Novel Tetracyclines Versus Alternative Antibiotics for Treating Acute Bacterial Infection: A Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
2. Methods
2.1. Study Search and Selection
2.2. Outcome Measurement
2.3. Data Analysis
3. Results
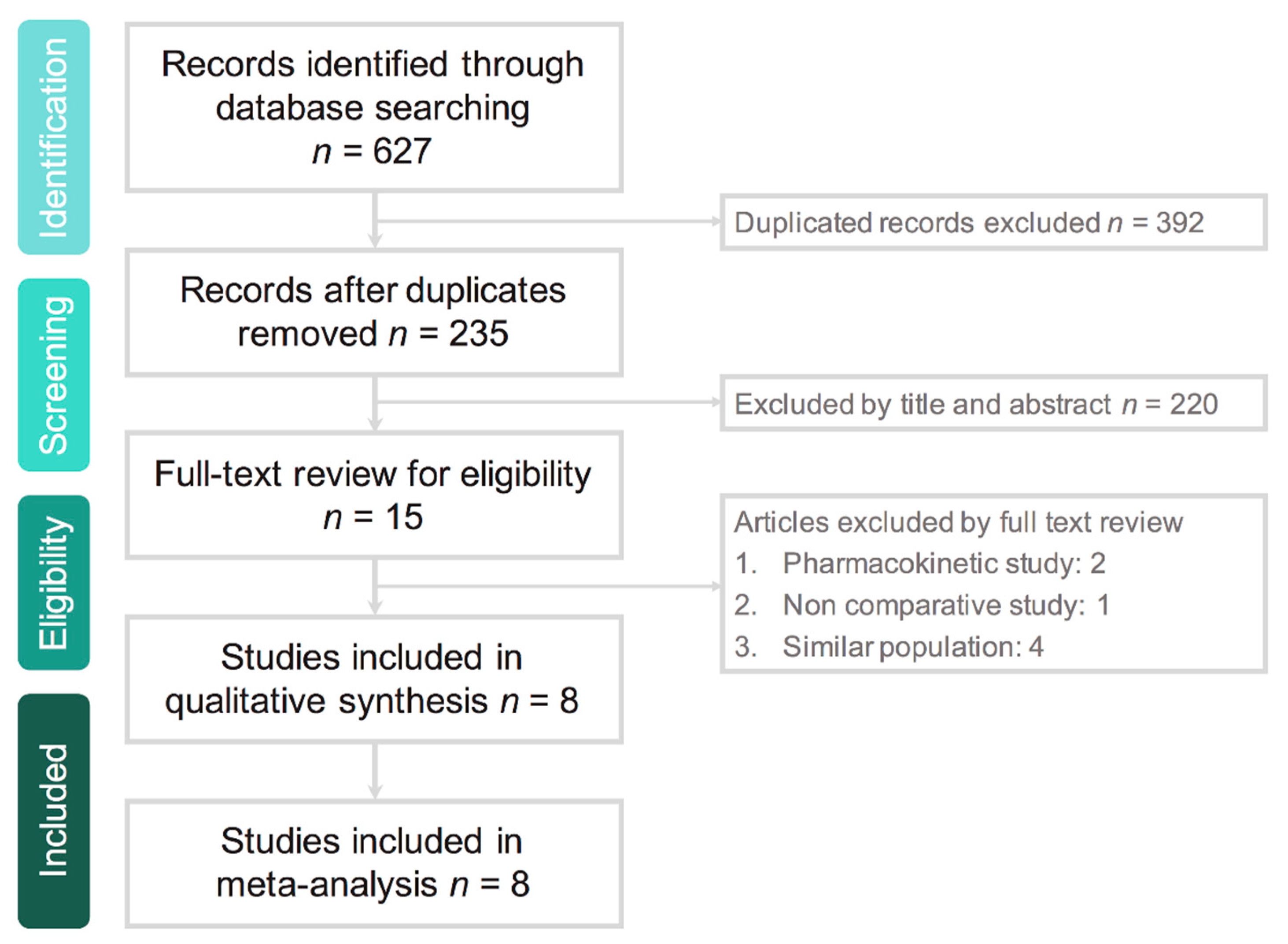
3.1. Study Selection
3.2. Study Characteristics
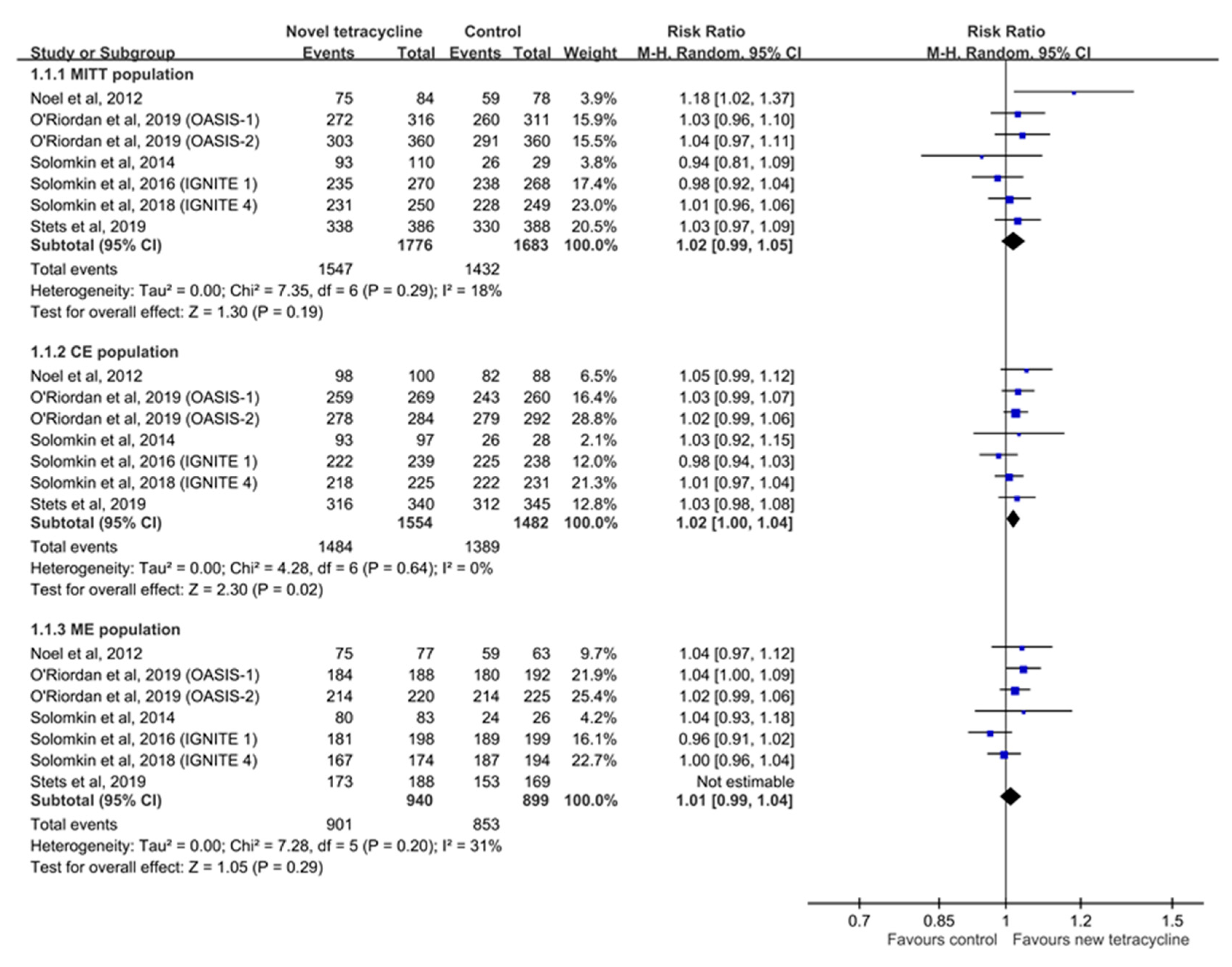
3.3. Clinical Efficacy
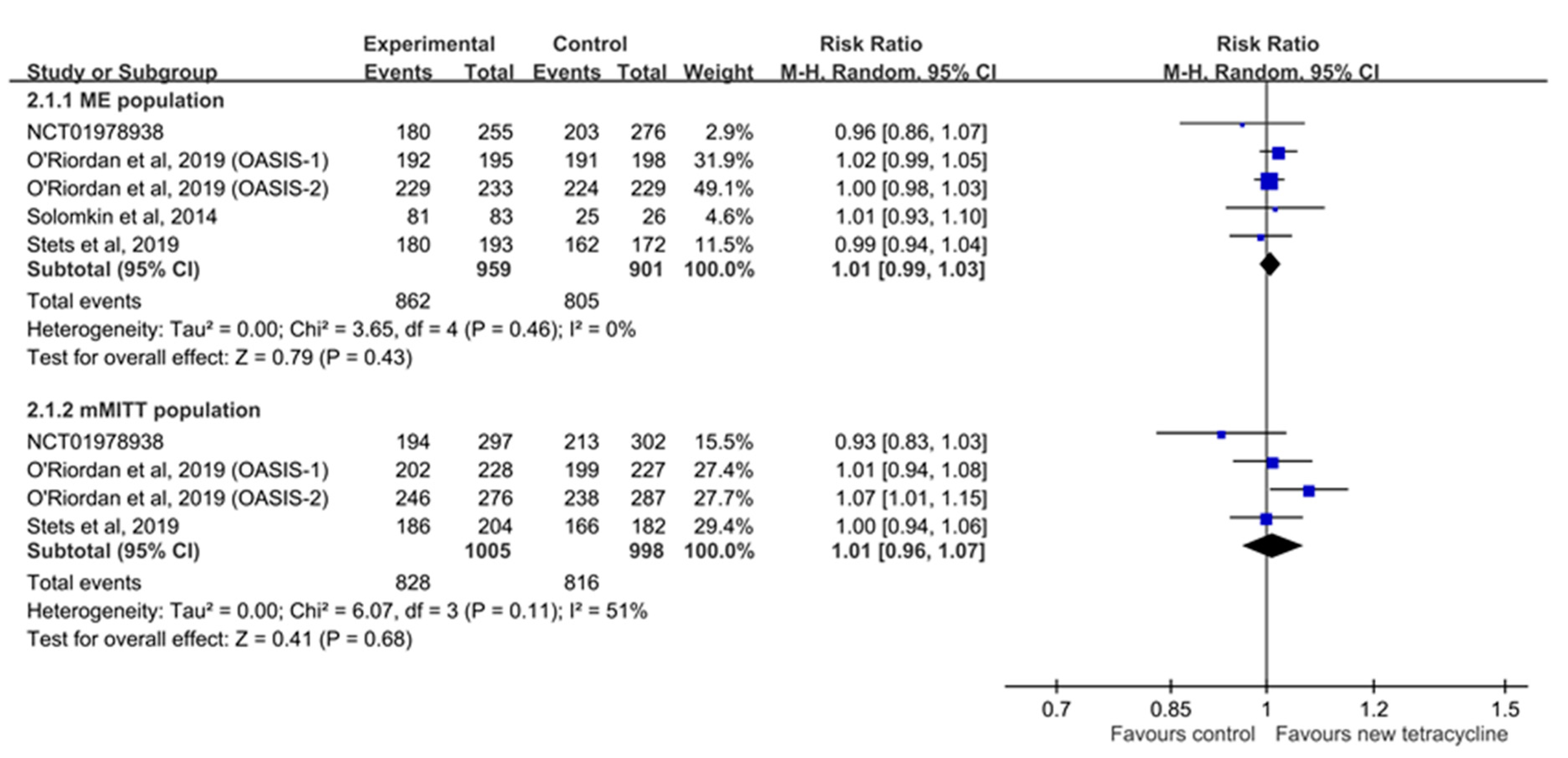
3.4. Microbiological Response
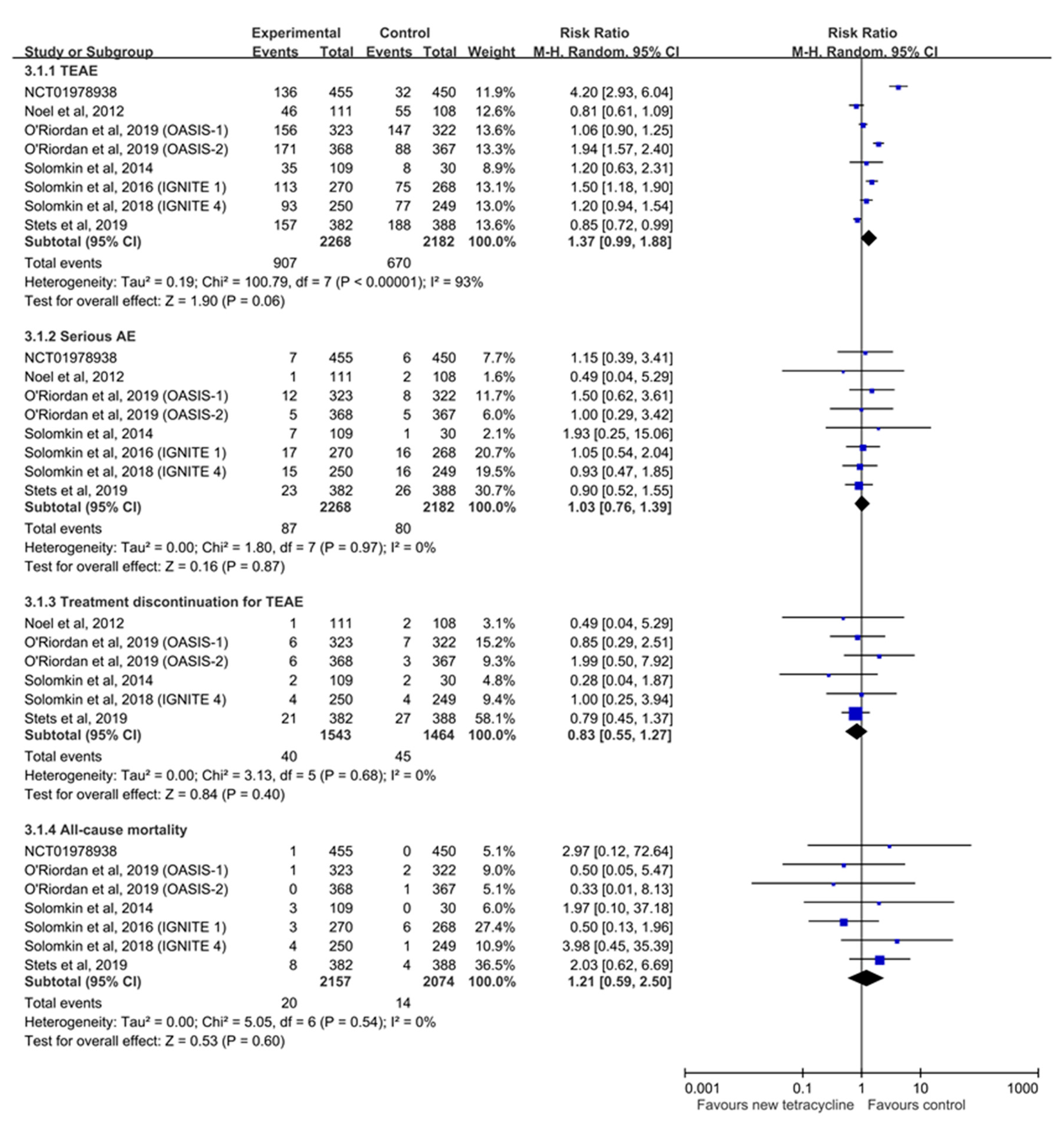
3.5. Risk of AEs
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
Appendix A
| PubMed search strategy—last searched on 11 July 2019 | Results | |
| 1 | Search (((((Eravacycline[Title/Abstract]) OR Xerava[Title/Abstract]) OR TP-434[Title/Abstract]) OR Omadacycline[Title/Abstract]) OR Nuzyra[Title/Abstract]) OR PTK-0796[Title/Abstract] | 172 |
| 2 | Search Infection*[Title/Abstract] | 1,343,862 |
| 3 | Search (Infection*[Title/Abstract]) AND ((((((Eravacycline[Title/Abstract]) OR Xerava[Title/Abstract]) OR TP-434[Title/Abstract]) OR Omadacycline[Title/Abstract]) OR Nuzyra[Title/Abstract]) OR PTK-0796[Title/Abstract]) | 124 |
| Web of Science search strategy—last searched on 11 July 2019 | Results | |
| 1 | Topic: (Omadacycline) OR Topic: (Nuzyra) OR Topic: (PTK-0796) OR Topic: (Eravacycline) OR Topic: (Xerava) OR Topic: (TP-434) | 172 |
| 2 | Topic: (Infection*) | 1,382,483 |
| 3 | #1 AND #2 | 127 |
| EBSCO search strategy—last searched on 11 July 2019 | Results | |
| 1 | AB Eravacycline OR AB Xerava OR AB TP-434 OR AB Omadacycline OR AB Nuzyra OR AB PTK-0796 | 68 |
| 2 | AB Infection * | 489,510 |
| 3 | S1 AND S2 | 43 |
| Cochrane Library search strategy—last searched on 11 July 2019 | Results | |
| 1 | (Omadacycline):ti,ab,kw OR (PTK-0796):ti,ab,kw OR (Nuzyra):ti,ab,kw | 33 |
| 2 | (Eravacycline):ti,ab,kw OR (Xerava):ti,ab,kw OR (TP-434):ti,ab,kw | 14 |
| 3 | (Infection*):ti,ab,kw | 108,094 |
| 4 | (#1 OR #2) AND #3 | 42 |
| Ovid Medline search strategy—last searched on 11 July 2019 | Results | |
| 1 | (Omadacycline or Nuzyra or PTK-0796 or Eravacycline or Xerava or TP-434).ab | 174 |
| 2 | Infection *.ab | 1,468,481 |
| 3 | 1 and 2 | 128 |
| Embase search strategy—last searched on 11 July 2019 | Results | |
| 1 | omadacycline:ti,ab,kw OR nuzyra:ti,ab,kw OR ‘ptk 0796’:ti,ab,kw OR eravacycline:ti,ab,kw OR xerava:ti,ab,kw OR ‘tp 434’:ti,ab,kw | 217 |
| 2 | infection*:ti,ab,kw | 1,726,005 |
| 3 | #1 AND #2 | 163 |
References
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock. Intensive Care Med. 2017, 43, 304–377. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-W.; Tang, H.-J.; Chen, C.-C.; Lu, Y.-C.; Chen, H.-J.; Su, B.-A.; Weng, T.-C.; Chuang, Y.-C.; Lai, C.-C. The Microbiological Characteristics of Carbapenem-Resistant Enterobacteriaceae Carrying the mcr-1 Gene. J. Clin. Med. 2019, 8, 261. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-T.; Chao, C.-M.; Lin, H.-L.; Hung, M.-C.; Lai, C.-C. Emergence of Antibiotic-Resistant Bacteria in Patients with Fournier Gangrene. Surg. Infect. 2015, 16, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-C.; Chen, Y.-S.; Lee, N.-Y.; Tang, H.-J.; Lee, S.S.-J.; Lin, C.-F.; Lu, P.-L.; Wu, J.-J.; Ko, W.-C.; Lee, W.-S.; et al. Susceptibility rates of clinically important bacteria collected from intensive care units against colistin, carbapenems, and other comparative agents: Results from Surveillance of Multicenter Antimicrobial Resistance in Taiwan (SMART). Infect. Drug Resist. 2019, 12, 627–640. [Google Scholar] [CrossRef] [PubMed]
- Karlowsky, J.A.; Lob, S.H.; Young, K.; Motyl, M.R.; Sahm, D.F. Activity of imipenem/relebactam against Pseudomonas aeruginosa with antimicrobial-resistant phenotypes from seven global regions: SMART 2015–2016. J. Glob. Antimicrob. Resist. 2018, 15, 140–147. [Google Scholar] [CrossRef]
- Ponce-De-León, A.; Rodriguez-Noriega, E.; Morfin-Otero, R.; Cornejo-Juárez, D.P.; Tinoco, J.C.; Martínez-Gamboa, A.; Gaona-Tapia, C.J.; Guerrero-Almeida, M.L.; Martín-Onraet, A.; Cervantes, J.L.V.; et al. Antimicrobial susceptibility of gram-negative bacilli isolated from intra-abdominal and urinary-tract infections in Mexico from 2009 to 2015: Results from the Study for Monitoring Antimicrobial Resistance Trends (SMART). PLoS ONE 2018, 13, e0198621. [Google Scholar] [CrossRef]
- Tärnberg, M.; Nilsson, L.E.; Dowzicky, M.J. Antimicrobial activity against a global collection of skin and skin structure pathogens: Results from the Tigecycline Evaluation and Surveillance Trial (T.E.S.T.), 2010–2014. Int. J. Infect. Dis. 2016, 49, 141–148. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Novel Drug Approvals for 2018. Available online: https://www.fda.gov/drugs/developmentapprovalprocess/druginnovation/ucm592464.htm (accessed on 8 July 2019).
- Villano, S.; Steenbergen, J.; Loh, E. Omadacycline: Development of a novel aminomethylcycline antibiotic for treating drug-resistant bacterial infections. Future Microbiol. 2016, 11, 1421–1434. [Google Scholar] [CrossRef]
- Lee, Y.R.; Burton, C.E. Eravacycline, a newly approved fluorocycline. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1787–1794. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Efficacy and Safety Study of Eravacycline Compared with Levofloxacin in Complicated Urinary Tract Infections. Available online: https://clinicaltrials.gov/ct2/show/NCT01978938?term=eravacycline&rank=4 (accessed on 11 July 2019).
- Noel, G.J.; Draper, M.P.; Hait, H.; Tanaka, S.K.; Arbeit, R.D. A Randomized, Evaluator-Blind, Phase 2 Study Comparing the Safety and Efficacy of Omadacycline to Those of Linezolid for Treatment of Complicated Skin and Skin Structure Infections. Antimicrob. Agents Chemother. 2012, 56, 5650–5654. [Google Scholar] [CrossRef]
- O’Riordan, W.; Green, S.; Overcash, J.S.; Puljiz, I.; Metallidis, S.; Gardovskis, J.; Garrity-Ryan, L.; Das, A.F.; Tzanis, E.; Eckburg, P.B.; et al. Omadacycline for Acute Bacterial Skin and Skin-Structure Infections. N. Engl. J. Med. 2019, 380, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Solomkin, J.; Evans, D.; Slepavicius, A.; Lee, P.; Marsh, A.; Tsai, L.; Sutcliffe, J.A.; Horn, P. Assessing the Efficacy and Safety of Eravacycline vs Ertapenem in Complicated Intra-Abdominal Infections in the Investigating Gram-Negative Infections Treated with Eravacycline (IGNITE 1) Trial: A Randomized Clinical Trial. JAMA Surg. 2017, 152, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Solomkin, J.S.; Gardovskis, J.; Lawrence, K.; Solomkin, J.S.; Gardovskis, J.; Lawrence, K.; Montravers, P.; Sway, A.; Evans, D.; Tsai, L. IGNITE4: Results of a Phase 3, Randomized, Multicenter, Prospective Trial of Eravacycline vs. Meropenem in the Treatment of Complicated Intra-Abdominal Infections. Clin. Infect. Dis. 2018, 69, 921–929. [Google Scholar]
- Solomkin, J.S.; Ramesh, M.K.; Cesnauskas, G.; Novikovs, N.; Stefanova, P.; Sutcliffe, J.A.; Walpole, S.M.; Horn, P.T. Phase 2, randomized, double-blind study of the efficacy and safety of two dose regimens of eravacycline versus ertapenem for adult community-acquired complicated intra-abdominal infections. Antimicrob. Agents Chemother. 2014, 58, 1847–1854. [Google Scholar] [CrossRef] [PubMed]
- Stets, R.; Popescu, M.; Gonong, J.R.; Mitha, I.; Nseir, W.; Madej, A.; Kirsch, C.; Das, A.F.; Garrity-Ryan, L.; Steenbergen, J.N.; et al. Omadacycline for Community-Acquired Bacterial Pneumonia. N. Engl. J. Med. 2019, 380, 517–527. [Google Scholar] [CrossRef] [PubMed]
- O’Riordan, W.; Cardenas, C.; Shin, E.; Sirbu, A.; Garrity-Ryan, L.; Das, A.F.; Eckburg, P.B.; Manley, A.; Steenbergen, J.N.; Tzanis, E.; et al. Once-daily oral omadacycline versus twice-daily oral linezolid for acute bacterial skin and skin structure infections (OASIS-2): A phase 3, double-blind, multicentre, randomised, controlled, non-inferiority trial. Lancet Infect. Dis. 2019, 19, 1080–1090. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Abrahamian, F.M.; Sakoulas, G.; Tzanis, E.; Manley, A.; Steenbergen, J.; Das, A.F.; Eckburg, P.B.; McGovern, P.C. Omadacycline for Acute Bacterial Skin and Skin Structure Infections. Clin. Infect. Dis. 2019, 69, S23–S32. [Google Scholar] [CrossRef]
- Lan, S.-H.; Chang, S.-P.; Lai, C.-C.; Lu, L.-C.; Chao, C.-M. The Efficacy and Safety of Eravacycline in the Treatment of Complicated Intra-Abdominal Infections: A Systemic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2019, 8, 866. [Google Scholar] [CrossRef]
- Carvalhaes, C.G.; Huband, M.D.; Reinhart, H.H.; Flamm, R.K.; Sader, H.S. Antimicrobial Activity of Omadacycline Tested against Clinical Bacterial Isolates from Hospitals in Mainland China, Hong Kong, and Taiwan: Results from the SENTRY Antimicrobial Surveillance Program (2013 to 2016). Antimicrob. Agents Chemother. 2019, 63, e02262-18. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Rhomberg, P.R.; Huband, M.D.; Flamm, R.K. Activity of omadacycline tested against Streptococcus pneumoniae from a global surveillance program (2014). Diagn. Microbiol. Infect. Dis. 2018, 90, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Huband, M.D.; Rhomberg, P.R.; Flamm, R.K. Surveillance of Omadacycline Activity against Clinical Isolates from a Global Collection (North America, Europe, Latin America, Asia-Western Pacific), 2010–2011. Antimicrob. Agents Chemother. 2017, 61, e00018-17. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Rhomberg, P.R.; Huband, M.D.; Flamm, R.K. Activities of Omadacycline and Comparator Agents against Staphylococcus Aureus Isolates from a Surveillance Program Conducted in North America and Europe. Antimicrob. Agents Chemother. 2017, 61, e02411-16. [Google Scholar] [CrossRef] [PubMed]
- Zhanel, G.G.; Baxter, M.R.; Adam, H.J.; Sutcliffe, J.; Karlowsky, J.A. In vitro activity of eravacycline against 2213 Gram-negative and 2424 Gram-positive bacterial pathogens isolated in Canadian hospital laboratories: CANWARD surveillance study 2014–2015. Diagn. Microbiol. Infect. Dis. 2018, 91, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Seifert, H.; Stefanik, D.; Sutcliffe, J.A.; Higgins, P.G. In-vitro activity of the novel fluorocycline eravacycline against carbapenem non-susceptible Acinetobacter baumannii. Int. J. Antimicrob. Agents 2018, 51, 62–64. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Wang, X.; Zhang, Y.; Wang, R.; Wang, Q.; Li, H.; Wang, H. In vitro activities of Eravacycline against 336 isolates collected from 2012 to 2016 from 11 teaching hospitals in China. BMC Infect. Dis. 2019, 19, 508. [Google Scholar] [CrossRef]
- Livermore, D.M.; Mushtaq, S.; Warner, M.; Woodford, N. In Vitro Activity of Eravacycline against Carbapenem-Resistant Enterobacteriaceae and Acinetobacter baumannii. Antimicrob. Agents Chemother. 2016, 60, 3840–3844. [Google Scholar] [CrossRef]

| Study, Published Year | Study Design | Study Site | Study Period | Type of Infection | No of Patients | Dose Regimen | ||
|---|---|---|---|---|---|---|---|---|
| Study | Comparator | Study | Comparator | |||||
| Omadacycline | ||||||||
| Noel et al., 2012 | Randomized, controlled, evaluator-blinded study | 11 sites in US | 2007–2008 | Complicated skin and skin structure infection | 118 | 116 | 100 mg qd | Linezolid 600 mg q12h ± aztreonam |
| O’Riordan et al., 2019 (OASIS-1) | Double blind, randomized controlled trial | 55 sites in US, Peru, South Africa and Europe | 2015–2016 | Acute bacterial skin and skin-structure infection | 323 | 322 | 100 mg q12h x 2 doses than 100 mg qd | Linezolid 600 mg q12h |
| Stets et al., 2019 | Double blind, randomized controlled trial | 86 sites in Europe, North America, South America, the Middle East, Africa, and Asia | 2015–2017 | Community-acquired pneumonia in PSI risk II, III or IV | 386 | 388 | 100 mg q12h x 2 doses than 100 mg qd | Moxifloxacin 400 mg |
| O’Riordan et al., 2019 (OASIS-2) | Double0blind, randomized controlled trial | 33 sites in US | May 2017–June 2017 | Acute bacterial skin and skin-structure infection | 368 | 367 | 450 mg (oral) qd x 2 doses then 300 mg qd | Linezolid 600 mg (oral) q12h |
| Eravacycline | ||||||||
| Solomkin et al., 2014 | Randomized, double-blind trial | 19 sites in 6 countries | 2011–2012 | Complicated intra-abdominal infection | 56 (1.5 mg/kg), 57 (1.0 mg/kg) | 30 | 1.5 mg/kg or 1.0 mg/kg q24h | Ertapenem 1 g q24h |
| Solomkin et al., 2017 | Randomized, double-blind trial | 66 sites in 11 countries | 2013–2014 | Complicated intra-abdominal infection | 270 | 271 | 1.0 mg/kg q12h | Ertapenem 1 g q24h |
| Solomkin et al., 2018 | Randomized, double-blind trial | 65 sites in 11 countries | 2016–2017 | Complicated intra-abdominal infection | 250 | 250 | 1.0 mg/kg q12h | Meropenem 1 g q8h |
| NCT01978938 | Randomized, double-blind, double-dummy, prospective study | 99 sites in 18 countries | 2014–2015 | Complicated urinary tract infection | 455 | 453 | 1.5 mg/kg q24h | Levofloxacin 750 mg q24h |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, S.-H.; Lin, W.-T.; Chang, S.-P.; Lu, L.-C.; Lai, C.-C.; Wang, J.-H.; Chao, C.-M. Novel Tetracyclines Versus Alternative Antibiotics for Treating Acute Bacterial Infection: A Meta-Analysis of Randomized Controlled Trials. Antibiotics 2019, 8, 233. https://doi.org/10.3390/antibiotics8040233
Lan S-H, Lin W-T, Chang S-P, Lu L-C, Lai C-C, Wang J-H, Chao C-M. Novel Tetracyclines Versus Alternative Antibiotics for Treating Acute Bacterial Infection: A Meta-Analysis of Randomized Controlled Trials. Antibiotics. 2019; 8(4):233. https://doi.org/10.3390/antibiotics8040233
Chicago/Turabian StyleLan, Shao-Huan, Wei-Ting Lin, Shen-Peng Chang, Li-Chin Lu, Chih-Cheng Lai, Jui-Hsiang Wang, and Chien-Ming Chao. 2019. "Novel Tetracyclines Versus Alternative Antibiotics for Treating Acute Bacterial Infection: A Meta-Analysis of Randomized Controlled Trials" Antibiotics 8, no. 4: 233. https://doi.org/10.3390/antibiotics8040233
APA StyleLan, S.-H., Lin, W.-T., Chang, S.-P., Lu, L.-C., Lai, C.-C., Wang, J.-H., & Chao, C.-M. (2019). Novel Tetracyclines Versus Alternative Antibiotics for Treating Acute Bacterial Infection: A Meta-Analysis of Randomized Controlled Trials. Antibiotics, 8(4), 233. https://doi.org/10.3390/antibiotics8040233





