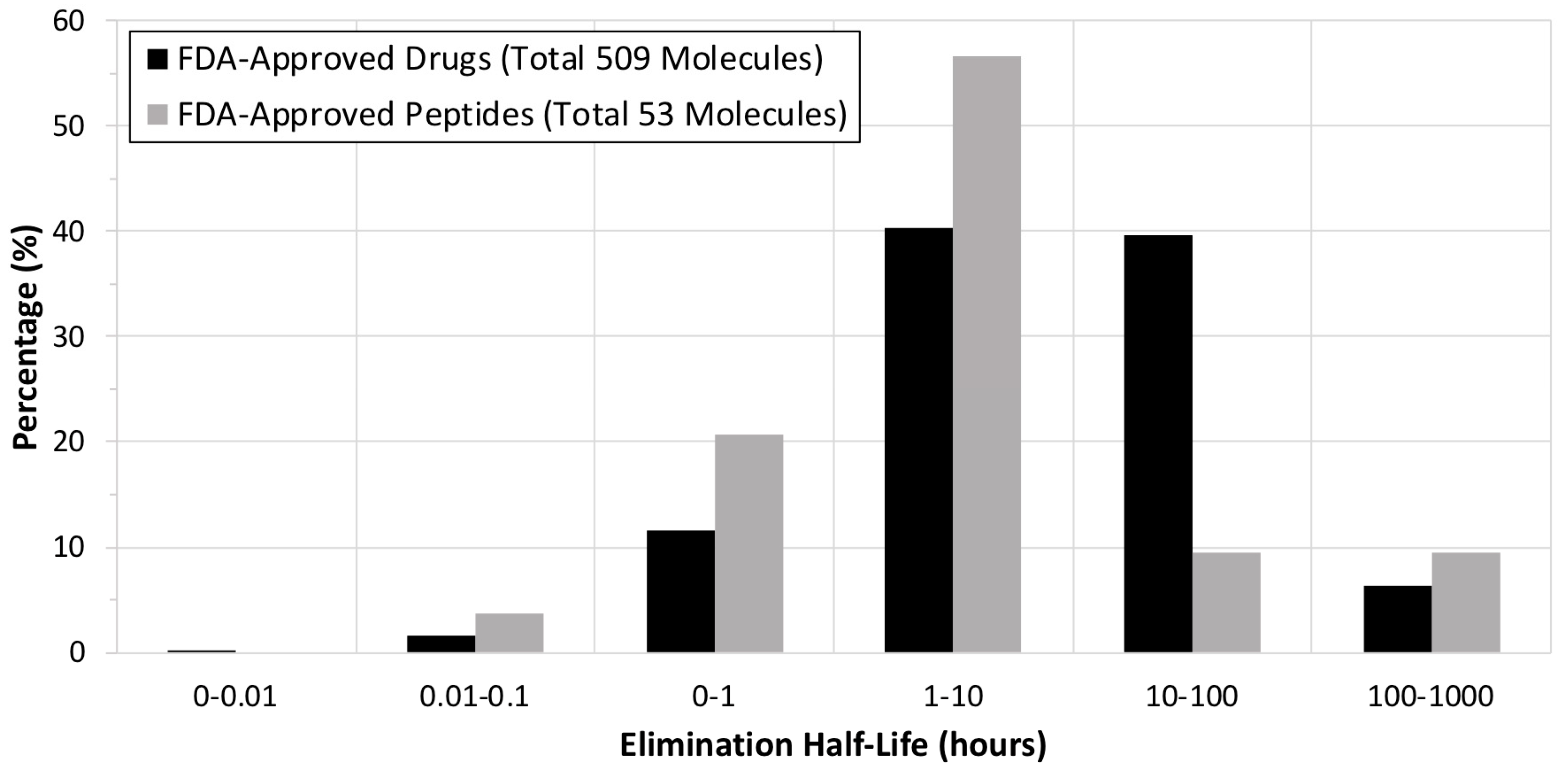
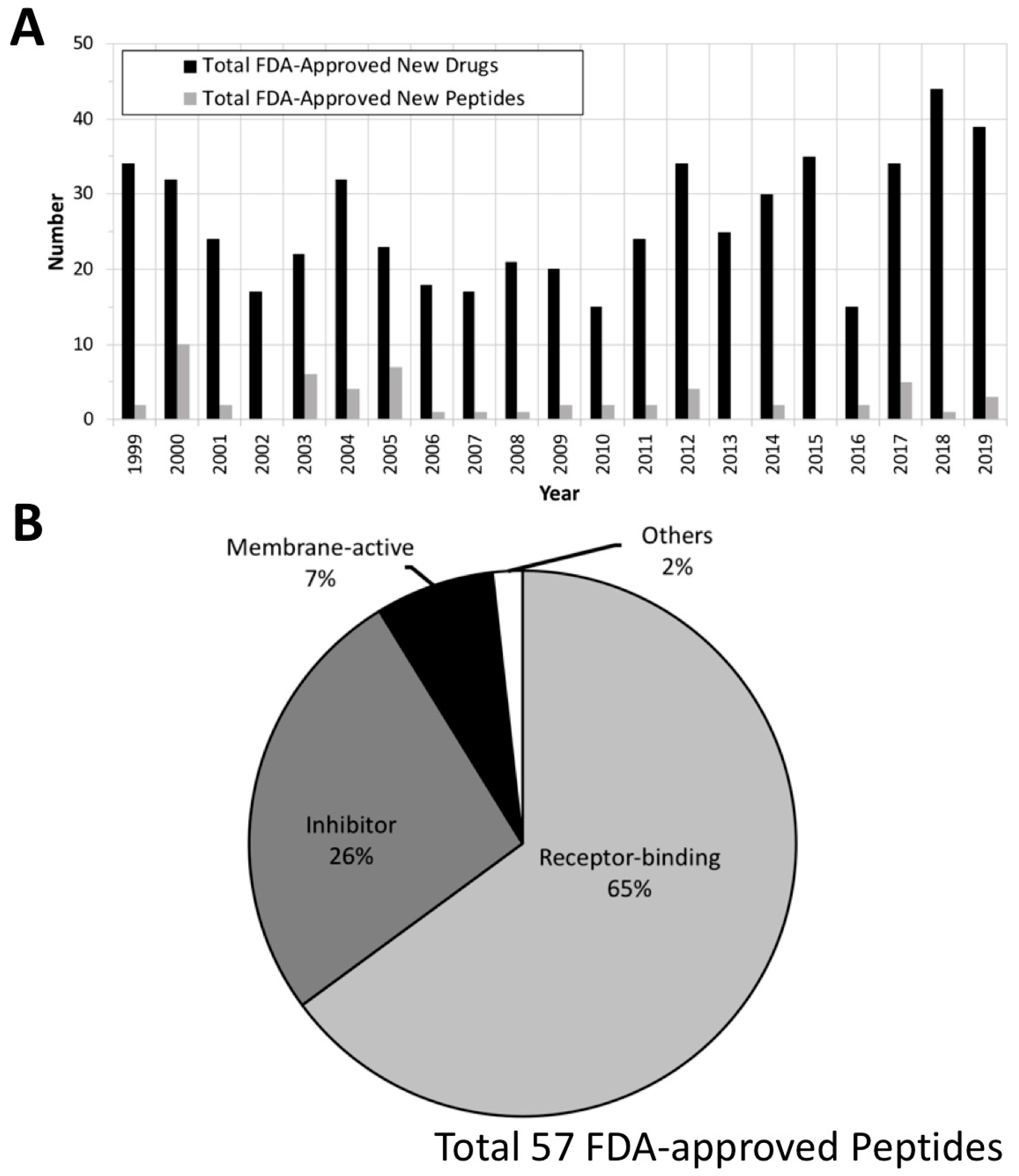
2. FDA Drug Approvals and Databases
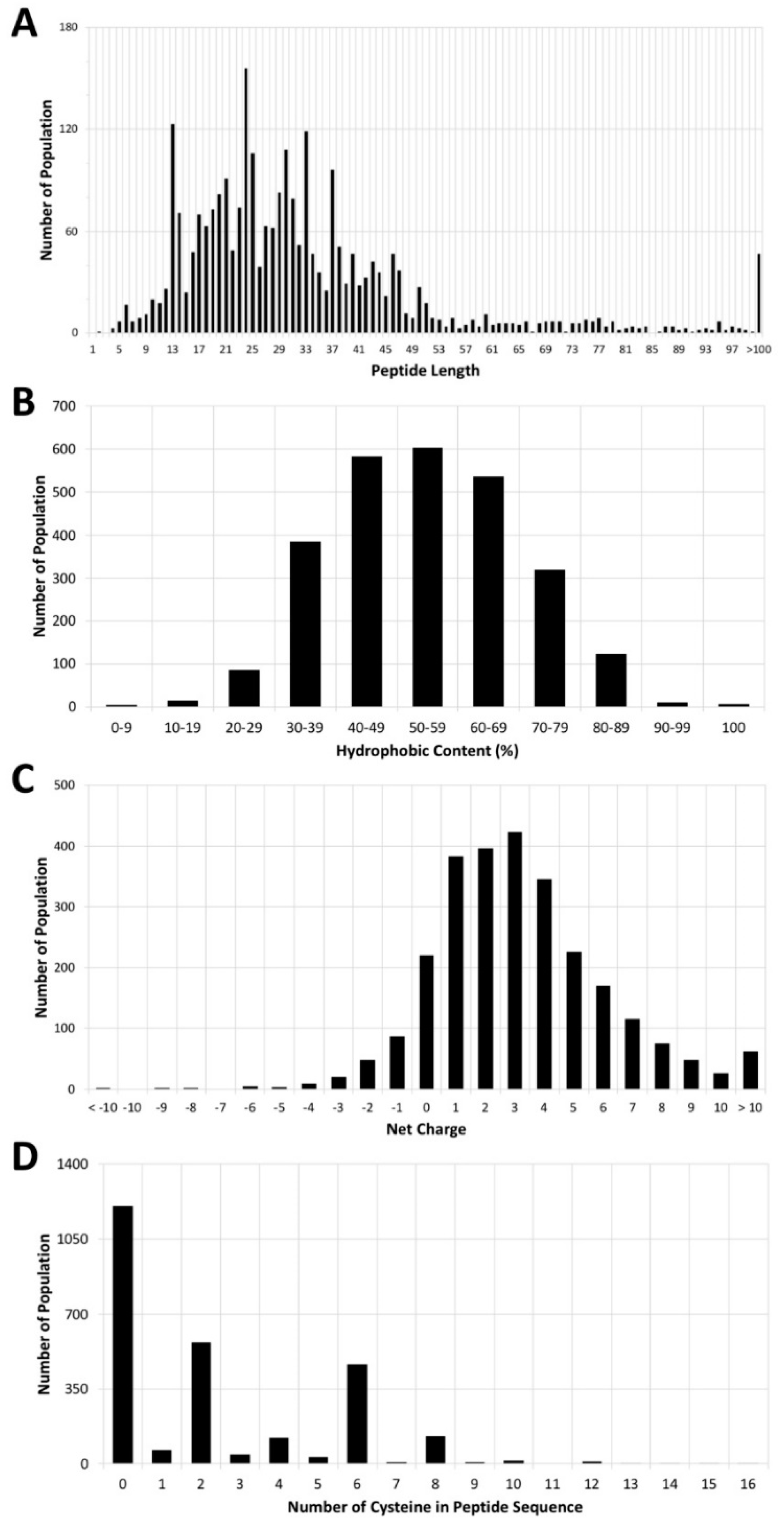
3. Antimicrobial Peptide Database
4. Current Development of Peptide Drugs
4.1. Receptor-Binding Peptides
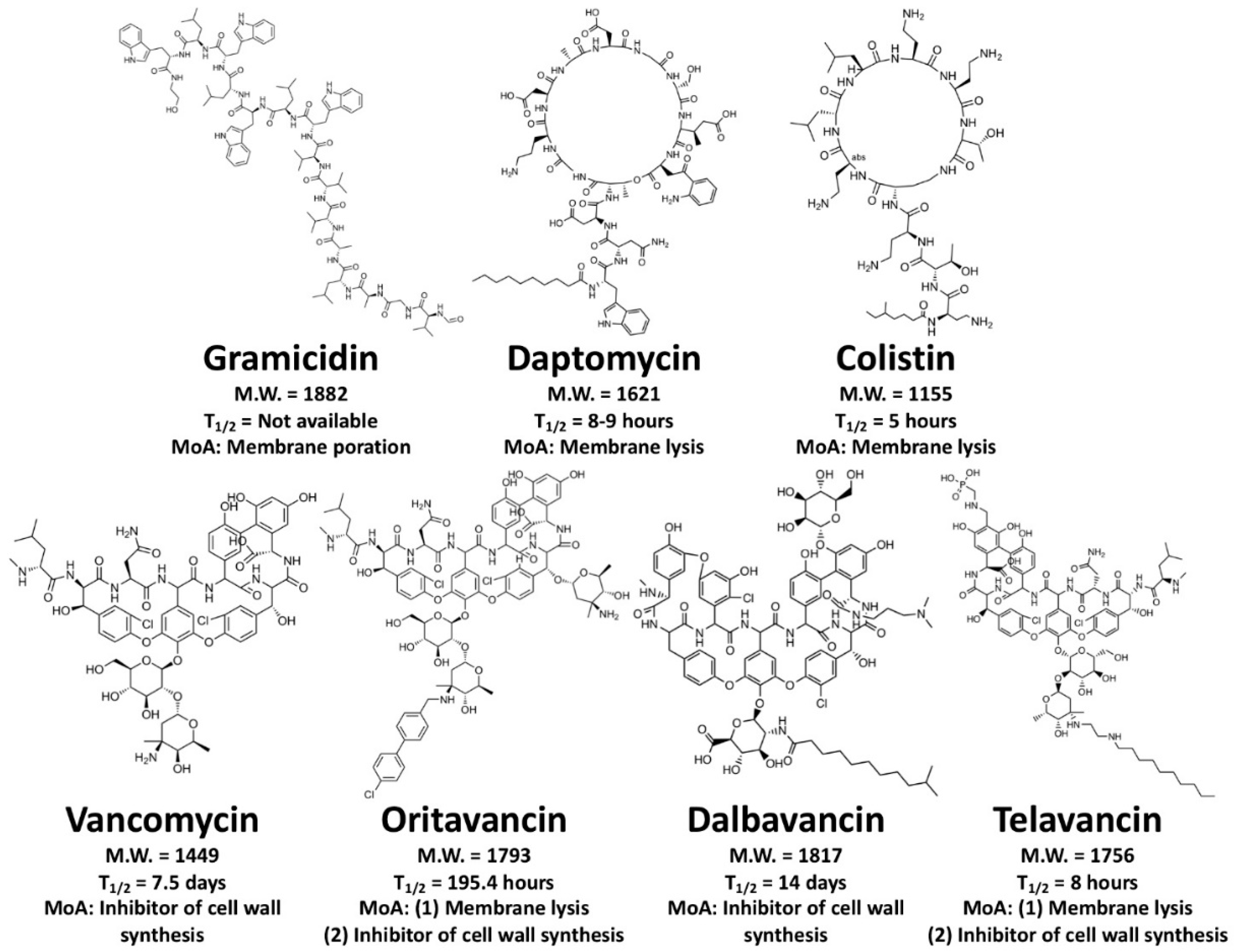
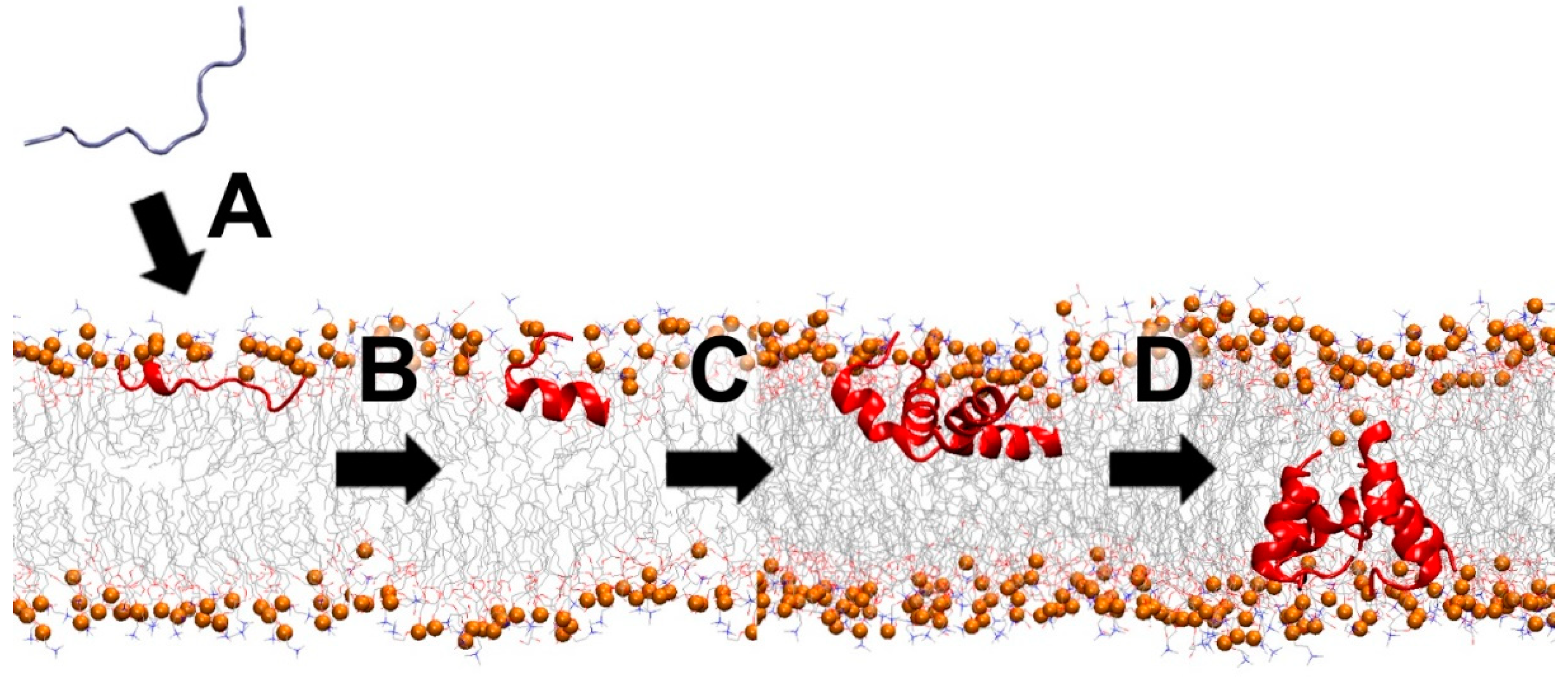
4.2. Membrane-Active Peptides (MAPs)
4.3. Cell Wall-Inhibiting Peptides
4.4. Peptides Having Other Inhibitory Mechanisms
5. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gold, H.S.; Moellering, R.C. Antimicrobial-drug resistance. N. Engl. J. Med. 1996, 335, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Willyard, C. The drug-resistant bacteria that pose the greatest health threats. Nature 2017, 543, 15. [Google Scholar] [CrossRef] [PubMed]
- Alghoribi, M.F.; Gibreel, T.M.; Farnham, G.; Al Johani, S.M.; Balkhy, H.H.; Upton, M. Antibiotic-resistant ST38, ST131 and ST405 strains are the leading uropathogenic Escherichia coli clones in Riyadh, Saudi Arabia. J. Antimicrob. Chemother. 2015, 70, 2757–2762. [Google Scholar] [CrossRef] [PubMed]
- Petty, N.K.; Ben Zakour, N.L.; Stanton-Cook, M.; Skippington, E.; Totsika, M.; Forde, B.M.; Phan, M.D.; Gomes Moriel, D.; Peters, K.M.; Davies, M.; et al. Global dissemination of a multidrug resistant Escherichia coli clone. Proc. Natl. Acad. Sci. USA 2014, 111, 5694–5699. [Google Scholar] [CrossRef]
- Silver, L.L. Challenges of antibacterial discovery. Clin. Microbiol. Rev. 2011, 24, 71–109. [Google Scholar] [CrossRef]
- Conly, J.; Johnston, B. Where are all the new antibiotics? The new antibiotic paradox. Can. J. Infect. Dis. Med. Microbiol. 2005, 16, 159–160. [Google Scholar] [CrossRef]
- Hofer, U. The cost of antimicrobial resistance. Nat. Rev. Microbiol. 2019, 17, 3. [Google Scholar] [CrossRef]
- Collignon, P.; Beggs, J.J.; Walsh, T.R.; Gandra, S.; Laxminarayan, R. Anthropological and socioeconomic factors contributing to global antimicrobial resistance: A univariate and multivariable analysis. Lancet Planet Health 2018, 2, e398–e405. [Google Scholar] [CrossRef]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef]
- Wimley, W.C.; Hristova, K. Antimicrobial peptides: Successes, challenges and unanswered questions. J. Membr. Biol. 2011, 239, 27–34. [Google Scholar] [CrossRef]
- Torres, M.D.T.; Sothiselvam, S.; Lu, T.K.; de la Fuente-Nunez, C. Peptide design principles for antimicrobial applications. J. Mol. Biol. 2019, 431, 3547–3567. [Google Scholar] [CrossRef]
- Upton, M.; Cotter, P.; Tagg, J. Antimicrobial peptides as therapeutic agents. Int. J. Microbiol. 2012, 2012, 326503. [Google Scholar] [CrossRef]
- Wang, G.; Li, X.; Wang, Z. APD3: The antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2016, 44, D1087–D1093. [Google Scholar] [CrossRef]
- Gordon, Y.J.; Romanowski, E.G.; McDermott, A.M. A review of antimicrobial peptides and their therapeutic potential as anti-infective drugs. Curr. Eye Res. 2005, 30, 505–515. [Google Scholar] [CrossRef]
- Usmani, S.S.; Bedi, G.; Samuel, J.S.; Singh, S.; Kalra, S.; Kumar, P.; Ahuja, A.A.; Sharma, M.; Gautam, A.; Raghava, G.P.S. THPdb: Database of FDA-approved peptide and protein therapeutics. PLoS ONE 2017, 12, e0181748. [Google Scholar] [CrossRef]
- Wallace, B.A. Gramicidin channels and pores. Annu. Rev. Biophys. Biophys. Chem. 1990, 19, 127–157. [Google Scholar] [CrossRef]
- Hotchkiss, R.D.; Dubos, R.J. The isolation of bactericidal substances from cultures of bacillus brevis. J. Biol. Chem. 1941, 141, 155–162. [Google Scholar]
- Lipmann, F.; Hotchkiss, R.D.; Dubos, R.J. The occurrence of d-amino acids in gramicidin and tyrocidine. J. Biol. Chem. 1941, 141, 163–170. [Google Scholar]
- Hotchkiss, R.D. The chemical nature of gramicidin and tyrocidine. J. Biol. Chem. 1941, 141, 171–186. [Google Scholar]
- Christensen, H.N.; Edwards, R.R.; Piersma, H.D. The composition of gramicidin and tyrocidine. J. Biol. Chem. 1941, 141, 187–196. [Google Scholar]
- Tishler, M.; Stokes, J.L.; Trenner, N.R.; Conn, J.B. Some properties of gramicidin. J. Biol. Chem. 1941, 141, 197–206. [Google Scholar]
- Hallett, J.W.; Wolkowicz, M.I.; Leopold, I.H. Ophthalmic use of neosporin. Am. J. Ophthalmol. 1956, 41, 850–853. [Google Scholar] [CrossRef]
- Lee, M.T.; Yang, P.Y.; Charron, N.E.; Hsieh, M.H.; Chang, Y.Y.; Huang, H.W. Comparison of the effects of daptomycin on bacterial and model membranes. Biochemistry 2018, 57, 5629–5639. [Google Scholar] [CrossRef]
- Kreutzberger, M.A.; Pokorny, A.; Almeida, P.F. Daptomycin-phosphatidylglycerol domains in lipid membranes. Langmuir 2017, 33, 13669–13679. [Google Scholar] [CrossRef]
- Eliopoulos, G.M.; Willey, S.; Reiszner, E.; Spitzer, P.G.; Caputo, G.; Moellering, R.C. In vitro and in vivo activity of LY 146032, a new cyclic lipopeptide antibiotic. Antimicrob. Agents Chemother. 1986, 30, 532–535. [Google Scholar] [CrossRef]
- Carpenter, C.F.; Chambers, H.F. Daptomycin: Another novel agent for treating infections due to drug-resistant gram-positive pathogens. Clin. Infect. Dis. 2004, 38, 994–1000. [Google Scholar] [CrossRef]
- Chen, A.Y.; Zervos, M.J.; Vazquez, J.A. Dalbavancin: A novel antimicrobial. Int. J. Clin. Pract. 2007, 61, 853–863. [Google Scholar] [CrossRef]
- Zhanel, G.G.; Calic, D.; Schweizer, F.; Zelenitsky, S.; Adam, H.; Lagacé-Wiens, P.R.; Rubinstein, E.; Gin, A.S.; Hoban, D.J.; Karlowsky, J.A. New lipoglycopeptides: A comparative review of dalbavancin, oritavancin and telavancin. Drugs 2010, 70, 859–886. [Google Scholar] [CrossRef]
- Saravolatz, L.D.; Stein, G.E.; Johnson, L.B. Telavancin: A novel lipoglycopeptide. Clin. Infect. Dis. 2009, 49, 1908–1914. [Google Scholar] [CrossRef]
- Higgins, D.L.; Chang, R.; Debabov, D.V.; Leung, J.; Wu, T.; Krause, K.M.; Sandvik, E.; Hubbard, J.M.; Kaniga, K.; Schmidt, D.E.; et al. Telavancin, a multifunctional lipoglycopeptide, disrupts both cell wall synthesis and cell membrane integrity in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2005, 49, 1127–1134. [Google Scholar] [CrossRef]
- Hamamoto, K.; Kida, Y.; Zhang, Y.; Shimizu, T.; Kuwano, K. Antimicrobial activity and stability to proteolysis of small linear cationic peptides with D-amino acid substitutions. Microbiol. Immunol. 2002, 46, 741–749. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Chau, J.K.; Perry, N.A.; de Boer, L.; Zaat, S.A.; Vogel, H.J. Serum stabilities of short tryptophan- and arginine-rich antimicrobial peptide analogs. PLoS ONE 2010, 5, e12684. [Google Scholar] [CrossRef]
- Wang, J.; Yadav, V.; Smart, A.L.; Tajiri, S.; Basit, A.W. Toward oral delivery of biopharmaceuticals: An assessment of the gastrointestinal stability of 17 peptide drugs. Mol. Pharm. 2015, 12, 966–973. [Google Scholar] [CrossRef]
- Wang, J.; Yadav, V.; Smart, A.L.; Tajiri, S.; Basit, A.W. Stability of peptide drugs in the colon. Eur. J. Pharm. Sci. 2015, 78, 31–36. [Google Scholar] [CrossRef]
- Johnson, I.S. Human insulin from recombinant DNA technology. Science 1983, 219, 632–637. [Google Scholar] [CrossRef]
- Duckworth, W.C.; Bennett, R.G.; Hamel, F.G. Insulin degradation: Progress and potential. Endocr. Rev. 1998, 19, 608–624. [Google Scholar]
- Zorzi, A.; Deyle, K.; Heinis, C. Cyclic peptide therapeutics: Past, present and future. Curr. Opin. Chem. Biol. 2017, 38, 24–29. [Google Scholar] [CrossRef]
- Lau, J.L.; Dunn, M.K. Therapeutic peptides: Historical perspectives, current development trends, and future directions. Bioorg. Med. Chem. 2018, 26, 2700–2707. [Google Scholar] [CrossRef]
- Lee, A.C.; Harris, J.L.; Khanna, K.K.; Hong, J.H. A comprehensive review on current advances in peptide drug development and design. Int. J. Mol. Sci. 2019, 20, 2383. [Google Scholar] [CrossRef]
- Bogdanowich-Knipp, S.J.; Chakrabarti, S.; Williams, T.D.; Dillman, R.K.; Siahaan, T.J. Solution stability of linear vs. cyclic RGD peptides. J. Pept. Res. 1999, 53, 530–541. [Google Scholar] [CrossRef]
- Rink, R.; Arkema-Meter, A.; Baudoin, I.; Post, E.; Kuipers, A.; Nelemans, S.A.; Akanbi, M.H.; Moll, G.N. To protect peptide pharmaceuticals against peptidases. J. Pharmacol. Toxicol. Methods 2010, 61, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Guha, S.; Ghimire, J.; Wu, E.; Wimley, W.C. Mechanistic landscape of membrane-permeabilizing peptides. Chem Rev. 2019, 119, 6040–6085. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Hofferek, V.; Separovic, F.; Reid, G.E.; Aguilar, M.I. The role of bacterial lipid diversity and membrane properties in modulating antimicrobial peptide activity and drug resistance. Curr. Opin. Chem. Biol. 2019, 52, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Starr, C.G.; Troendle, E.; Wiedman, G.; Wimley, W.C.; Ulmschneider, J.P.; Ulmschneider, M.B. Simulation-guided rational de novo design of a small pore-forming antimicrobial peptide. J. Am. Chem. Soc. 2019, 141, 4839–4848. [Google Scholar] [CrossRef] [PubMed]
- Hollmann, A.; Martinez, M.; Maturana, P.; Semorile, L.C.; Maffia, P.C. Antimicrobial peptides: Interaction with model and biological membranes and synergism with chemical antibiotics. Front. Chem. 2018, 6, 204. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, K. Control of cell selectivity of antimicrobial peptides. Biochim. Biophys. Acta 2009, 1788, 1687–1692. [Google Scholar] [CrossRef]
- Matsuzaki, K. Why and how are peptide-lipid interactions utilized for self-defense? Magainins and tachyplesins as archetypes. Biochim. Biophys. Acta 1999, 1462, 1–10. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Sugishita, K.; Harada, M.; Fujii, N.; Miyajima, K. Interactions of an antimicrobial peptide, magainin 2, with outer and inner membranes of Gram-negative bacteria. Biochim. Biophys. Acta 1997, 1327, 119–130. [Google Scholar] [CrossRef]
- Henderson, J.M.; Iyengar, N.S.; Lam, K.L.H.; Maldonado, E.; Suwatthee, T.; Roy, I.; Waring, A.J.; Lee, K.Y.C. Beyond electrostatics: Antimicrobial peptide selectivity and the influence of cholesterol-mediated fluidity and lipid chain length on protegrin-1 activity. Biochim. Biophys. Acta Biomembr. 2019, 1861, 182977. [Google Scholar] [CrossRef] [PubMed]
- Law, V.; Knox, C.; Djoumbou, Y.; Jewison, T.; Guo, A.C.; Liu, Y.; Maciejewski, A.; Arndt, D.; Wilson, M.; Neveu, V.; et al. DrugBank 4.0: Shedding new light on drug metabolism. Nucleic Acids Res. 2014, 42, D1091–D1097. [Google Scholar] [CrossRef] [PubMed]
- Mahlapuu, M.; Håkansson, J.; Ringstad, L.; Björn, C. Antimicrobial peptides: An emerging category of therapeutic agents. Front. Cell. Infect. Microbiol. 2016, 6, 194. [Google Scholar] [CrossRef] [PubMed]
- Klein, T.; Henn, C.; de Jong, J.C.; Zimmer, C.; Kirsch, B.; Maurer, C.K.; Pistorius, D.; Müller, R.; Steinbach, A.; Hartmann, R.W. Identification of small-molecule antagonists of the Pseudomonas aeruginosa transcriptional regulator PqsR: Biophysically guided hit discovery and optimization. ACS Chem. Biol. 2012, 7, 1496–1501. [Google Scholar] [CrossRef]
- Worthington, R.J.; Blackledge, M.S.; Melander, C. Small-molecule inhibition of bacterial two-component systems to combat antibiotic resistance and virulence. Future Med. Chem. 2013, 5, 1265–1284. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Smith, C.; Yin, H. Targeting Toll-like receptors with small molecule agents. Chem. Soc. Rev. 2013, 42, 4859–4866. [Google Scholar] [CrossRef] [PubMed]
- Easton, D.M.; Nijnik, A.; Mayer, M.L.; Hancock, R.E. Potential of immunomodulatory host defense peptides as novel anti-infectives. Trends Biotechnol. 2009, 27, 582–590. [Google Scholar] [CrossRef]
- Haney, E.F.; Hancock, R.E. Peptide design for antimicrobial and immunomodulatory applications. Biopolymers 2013, 100, 572–583. [Google Scholar] [CrossRef] [PubMed]
- Nijnik, A.; Hancock, R. Host defence peptides: Antimicrobial and immunomodulatory activity and potential applications for tackling antibiotic-resistant infections. Emerg. Health Threats J. 2009, 2, e1. [Google Scholar]
- Lee, E.Y.; Lee, M.W.; Wong, G.C.L. Modulation of toll-like receptor signaling by antimicrobial peptides. Semin. Cell Dev. Biol. 2019, 88, 173–184. [Google Scholar] [CrossRef]
- Van Harten, R.M.; van Woudenbergh, E.; van Dijk, A.; Haagsman, H.P. Cathelicidins: Immunomodulatory antimicrobials. Vaccines 2018, 6, 63. [Google Scholar] [CrossRef]
- Takahashi, T.; Kulkarni, N.N.; Lee, E.Y.; Zhang, L.J.; Wong, G.C.L.; Gallo, R.L. Cathelicidin promotes inflammation by enabling binding of self-RNA to cell surface scavenger receptors. Sci. Rep. 2018, 8, 4032. [Google Scholar] [CrossRef]
- Grönberg, A.; Mahlapuu, M.; Ståhle, M.; Whately-Smith, C.; Rollman, O. Treatment with LL-37 is safe and effective in enhancing healing of hard-to-hear venous leg ulcers: A randomized, placebo-controlled clinical trial. Wound Repair Regen. 2014, 22, 613–621. [Google Scholar]
- Kelkar, D.A.; Chattopadhyay, A. The gramicidin ion channel: A model membrane protein. Biochim. Biophys. Acta 2007, 1768, 2011–2025. [Google Scholar] [CrossRef] [PubMed]
- Seydlová, G.; Sokol, A.; Lišková, P.; Konopásek, I.; Fišer, R. Daptomycin pore formation and stoichiometry depend on membrane potential of target membrane. Antimicrob. Agents Chemother. 2019, 63, e01589-18. [Google Scholar] [CrossRef] [PubMed]
- Barboiu, M.; Le Duc, Y.; Gilles, A.; Cazade, P.A.; Michau, M.; Marie Legrand, Y.; van der Lee, A.; Coasne, B.; Parvizi, P.; Post, J.; et al. An artificial primitive mimic of the Gramicidin-A channel. Nat. Commun. 2014, 5, 4142. [Google Scholar] [CrossRef]
- Zhanel, G.G.; Trapp, S.; Gin, A.S.; DeCorby, M.; Lagacé-Wiens, P.R.; Rubinstein, E.; Hoban, D.J.; Karlowsky, J.A. Dalbavancin and telavancin: Novel lipoglycopeptides for the treatment of Gram-positive infections. Expert Rev. Anti-Infect. Ther. 2008, 6, 67–81. [Google Scholar] [CrossRef]
- Velkov, T.; Thompson, P.E.; Nation, R.L.; Li, J. Structure—Activity relationships of polymyxin antibiotics. J. Med. Chem. 2010, 53, 1898–1916. [Google Scholar] [CrossRef]
- Mohamed, Y.F.; Abou-Shleib, H.M.; Khalil, A.M.; El-Guink, N.M.; El-Nakeeb, M.A. Membrane permeabilization of colistin toward pan-drug resistant Gram-negative isolates. Braz. J. Microbiol. 2016, 47, 381–388. [Google Scholar] [CrossRef]
- Huang, H.W. Deformation free energy of bilayer membrane and its effect on gramicidin channel lifetime. Biophys. J. 1986, 50, 1061–1070. [Google Scholar] [CrossRef]
- Raghuraman, H.; Chattopadhyay, A. Melittin: A membrane-active peptide with diverse functions. Biosci. Rep. 2007, 27, 189–223. [Google Scholar] [CrossRef]
- Leveritt, J.M.; Pino-Angeles, A.; Lazaridis, T. The structure of a melittin-stabilized pore. Biophys. J. 2015, 108, 2424–2426. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, C.H.; Hu, D.; Ulmschneider, M.B.; Ulmschneider, J.P. Spontaneous formation of structurally diverse membrane channel architectures from a single antimicrobial peptide. Nat. Commun. 2016, 7, 13535. [Google Scholar] [CrossRef] [PubMed]
- Fox, R.O.; Richards, F.M. A voltage-gated ion channel model inferred from the crystal structure of alamethicin at 1.5-A resolution. Nature 1982, 300, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Harroun, T.A.; Weiss, T.M.; Ding, L.; Huang, H.W. Barrel-stave model or toroidal model? A case study on melittin pores. Biophys. J. 2001, 81, 1475–1485. [Google Scholar] [CrossRef]
- Wiedman, G.; Fuselier, T.; He, J.; Searson, P.C.; Hristova, K.; Wimley, W.C. Highly efficient macromolecule-sized poration of lipid bilayers by a synthetically evolved peptide. J. Am. Chem. Soc. 2014, 136, 4724–4731. [Google Scholar] [CrossRef]
- Li, S.; Kim, S.Y.; Pittman, A.E.; King, G.M.; Wimley, W.C.; Hristova, K. Potent macromolecule-sized poration of lipid bilayers by the macrolittins, a synthetically evolved family of pore-forming peptides. J. Am. Chem. Soc. 2018, 140, 6441–6447. [Google Scholar] [CrossRef]
- Wiedman, G.; Herman, K.; Searson, P.; Wimley, W.C.; Hristova, K. The electrical response of bilayers to the bee venom toxin melittin: Evidence for transient bilayer permeabilization. Biochim. Biophys. Acta 2013, 1828, 1357–1364. [Google Scholar] [CrossRef]
- Harriss, L.M.; Cronin, B.; Thompson, J.R.; Wallace, M.I. Imaging multiple conductance states in an alamethicin pore. J. Am. Chem. Soc. 2011, 133, 14507–14509. [Google Scholar] [CrossRef]
- LaPlante, K.L.; Rybak, M.J. Daptomycin—A novel antibiotic against Gram-positive pathogens. Expert Opin. Pharmacother. 2004, 5, 2321–2331. [Google Scholar] [CrossRef]
- Alder, J.D. Daptomycin: A new drug class for the treatment of Gram-positive infections. Drugs Today 2015, 41, 81–90. [Google Scholar] [CrossRef]
- Neville, F.; Cahuzac, M.; Konovalov, O.; Ishitsuka, Y.; Lee, K.Y.; Kuzmenko, I.; Kale, G.M.; Gidalevitz, D. Lipid headgroup discrimination by antimicrobial peptide LL-37: Insight into mechanism of action. Biophys. J. 2006, 90, 1275–1287. [Google Scholar] [CrossRef]
- Fernandez, D.I.; Le Brun, A.P.; Whitwell, T.C.; Sani, M.A.; James, M.; Separovic, F. The antimicrobial peptide aurein 1.2 disrupts model membranes via the carpet mechanism. Phys. Chem. Chem. Phys. 2012, 14, 15739–15751. [Google Scholar] [CrossRef] [PubMed]
- Ambroggio, E.E.; Separovic, F.; Bowie, J.H.; Fidelio, G.D.; Bagatolli, L.A. Direct visualization of membrane leakage induced by the antibiotic peptides: Maculatin, citropin, and aurein. Biophys. J. 2005, 89, 1874–1881. [Google Scholar] [CrossRef] [PubMed]
- Perrin, B.S.; Fu, R.; Cotton, M.L.; Pastor, R.W. Simulations of membrane-disrupting peptides II: AMP piscidin 1 favors surface defects over pores. Biophys. J. 2016, 111, 1258–1266. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.C.; Lee, S.H.; Hour, A.L.; Pan, C.Y.; Lee, L.H.; Chen, J.Y. Five different piscidins from Nile tilapia, Oreochromis niloticus: Analysis of their expressions and biological functions. PLoS ONE 2012, 7, e50263. [Google Scholar] [CrossRef]
- Ladokhin, A.S.; White, S.H. ‘Detergent-like’ permeabilization of anionic lipid vesicles by melittin. Biochim. Biophys. Acta 2001, 1514, 253–260. [Google Scholar] [CrossRef]
- Torres, M.D.T.; Pedron, C.N.; Higashikuni, Y.; Kramer, R.M.; Cardoso, M.H.; Oshiro, K.G.N.; Franco, O.L.; Silva, P.I., Jr.; Silva, F.D.; Oliveira, V.X., Jr.; et al. Structure-function-guided exploration of the antimicrobial peptide polybia-CP identifies activity determinants and generates synthetic therapeutic candidates. Commun. Biol. 2018, 1, 221. [Google Scholar] [CrossRef]
- Walther, T.H.; Ulrich, A.S. Transmembrane helix assembly and the role of salt bridges. Curr. Opin. Struct. Biol. 2014, 27, 63–68. [Google Scholar] [CrossRef]
- Ulmschneider, M.B.; Ulmschneider, J.P.; Schiller, N.; Wallace, B.A.; von Heijne, G.; White, S.H. Spontaneous transmembrane helix insertion thermodynamically mimics translocon-guided insertion. Nat. Commun. 2014, 5, 4863. [Google Scholar] [CrossRef]
- Pino-Angeles, A.; Lazaridis, T. Effects of peptide charge, orientation, and concentration on melittin transmembrane pores. Biophys. J. 2018, 114, 2865–2874. [Google Scholar] [CrossRef]
- Walker, L.R.; Marzluff, E.M.; Townsend, J.A.; Resager, W.C.; Marty, M.T. Native mass spectrometry of antimicrobial peptides in lipid nanodiscs elucidates complex assembly. Anal. Chem. 2019, 91, 9284–9291. [Google Scholar] [CrossRef]
- Epand, R.F.; Savage, P.B.; Epand, R.M. Bacterial lipid composition and the antimicrobial efficacy of cationic steroid compounds (Ceragenins). Biochim. Biophys. Acta 2007, 1768, 2500–2509. [Google Scholar] [CrossRef] [PubMed]
- Maxfield, F.R.; van Meer, G. Cholesterol, the central lipid of mammalian cells. Curr. Opin. Cell Biol. 2010, 22, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Goluszko, P.; Nowicki, B. Membrane cholesterol: A crucial molecule affecting interactions of microbial pathogens with mammalian cells. Infect. Immun. 2005, 73, 7791–7796. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rojas, A.; Moreno-Morales, J.; Mason, A.J.; Rolff, J. Cationic antimicrobial peptides do not change recombination frequency in Escherichia coli. Biol. Lett. 2018, 14, 20180006. [Google Scholar] [CrossRef] [PubMed]
- Candiani, G.; Abbondi, M.; Borgonovi, M.; Romanò, G.; Parenti, F. In-vitro and in-vivo antibacterial activity of BI 397, a new semi-synthetic glycopeptide antibiotic. J. Antimicrob. Chemother. 1999, 44, 179–192. [Google Scholar] [CrossRef][Green Version]
- Dinos, G.; Wilson, D.N.; Teraoka, Y.; Szaflarski, W.; Fucini, P.; Kalpaxis, D.; Nierhaus, K.H. Dissecting the ribosomal inhibition mechanisms of edeine and pactamycin: The universally conserved residues G693 and C795 regulate P-site RNA binding. Mol. Cell 2004, 13, 113–124. [Google Scholar] [CrossRef]
- Szer, W.; Kurylo-Borowska, Z. Interactions of edeine with bacterial ribosomal subunits. Selective inhibition of aminoacyl-tRNA binding sites. Biochim. Biophys. Acta 1972, 259, 357–368. [Google Scholar] [CrossRef]
- Wank, H.; Rogers, J.; Davies, J.; Schroeder, R. Peptide antibiotics of the tuberactinomycin family as inhibitors of group I intron RNA splicing. J. Mol. Biol. 1994, 236, 1001–1010. [Google Scholar] [CrossRef]
- Bulkley, D.; Brandi, L.; Polikanov, Y.S.; Fabbretti, A.; O’Connor, M.; Gualerzi, C.O.; Steitz, T.A. The antibiotics dityromycin and GE82832 bind protein S12 and block EF-G-catalyzed translocation. Cell Rep. 2014, 6, 357–365. [Google Scholar] [CrossRef]
- Nijnik, A.; Hancock, R.E. The roles of cathelicidin LL-37 in immune defences and novel clinical applications. Curr. Opin. Hematol. 2009, 16, 41–47. [Google Scholar] [CrossRef]
- Mangoni, M.L.; McDermott, A.M.; Zasloff, M. Antimicrobial peptides and wound healing: Biological and therapeutic considerations. Exp. Dermatol. 2016, 25, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Vlieghe, P.; Lisowski, V.; Martinez, J.; Khrestchatisky, M. Synthetic therapeutic peptides: Science and market. Drug Discov. Today 2010, 15, 40–56. [Google Scholar] [CrossRef] [PubMed]
- Scheinfeld, N. Dalbavancin: A review. Drugs Today (Barc) 2007, 43, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, S.; Decano, A.G.; Bandali, A.; Lai, D.; Malat, G.E.; Bias, T.E. Oritavancin (Orbactiv): A new-generation lipoglycopeptide for the treatment of acute bacterial skin and skin structure infections. Pharm. Ther. 2018, 43, 143–179. [Google Scholar]
- Smith, J.R.; Roberts, K.D.; Rybak, M.J. Dalbavancin: A novel lipoglycopeptide antibiotic with extended activity against gram-positive infections. Infect. Dis. Ther. 2015, 4, 245–258. [Google Scholar] [CrossRef]
- Cavanaugh, C.; Moeckel, G.W.; Perazella, M.A. Telavancin-associated acute kidney injury. Clin. Nephrol. 2019, 91, 187–191. [Google Scholar] [CrossRef]
- Nnedu, O.N.; Pankey, G.A. Update on the emerging role of telavancin in hospital-acquired infections. Ther. Clin. Risk Manag. 2015, 11, 605–610. [Google Scholar] [CrossRef][Green Version]
- Wolinsky, E.; Hines, J.D. Neurotoxic and nephrotoxic effects of colistin in patients with renal disease. N. Engl. J. Med. 1962, 266, 759–762. [Google Scholar] [CrossRef]
- Lim, L.M.; Ly, N.; Anderson, D.; Yang, J.C.; Macander, L.; Jarkowski, A.; Forrest, A.; Bulitta, J.B.; Tsuji, B.T. Resurgence of colistin: A review of resistance, toxicity, pharmacodynamics, and dosing. Pharmacotherapy 2010, 30, 1279–1291. [Google Scholar] [CrossRef]
- Poirel, L.; Jayol, A.; Nordmann, P. Polymyxins: Antibacterial activity, susceptibility testing, and resistance mechanisms encoded by plasmids or chromosomes. Clin. Microbiol. Rev. 2017, 30, 557–596. [Google Scholar] [CrossRef]
- Bechinger, B. Structure and functions of channel-forming peptides: Magainins, cecropins, melittin and alamethicin. J. Membr. Biol. 1997, 156, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Leitgeb, B.; Szekeres, A.; Manczinger, L.; Vágvölgyi, C.; Kredics, L. The history of alamethicin: A review of the most extensively studied peptaibol. Chem. Biodivers. 2007, 4, 1027–1051. [Google Scholar] [CrossRef] [PubMed]
- Ladram, A.; Nicolas, P. Antimicrobial peptides from frog skin: Biodiversity and therapeutic promises. Front. Biosci. 2016, 21, 1341–1371. [Google Scholar] [CrossRef] [PubMed]
- Conlon, J.M.; Mechkarska, M. Host-defense peptides with therapeutic potential from skin secretions of frogs from the family pipidae. Pharmaceuticals 2014, 7, 58–77. [Google Scholar] [CrossRef]
- Wu, J.; Liu, H.; Yang, H.; Yu, H.; You, D.; Ma, Y.; Ye, H.; Lai, R. Proteomic analysis of skin defensive factors of tree frog Hyla simplex. J. Proteome Res. 2011, 10, 4230–4240. [Google Scholar] [CrossRef]
- Wehbe, R.; Frangieh, J.; Rima, M.; El Obeid, D.; Sabatier, J.M.; Fajloun, Z. Bee venom: Overview of main compounds and bioactivities for therapeutic interests. Molecules 2019, 24, 2997. [Google Scholar] [CrossRef]
- Munawar, A.; Ali, S.A.; Akrem, A.; Betzel, C. Snake venom peptides: Tools of biodiscovery. Toxins (Basel) 2018, 10, 474. [Google Scholar] [CrossRef]
- Cochrane, S.A.; Vederas, J.C. Lipopeptides from bacillus and paenibacillus spp.: A gold mine of antibiotic candidates. Med. Res. Rev. 2016, 36, 4–31. [Google Scholar] [CrossRef]
- Ballantine, R.D.; Li, Y.X.; Qian, P.Y.; Cochrane, S.A. Rational design of new cyclic analogues of the antimicrobial lipopeptide tridecaptin A1. Chem. Commun. 2018, 54, 10634–10637. [Google Scholar] [CrossRef]
- Ballantine, R.D.; McCallion, C.E.; Nassour, E.; Tokajian, S.; Cochrane, S.A. Tridecaptin-inspired antimicrobial peptides with activity against multidrug-resistant Gram-negative bacteria. MedChemComm 2019, 10, 484–487. [Google Scholar] [CrossRef]
- Krauson, A.J.; He, J.; Wimley, W.C. Gain-of-function analogues of the pore-forming peptide melittin selected by orthogonal high-throughput screening. J. Am. Chem. Soc. 2012, 134, 12732–12741. [Google Scholar] [CrossRef] [PubMed]
- Touti, F.; Gates, Z.P.; Bandyopadhyay, A.; Lautrette, G.; Pentelute, B.L. In-solution enrichment identifies peptide inhibitors of protein-protein interactions. Nat. Chem. Biol. 2019, 15, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Gates, Z.P.; Vinogradov, A.A.; Quartararo, A.J.; Bandyopadhyay, A.; Choo, Z.N.; Evans, E.D.; Halloran, K.H.; Mijalis, A.J.; Mong, S.K.; Simon, M.D.; et al. Xenoprotein engineering via synthetic libraries. Proc. Natl. Acad. Sci. USA 2018, 115, E5298–E5306. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.; Wang, G. Ab initio design of potent anti-MRSA peptides based on database filtering technology. J. Am. Chem. Soc. 2012, 134, 12426–12429. [Google Scholar] [CrossRef] [PubMed]
- Porto, W.F.; Irazazabal, L.; Alves, E.S.F.; Ribeiro, S.M.; Matos, C.O.; Pires, Á.; Fensterseifer, I.C.M.; Miranda, V.J.; Haney, E.F.; Humblot, V.; et al. In silico optimization of a guava antimicrobial peptide enables combinatorial exploration for peptide design. Nat. Commun. 2018, 9, 1490. [Google Scholar] [CrossRef] [PubMed]
- Sani, M.A.; Lee, T.H.; Aguilar, M.I.; Separovic, F. Proline-15 creates an amphipathic wedge in maculatin 1.1 peptides that drives lipid membrane disruption. Biochim. Biophys. Acta 2015, 1848, 2277–2289. [Google Scholar] [CrossRef]
- Fernandez, D.I.; Lee, T.H.; Sani, M.A.; Aguilar, M.I.; Separovic, F. Proline facilitates membrane insertion of the antimicrobial peptide maculatin 1.1 via surface indentation and subsequent lipid disordering. Biophys. J. 2013, 104, 1495–1507. [Google Scholar] [CrossRef]
- Hilpert, K. High-throughput screening for antimicrobial peptides using the SPOT technique. Methods Mol. Biol. 2010, 618, 125–133. [Google Scholar]
- López-Pérez, P.M.; Grimsey, E.; Bourne, L.; Mikut, R.; Hilpert, K. Screening and optimizing antimicrobial peptides by using SPOT-synthesis. Front. Chem. 2017, 5, 25. [Google Scholar] [CrossRef]
- Hilpert, K.; Winkler, D.F.; Hancock, R.E. Peptide arrays on cellulose support: SPOT synthesis, a time and cost efficient method for synthesis of large numbers of peptides in a parallel and addressable fashion. Nat. Protoc. 2007, 2, 1333–1349. [Google Scholar] [CrossRef]
- Coley, C.W.; Thomas, D.A.; Lummiss, J.A.M.; Jaworski, J.N.; Breen, C.P.; Schultz, V.; Hart, T.; Fishman, J.S.; Rogers, L.; Gao, H.; et al. A robotic platform for flow synthesis of organic compounds informed by AI planning. Science 2019, 365, eaax1566. [Google Scholar] [CrossRef] [PubMed]
- Touti, F.; Lautrette, G.; Johnson, K.D.; Delaney, J.C.; Wollacott, A.; Tissire, H.; Viswanathan, K.; Shriver, Z.; Mong, S.K.; Mijalis, A.J.; et al. Antibody-bactericidal macrocyclic peptide conjugates to target gram-negative bacteria. ChemBioChem 2018, 19, 2039–2044. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.J.; O’Brien-Simpson, N.M.; Pantarat, N.; Sulistio, A.; Wong, E.H.; Chen, Y.Y.; Lenzo, J.C.; Holden, J.A.; Blencowe, A.; Reynolds, E.C.; et al. Combating multidrug-resistant Gram-negative bacteria with structurally nanoengineered antimicrobial peptide polymers. Nat. Microbiol. 2016, 1, 16162. [Google Scholar] [CrossRef] [PubMed]
- Biswaro, L.S.; da Costa Sousa, M.G.; Rezende, T.M.B.; Dias, S.C.; Franco, O.L. Antimicrobial peptides and nanotechnology, recent advances and challenges. Front. Microbiol. 2018, 9, 855. [Google Scholar] [CrossRef]
- Rajchakit, U.; Sarojini, V. Recent developments in antimicrobial-peptide-conjugated gold nanoparticles. Bioconjug. Chem. 2017, 28, 2673–2686. [Google Scholar] [CrossRef]
- Casciaro, B.; Moros, M.; Rivera-Fernández, S.; Bellelli, A.; de la Fuente, J.M.; Mangoni, M.L. Gold-nanoparticles coated with the antimicrobial peptide esculentin-1a(1-21)NH. Acta Biomater. 2017, 47, 170–181. [Google Scholar] [CrossRef]
- Deptuła, M.; Wardowska, A.; Dzierżyńska, M.; Rodziewicz-Motowidło, S.; Pikuła, M. Antibacterial peptides in dermatology-strategies for evaluation of allergic potential. Molecules 2018, 23, 414. [Google Scholar] [CrossRef]
- Pikuła, M.; Zieliński, M.; Specjalski, K.; Barańska-Rybak, W.; Dawgul, M.; Langa, P.; Jassem, E.; Kamysz, W.; Trzonkowski, P. In vitro evaluation of the allergic potential of antibacterial peptides: Camel and citropin. Chem. Biol. Drug Des. 2016, 87, 562–568. [Google Scholar] [CrossRef]
- Brunetti, J.; Roscia, G.; Lampronti, I.; Gambari, R.; Quercini, L.; Falciani, C.; Bracci, L.; Pini, A. Immunomodulatory and anti-inflammatory activity in vitro and in vivo of a novel antimicrobial candidate. J. Biol. Chem. 2016, 291, 25742–25748. [Google Scholar] [CrossRef]
- Pacor, S.; Giangaspero, A.; Bacac, M.; Sava, G.; Tossi, A. Analysis of the cytotoxicity of synthetic antimicrobial peptides on mouse leucocytes: Implications for systemic use. J. Antimicrob. Chemother. 2002, 50, 339–348. [Google Scholar] [CrossRef]
- Schein, C.H.; Ivanciuc, O.; Braun, W. Bioinformatics approaches to classifying allergens and predicting cross-reactivity. Immunol. Allergy Clin. N. Am. 2007, 27, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Ivanciuc, O.; Schein, C.H.; Braun, W. SDAP: Database and computational tools for allergenic proteins. Nucleic Acids Res. 2003, 31, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Ivanciuc, O.; Gendel, S.M.; Power, T.D.; Schein, C.H.; Braun, W. AllerML: Markup language for allergens. Regul. Toxicol. Pharmacol. 2011, 60, 151–160. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lei, J.; Sun, L.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, D.H.; He, Q. The antimicrobial peptides and their potential clinical applications. Am. J. Transl. Res. 2019, 11, 3919–3931. [Google Scholar] [PubMed]
| DRUG NAME | APPROVAL DATE | NDA NUMBER | ACTIVE INGREDIENTS | COMPANY | MW | PEPTIDE | APPLICATIONS | CATEGORY | ELIMINATION HALF-LIFE |
|---|---|---|---|---|---|---|---|---|---|
| SCENESSE | 10/8/2019 | 210797 | AFAMELANOTIDE | CLINUVEL INC | 1646.85 | Synthetic peptide | Prevents skin damage from the sun in patients with erythropoietic protoporphyria | Receptor binding | 30 min |
| GALLIUM DOTATOC GA68 | 8/21/2019 | 210828 | GALLIUM DOTATOC GA-68 | UNIV IOWA HOSPS AND CLINICS PET IMAGING CENTER | 1489.65 | Cyclic octapeptide | Neuroendocrine tumors (NETs) diagnosis | Receptor binding | 2–4 h |
| VYLEESI (AUTOINJECTOR) | 6/21/2019 | 210557 | BREMELANOTIDE ACETATE | AMAG PHARMS INC | 1025.2 | Cyclic heptapeptide | Hypoactive sexual desire disorder (HSDD) treatment | Receptor binding | 1.9–4 h |
| LUTATHERA | 1/26/2018 | 208700 | LUTETIUM DOTATATE LU-177 | AAA USA INC | 1609.6 | Cyclic peptide-radionuclide conjugate | Gastroenteropan-creatic neuroendocrine tumors (GEP-NETs) treatment | Receptor binding | 3.5–71 h |
| GIAPREZA | 12/21/2017 | 209360 | ANGIOTENSIN II ACETATE | LA JOLLA PHARMA | 1046.2 | Synthetic peptide | Treatment of sepsis, septic shock, diabetes mellitus, and acute renal failure | Receptor binding | <1 min |
| OZEMPIC | 12/5/2017 | 209637 | SEMAGLUTIDE | NOVO NORDISK INC | 4113.58 | Chemically modified peptide | Improving glycemic control in patients with type 2 diabetes mellitus | Receptor binding | 7 days |
| TYMLOS | 4/28/2017 | 208743 | ABALOPARATIDE | RADIUS HEALTH INC | 3961 | Synthetic peptide | Osteoporosis treatment | Receptor binding | 1.7 h |
| PARSABIV | 2/7/2017 | 208325 | ETELCALCETIDE | KAI PHARMS INC | 1047.5 | Synthetic peptide | Treatment of secondary hyperparathyroidism | Receptor binding | 3–4 days |
| TRULANCE | 1/19/2017 | 208745 | PLECANATIDE | SALIX | 1682 | Cyclic peptide | Chronic idiopathic constipation (CIC) treatment | Receptor binding | N/A |
| ADLYXIN | 7/27/2016 | 208471 | LIXISENATIDE | SANOFI-AVENTIS US | 4858.5 | Synthetic peptide | Type 2 diabetes mellitus (T2DM) treatment | Receptor binding | 1–3.5 h |
| NETSPOT | 6/1/2016 | 208547 | GALLIUM DOTATATE GA-68 | AAA USA INC | 1435.6 | Cyclic peptide-radionuclide conjugate | Neuroendocrine tumors (NETs) diagnosis | Receptor binding | 1 h |
| ORBACTIV | 8/6/2014 | 206334 | ORITAVANCIN DIPHOSPHATE | MELINTA THERAP | 1989.09 | Lipoglycopeptide | Treatment of complicated skin and skin structure infections (cSSSI) caused by gram-positive bacteria | MAP | 195.4 h |
| DALVANCE | 5/23/2014 | 021883 | DALBAVANCIN HYDROCHLORIDE | ALLERGAN SALES LLC | ~1800 | Lipoglycopeptide | Treatment of complicated skin and skin structure infections (cSSSI) caused by gram-positive bacteria | Inhibitor | 346 h |
| GATTEX KIT | 12/21/2012 | 203441 | TEDUGLUTIDE RECOMBINANT | NPS PHARMS INC | 3752 | Glucagon-like peptide-2 | Short bowel syndrome (SBS) treatment | Receptor binding | 1.3–2 h |
| SIGNIFOR | 12/14/2012 | 200677 | PASIREOTIDE DIASPARTATE | NOVARTIS | 1313.41 | Cyclohexapeptide | Treatment of Cushing’s disease | Receptor binding | 10–13 h |
| LINZESS | 8/30/2012 | 202811 | LINACLOTIDE | ALLERGAN SALES LLC | 1526.8 | Cyclic peptide | Treatment of irritable bowel syndrome | Receptor binding | N/A |
| KYPROLIS | 7/20/2012 | 202714 | CARFILZOMIB | ONYX THERAP | 719.9 | Modified tetrapeptidyl epoxide | Multiple myeloma treatment | Inhibitor | ≤1 h |
| FIRAZYR | 8/25/2011 | 022150 | ICATIBANT ACETATE | SHIRE ORPHAN THERAP | 1304.5 | Synthetic peptide | Treatment of angioedema, liver disease, burns, and burn infections | Receptor binding | 1.4 h |
| INCIVEK | 5/23/2011 | 201917 | TELAPREVIR | VERTEX PHARMS | 679.85 | Chemically modified peptide | Treatment of chronic Hepatitis C | Inhibitor | 4–11 h |
| EGRIFTA | 11/10/2010 | 022505 | TESAMORELIN ACETATE | THERATECHNOLOGIES | 5135.9 | Synthetic peptide | Human immunodeficiency virus (HIV) treatment | Receptor binding | 26–38 min |
| VICTOZA | 1/25/2010 | 022341 | LIRAGLUTIDE RECOMBINANT | NOVO NORDISK INC | 3751.2 | Synthetic peptide | Improving glycemic control in patients with type 2 diabetes mellitus | Receptor binding | 13 h |
| ISTODAX | 11/5/2009 | 022393 | ROMIDEPSIN | CELGENE | 540.71 | Bicyclic peptide | Treatment of cutaneous T-cell lymphoma (CTCL) and/or peripheral T-cell lymphoma (PTCL) | Inhibitor | 3 h |
| VIBATIV | 9/11/2009 | 022110 | TELAVANCIN HYDROCHLORIDE | CUMBERLAND PHARMS | 1755.6 | Lipoglycopeptide | Treatment of complicated skin and skin structure infections (cSSSI) caused by Gram-positive bacteria | MAP | 8 h |
| FIRMAGON | 12/24/2008 | 022201 | DEGARELIX ACETATE | FERRING | 1632.3 | Synthetic peptide | Prostate cancer treatment | Receptor binding | 53 h |
| SOMATULINE DEPOT | 30/08/2007 | 022074 | LANREOTIDE ACETATE | IPSEN PHARMA | 1096.34 | Cyclical octapeptide | Treatment of neuroendocrine tumors (NETs) and acromegaly | Receptor binding | 22 days |
| ERAXIS | 2/17/2006 | 021632 | ANIDULAFUNGIN | VICURON | 1140.3 | Lipopeptide | Anti-fungal drug | Inhibitor | 40–50 h |
| LEVEMIR | 6/16/2005 | 021536 | INSULIN DETEMIR RECOMBINANT | NOVO NORDISK INC | 5916.9 | A long-acting basal insulin analog | Treatment of hyperglycemia caused by type 1 and type 2 diabetes | Receptor binding | 57 h |
| LEVEMIR FLEXPEN | 6/16/2005 | 021536 | INSULIN DETEMIR RECOMBINANT | NOVO NORDISK INC | 5916.9 | A long-acting basal insulin analog | Treatment of hyperglycemia caused by type 1 and type 2 diabetes | Receptor binding | 5–7 h |
| LEVEMIR FLEXTOUCH | 6/16/2005 | 021536 | INSULIN DETEMIR RECOMBINANT | NOVO NORDISK INC | 5916.9 | A long-acting basal insulin analog | Treatment of hyperglycemia caused by type 1 and type 2 diabetes | Receptor binding | 5–7 h |
| LEVEMIR INNOLET | 6/16/2005 | 021536 | INSULIN DETEMIR RECOMBINANT | NOVO NORDISK INC | 5916.9 | A long-acting basal insulin analog | Treatment of hyperglycemia caused by type 1 and type 2 diabetes | Receptor binding | 5–7 h |
| LEVEMIR PENFILL | 6/16/2005 | 021536 | INSULIN DETEMIR RECOMBINANT | NOVO NORDISK INC | 5916.9 | A long-acting basal insulin analog | Treatment of hyperglycemia caused by type 1 and type 2 diabetes | Receptor binding | 5–7 h |
| BYETTA | 4/28/2005 | 021773 | EXENATIDE SYNTHETIC | ASTRAZENECA AB | 4186.6 | Synthetic peptide | Improving glycemic control in patients with type 2 diabetes mellitus | Receptor binding | 2.4 h |
| SYMLIN | 3/16/2005 | 021332 | PRAMLINTIDE ACETATE | ASTRAZENECA AB | 3949.4 | Peptide hormone | Treatment of type 1 and type 2 diabetes mellitus | Receptor binding | 48 min |
| PRIALT | 12/28/2004 | 021060 | ZICONOTIDE ACETATE | TERSERA THERAPS LLC | 2639 | Synthetic peptide | Chronic pain treatment | Inhibitor | 2.9–6.5 h |
| APIDRA | 4/16/2004 | 021629 | INSULIN GLULISINE RECOMBINANT | SANOFI AVENTIS US | 5823 | Human insulin analog | Treatment of hyperglycemia caused by type 1 and type 2 diabetes | Inhibitor | 13–86 min |
| APIDRA SOLOSTAR | 4/16/2004 | 021629 | INSULIN GLULISINE RECOMBINANT | SANOFI AVENTIS US | 5823 | Human insulin analog | Treatment of hyperglycemia caused by type 1 and type 2 diabetes | Inhibitor | 13–86 min |
| CHIRHOSTIM | 4/9/2004 | 021256 | SECRETIN SYNTHETIC HUMAN | CHIRHOCLIN | 3039.44 | Gastrointestinal peptide hormone | (1) Pancreatic secretions to aid in the diagnosis of pancreatic exocrine dysfunction(2) Gastrin secretion to aid in the diagnosis of gastrinoma(3) Pancreatic secretions to facilitate the identification of the ampulla of Vater and accessory papilla during ERCP | Inhibitor | 45 min |
| PLENAXIS | 11/25/2003 | 021320 | ABARELIX | SPECIALTY EUROPEAN | 1416.06 | Synthetic peptide | Prostate cancer treatment | Inhibitor | 13.2 days |
| CUBICIN | 9/12/2003 | 021572 | DAPTOMYCIN | CUBIST PHARMS LLC | 1620.67 | Cyclic lipopeptide | Treatment of complicated skin and skin structure infections (cSSSI) caused by Gram-positive bacteria | MAP | 8.1–9 h |
| CUBICIN RF | 9/12/2003 | 021572 | DAPTOMYCIN | CUBIST PHARMS LLC | 1620.67 | Cyclic lipopeptide | Treatment of complicated skin and skin structure infections (cSSSI) caused by Gram-positive bacteria | MAP | 8.1–9 h |
| REYATAZ | 6/20/2003 | 021567 | ATAZANAVIR SULFATE | BRISTOL MYERS | 704.9 | Azapeptide | Human immunodeficiency virus (HIV) treatment | Inhibitor | 6.5–7.9 h |
| VELCADE | 5/13/2003 | 021602 | BORTEZOMIB | MILLENNIUM PHARMS | 384.24 | Chemically modified peptide | Multiple myeloma treatment | Inhibitor | 9–15 h |
| FUZEON | 3/13/2003 | 021481 | ENFUVIRTIDE | ROCHE | 4492 | Synthetic peptide | Human immunodeficiency virus (HIV) treatment | Inhibitor | 3.8 h |
| NATRECOR | 8/10/2001 | 020920 | NESIRITIDE RECOMBINANT | SCIOS LLC | 3464 | Cyclic peptide | Acute decompensated heart failure (ADHF) treatment | Receptor binding | 18 min |
| CANCIDAS | 1/26/2001 | 021227 | CASPOFUNGIN ACETATE | MERCK | 1213.42 | Cyclic lipopeptide | Anti-fungal drug | Inhibitor | 9–11 h |
| ANGIOMAX | 12/15/2000 | 020873 | BIVALIRUDIN | SANDOZ INC | 2180 | Synthetic peptide | Treatment of heparin-induced thrombocytopenia and for the prevention of thrombosis | Inhibitor | 22 min–3.5 h |
| CETROTIDE | 8/11/2000 | 021197 | CETRORELIX | EMD SERONO INC | 1431.06 | Synthetic peptide | For prevention of premature ovulation in women undergoing fertility treatments with controlled ovulation | Receptor binding | ~62.8 h |
| TRELSTAR | 6/15/2000 | 020715 | TRIPTORELIN PAMOATE | ALLERGAN SALES LLC | 1699.9 | Synthetic peptide | Prostate cancer treatment | Receptor binding | 6 min–3 h |
| NOVOLOG | 6/7/2000 | 020986 | INSULIN ASPART RECOMBINANT | NOVO NORDISK INC | 5825.8 | Peptide hormone | Treatment of hyperglycemia caused by type 1 and type 2 diabetes | Receptor binding | 81 min |
| NOVOLOG FLEXPEN | 6/7/2000 | 020986 | INSULIN ASPART RECOMBINANT | NOVO NORDISK INC | 5825.8 | Peptide hormone | Treatment of hyperglycemia caused by type 1 and type 2 diabetes | Receptor binding | 81 min |
| NOVOLOG FLEXTOUCH | 6/7/2000 | 020986 | INSULIN ASPART RECOMBINANT | NOVO NORDISK INC | 5825.8 | Peptide hormone | Treatment of hyperglycemia caused by type 1 and type 2 diabetes | Receptor binding | 81 min |
| NOVOLOG INNOLET | 6/7/2000 | 020986 | INSULIN ASPART RECOMBINANT | NOVO NORDISK INC | 5825.8 | Peptide hormone | Treatment of hyperglycemia caused by type 1 and type 2 diabetes | Receptor binding | 81 min |
| NOVOLOG PENFILL | 6/7/2000 | 020986 | INSULIN ASPART RECOMBINANT | NOVO NORDISK INC | 5825.8 | Peptide hormone | Treatment of hyperglycemia caused by type 1 and type 2 diabetes | Receptor binding | 81 min |
| LANTUS | 4/20/2000 | 021081 | INSULIN GLARGINE RECOMBINANT | SANOFI AVENTIS US | 6063 | Peptide hormone | Treatment of hyperglycemia caused by type 1 and type 2 diabetes | Receptor binding | N/A |
| LANTUS SOLOSTAR | 4/20/2000 | 021081 | INSULIN GLARGINE RECOMBINANT | SANOFI AVENTIS US | 6063 | Peptide hormone | Treatment of hyperglycemia caused by type 1 and type 2 diabetes | Receptor binding | N/A |
| NEO TECT KIT | 8/3/1999 | 021012 | TECHNETIUM TC-99M DEPREOTIDE | CIS BIO INTL SA | 486.14 | Cyclic peptide | (1) Detecting coronary artery disease(2) Evaluating myocardial function | Others | 6.02 h |
| GANIRELIX ACETATE | 7/29/1999 | 021057 | GANIRELIX ACETATE | ORGANON USA INC | 1570.35 | Peptide hormone | For inhibition of premature LH surges in women undergoing controlled ovarian hyperstimulation | Receptor binding | 16.2 h |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.H.; Lu, T.K. Development and Challenges of Antimicrobial Peptides for Therapeutic Applications. Antibiotics 2020, 9, 24. https://doi.org/10.3390/antibiotics9010024
Chen CH, Lu TK. Development and Challenges of Antimicrobial Peptides for Therapeutic Applications. Antibiotics. 2020; 9(1):24. https://doi.org/10.3390/antibiotics9010024
Chicago/Turabian StyleChen, Charles H., and Timothy K. Lu. 2020. "Development and Challenges of Antimicrobial Peptides for Therapeutic Applications" Antibiotics 9, no. 1: 24. https://doi.org/10.3390/antibiotics9010024
APA StyleChen, C. H., & Lu, T. K. (2020). Development and Challenges of Antimicrobial Peptides for Therapeutic Applications. Antibiotics, 9(1), 24. https://doi.org/10.3390/antibiotics9010024




