Development of a Novel Pharmaceutical Formula of Nanoparticle Lipid Carriers of Gentamicin/α-Tocopherol and In Vivo Assessment of the Antioxidant Protective Effect of α-Tocopherol in Gentamicin-Induced Nephrotoxicity
Abstract
:1. Introduction
2. Results
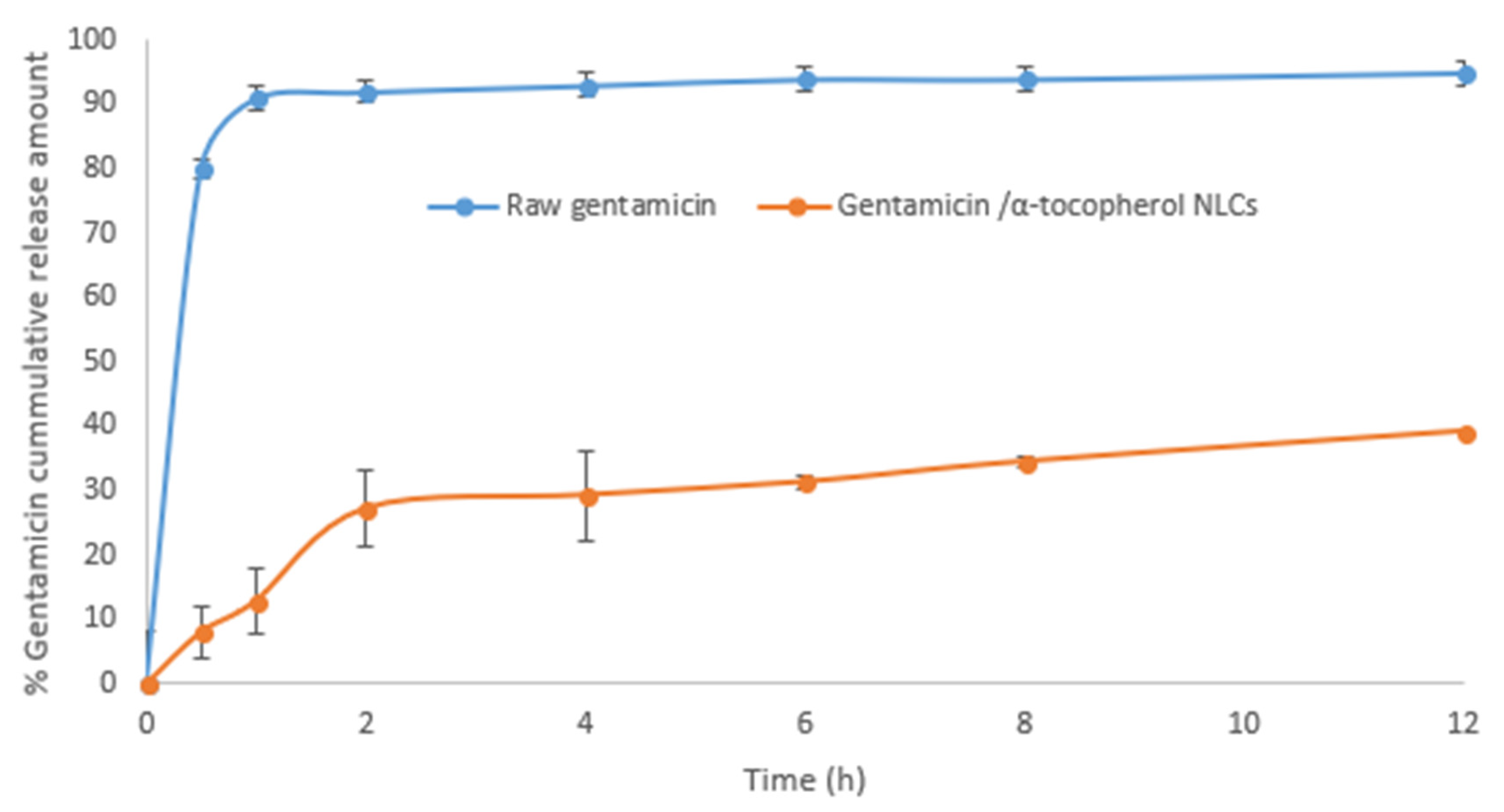
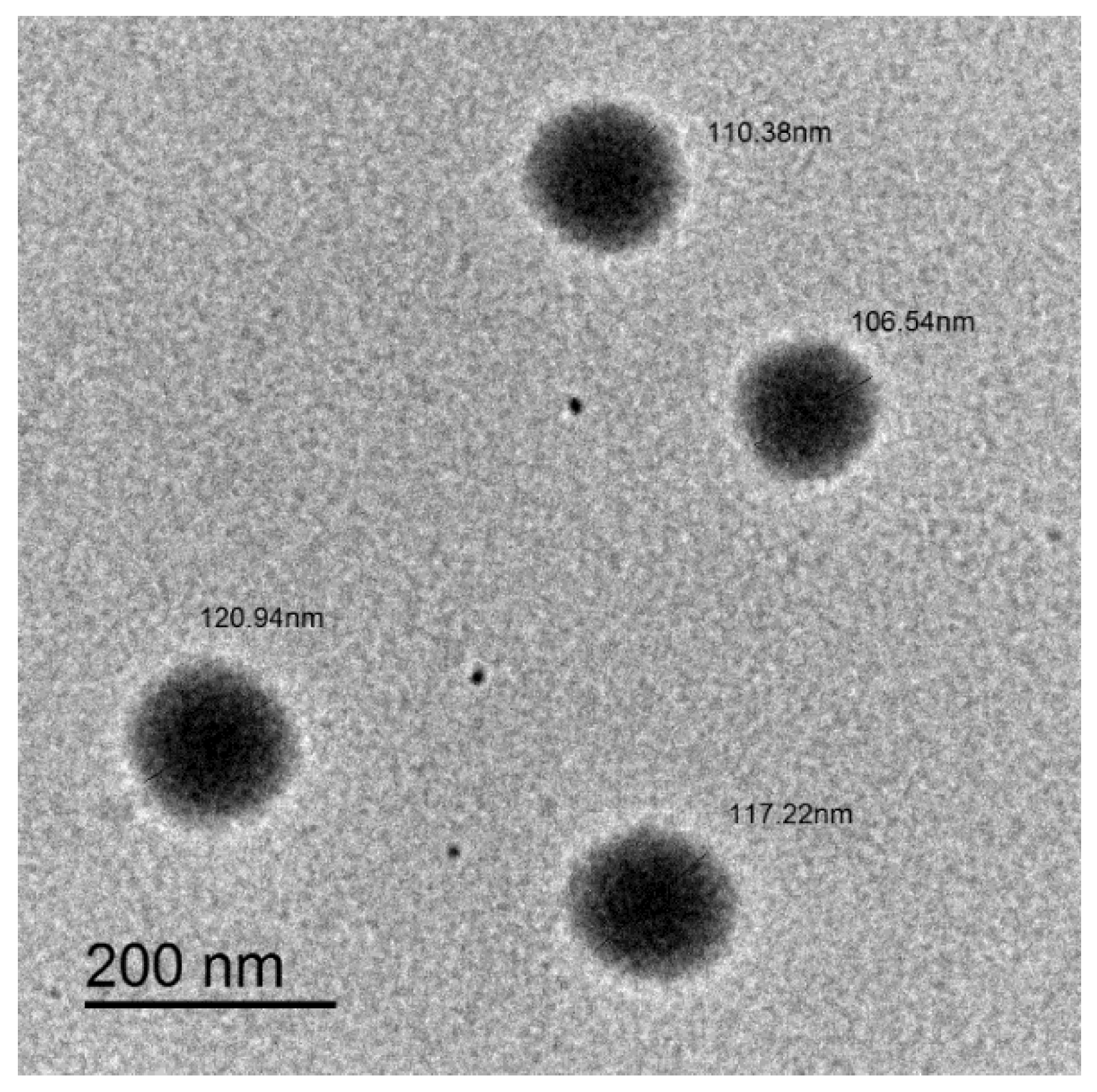
2.1. Characteristics of the Nanostructured Lipid Carriers
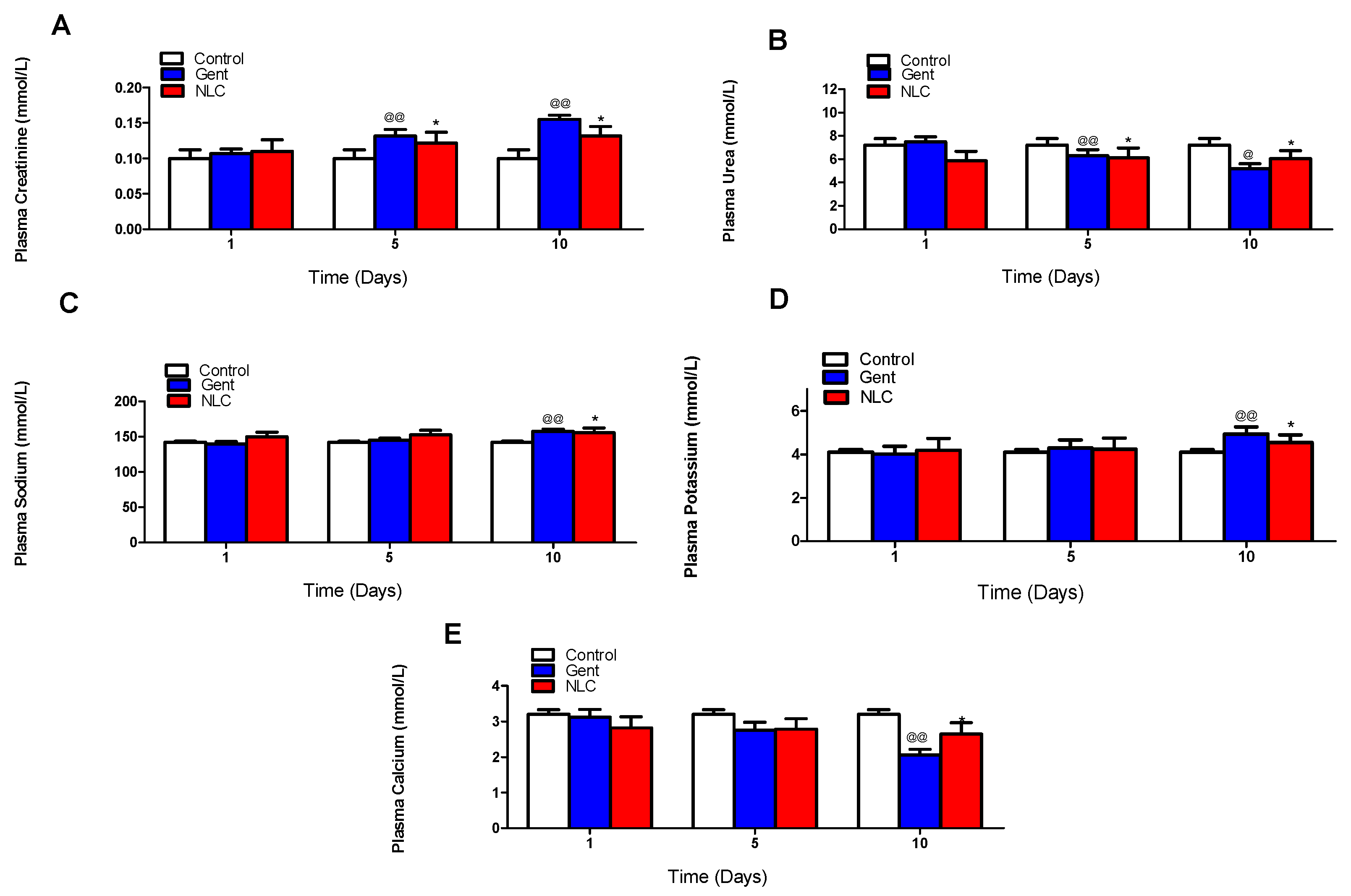
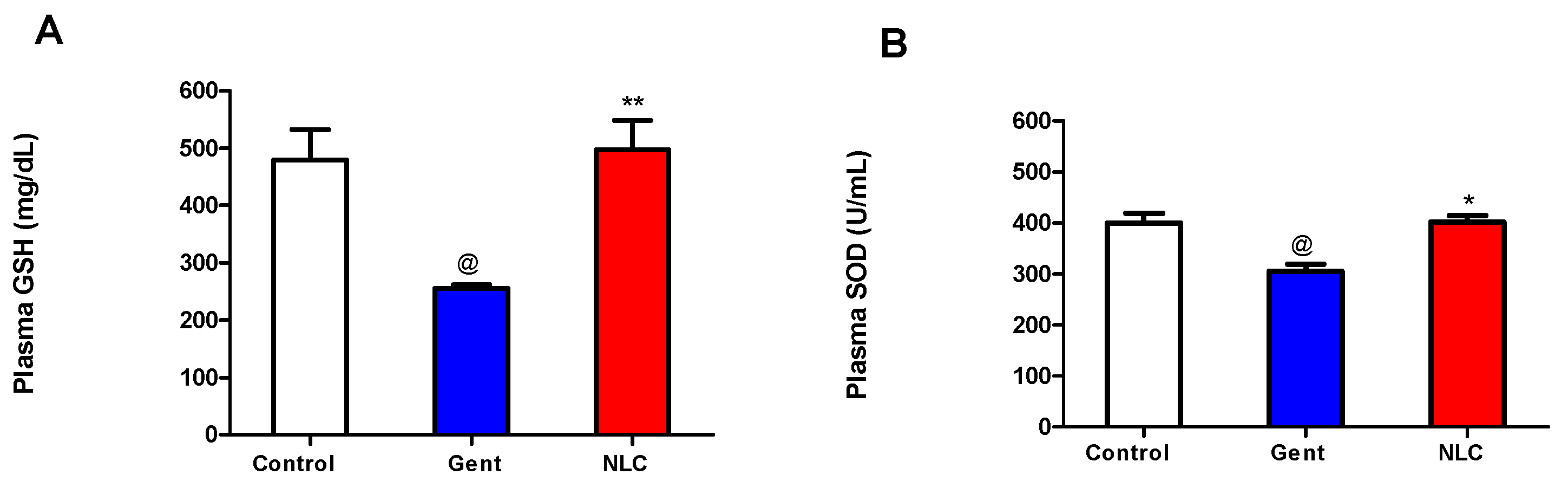
2.2. In Vivo Nephrotoxicity Study
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Nanostructured Lipid Carriers
4.3. Size and Zeta Potential of the NLC
4.4. Drug Content
4.5. Drug Release Profile
4.6. In Vivo Nephrotoxicity Study
4.7. Measurement of Lipid Peroxide
4.8. Measurement of Enzyme Activity of Superoxide Dismutase (SOD)
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, L.F.; Kaye, D. Current use for old antibacterial agents: Polymyxins, rifamycins, and aminoglycosides. Med. Clin. N. Am. 2011, 95, 819–842. [Google Scholar] [CrossRef] [PubMed]
- Bakker-Woudenberg, I.A.; Roosendaal, R. Impact of dosage schedule of antibiotics on the treatment of serious infections. Intensive Care Med. 1990, 16 (Suppl. 3), S229–S234. [Google Scholar] [CrossRef] [PubMed]
- Drusano, G.L. Role of pharmacokinetics in the outcome of infections. Antimicrob. Agents Chemother. 1988, 32, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Edson, R.S.; Terrell, C.L. The aminoglycosides. Mayo Clin. Proc. 1999, 74, 519–528. [Google Scholar] [CrossRef]
- Mingeot-Leclercq, M.P.; Tulkens, P.M. Aminoglycosides: Nephrotoxicity. Antimicrob. Agents Chemother. 1999, 43, 1003–1012. [Google Scholar] [CrossRef]
- Becker, B.; Cooper, M.A. Aminoglycoside antibiotics in the 21st century. ACS Chem. Biol. 2013, 8, 105–115. [Google Scholar] [CrossRef]
- Lopez-Novoa, J.M.; Quiros, Y.; Vicente, L.; Morales, A.I.; Lopez-Hernandez, F.J. New insights into the mechanism of aminoglycoside nephrotoxicity: An integrative point of view. Kidney Int. 2011, 79, 33–45. [Google Scholar] [CrossRef]
- Parsons, P.P.; Garland, H.O.; Harpur, E.S.; Old, S. Acute gentamicin-induced hypercalciuria and hypermagnesiuria in the rat: Dose-response relationship and role of renal tubular injury. Br. J. Pharmacol. 1997, 122, 570–576. [Google Scholar] [CrossRef]
- Banday, A.A.; Farooq, N.; Priyamvada, S.; Yusufi, A.N.; Khan, F. Time dependent effects of gentamicin on the enzymes of carbohydrate metabolism, brush border membrane and oxidative stress in rat kidney tissues. Life Sci. 2008, 82, 450–459. [Google Scholar] [CrossRef]
- Martinez-Salgado, C.; Lopez-Hernandez, F.J.; Lopez-Novoa, J.M. Glomerular nephrotoxicity of aminoglycosides. Toxicol. Appl. Pharmacol. 2007, 223, 86–98. [Google Scholar] [CrossRef]
- Klotman, P.E.; Yarger, W.E. Reduction of renal blood flow and proximal bicarbonate reabsorption in rats by gentamicin. Kidney Int. 1983, 24, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Cuzzocrea, S.; Mazzon, E.; Dugo, L.; Serraino, I.; Di Paola, R.; Britti, D.; De Sarro, A.; Pierpaoli, S.; Caputi, A.; Masini, E.; et al. A role for superoxide in gentamicin-mediated nephropathy in rats. Eur. J. Pharmacol. 2002, 450, 67–76. [Google Scholar] [CrossRef]
- Ali, B.H. Gentamicin nephrotoxicity in humans and animals: Some recent research. Gen. Pharmacol. 1995, 26, 1477–1487. [Google Scholar] [CrossRef]
- Adi, P.J.; Burra, S.P.; Vataparti, A.R.; Matcha, B. Calcium, zinc and vitamin E ameliorate cadmium-induced renal oxidative damage in albino Wistar rats. Toxicol. Rep. 2016, 3, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Begg, E.J.; Barclay, M.L. Aminoglycosides—50 years on. Br. J. Clin. Pharmacol. 1995, 39, 597–603. [Google Scholar] [PubMed]
- Kavutcu, M.; Canbolat, O.; Ozturk, S.; Olcay, E.; Ulutepe, S.; Ekinci, C.; Gokhun, I.H.; Durak, I. Reduced enzymatic antioxidant defense mechanism in kidney tissues from gentamicin-treated guinea pigs: Effects of vitamins E and C. Nephron 1996, 72, 269–274. [Google Scholar] [CrossRef]
- Kadkhodaee, M.; Khastar, H.; Faghihi, M.; Ghaznavi, R.; Zahmatkesh, M. Effects of co-supplementation of vitamins E and C on gentamicin-induced nephrotoxicity in rat. Exp. Physiol. 2005, 90, 571–576. [Google Scholar] [CrossRef]
- Rajpoot, K. Solid Lipid Nanoparticles: A Promising Nanomaterial in Drug Delivery. Curr. Pharm. Des. 2019. [Google Scholar] [CrossRef]
- Fang, C.L.; Al-Suwayeh, S.A.; Fang, J.Y. Nanostructured lipid carriers (NLCs) for drug delivery and targeting. Recent Pat. Nanotechnol. 2013, 7, 41–55. [Google Scholar] [CrossRef]
- Beloqui, A.; Solinis, M.A.; Rodriguez-Gascon, A.; Almeida, A.J.; Preat, V. Nanostructured lipid carriers: Promising drug delivery systems for future clinics. Nanomedicine 2016, 12, 143–161. [Google Scholar] [CrossRef]
- Eisenberg, J.M.; Koffer, H.; Glick, H.A.; Connell, M.L.; Loss, L.E.; Talbot, G.H.; Shusterman, N.H.; Strom, B.L. What is the cost of nephrotoxicity associated with aminoglycosides? Ann. Intern. Med. 1987, 107, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Stojiljkovic, N.; Ilic, S.; Veljkovic, M.; Todorovic, J.; Mladenovic, M. α-Tocopherol Reduces Morphological Changes and Oxidative Stress during Gentamicin-Induced Acute Renal Failure. Bull. Exp. Biol. Med. 2018, 164, 442–445. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Naim, A.B.; Abdel-Wahab, M.H.; Attia, F.F. Protective effects of vitamin e and probucol against gentamicin-induced nephrotoxicity in rats. Pharm. Res. 1999, 40, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Luciak, M. Antioxidants in the treatment of patients with renal failure. Rocz. Akad. Med. Bialymst. 2004, 49, 157–161. [Google Scholar] [PubMed]
- Ramsammy, L.; Ling, K.Y.; Josepovitz, C.; Levine, R.; Kaloyanides, G.J. Effect of gentamicin on lipid peroxidation in rat renal cortex. Biochem. Pharmacol. 1985, 34, 3895–3900. [Google Scholar] [CrossRef]
- Elsayed, M.G.; Elkomy, A.A.; Gaballah, M.S.; Elbadawy, M. Nephrotoxicity of cefepime: A new cephalosporin antibiotic in rats. J. Pharmacol. Pharmacother. 2014, 5, 33–38. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Nishikimi, M.; Appaji, N.; Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elfaky, M.A.; Thabit, A.K.; Sirwi, A.; Fahmy, U.A.; Bahabri, R.M.; Al-Awad, E.A.; Basaeed, L.F. Development of a Novel Pharmaceutical Formula of Nanoparticle Lipid Carriers of Gentamicin/α-Tocopherol and In Vivo Assessment of the Antioxidant Protective Effect of α-Tocopherol in Gentamicin-Induced Nephrotoxicity. Antibiotics 2019, 8, 234. https://doi.org/10.3390/antibiotics8040234
Elfaky MA, Thabit AK, Sirwi A, Fahmy UA, Bahabri RM, Al-Awad EA, Basaeed LF. Development of a Novel Pharmaceutical Formula of Nanoparticle Lipid Carriers of Gentamicin/α-Tocopherol and In Vivo Assessment of the Antioxidant Protective Effect of α-Tocopherol in Gentamicin-Induced Nephrotoxicity. Antibiotics. 2019; 8(4):234. https://doi.org/10.3390/antibiotics8040234
Chicago/Turabian StyleElfaky, Mahmoud A., Abrar K. Thabit, Alaa Sirwi, Usama A. Fahmy, Raghdah M. Bahabri, Eman A. Al-Awad, and Lamis F. Basaeed. 2019. "Development of a Novel Pharmaceutical Formula of Nanoparticle Lipid Carriers of Gentamicin/α-Tocopherol and In Vivo Assessment of the Antioxidant Protective Effect of α-Tocopherol in Gentamicin-Induced Nephrotoxicity" Antibiotics 8, no. 4: 234. https://doi.org/10.3390/antibiotics8040234
APA StyleElfaky, M. A., Thabit, A. K., Sirwi, A., Fahmy, U. A., Bahabri, R. M., Al-Awad, E. A., & Basaeed, L. F. (2019). Development of a Novel Pharmaceutical Formula of Nanoparticle Lipid Carriers of Gentamicin/α-Tocopherol and In Vivo Assessment of the Antioxidant Protective Effect of α-Tocopherol in Gentamicin-Induced Nephrotoxicity. Antibiotics, 8(4), 234. https://doi.org/10.3390/antibiotics8040234






