Exploring the Potential of High-Voltage Electric Field Cold Plasma (HVCP) Using a Dielectric Barrier Discharge (DBD) as a Plasma Source on the Quality Parameters of Carrot Juice
Abstract
1. Introduction
2. Material and Methods
2.1. Chemicals
2.2. Procurement of Raw Material, Blanching and Sample Preparation
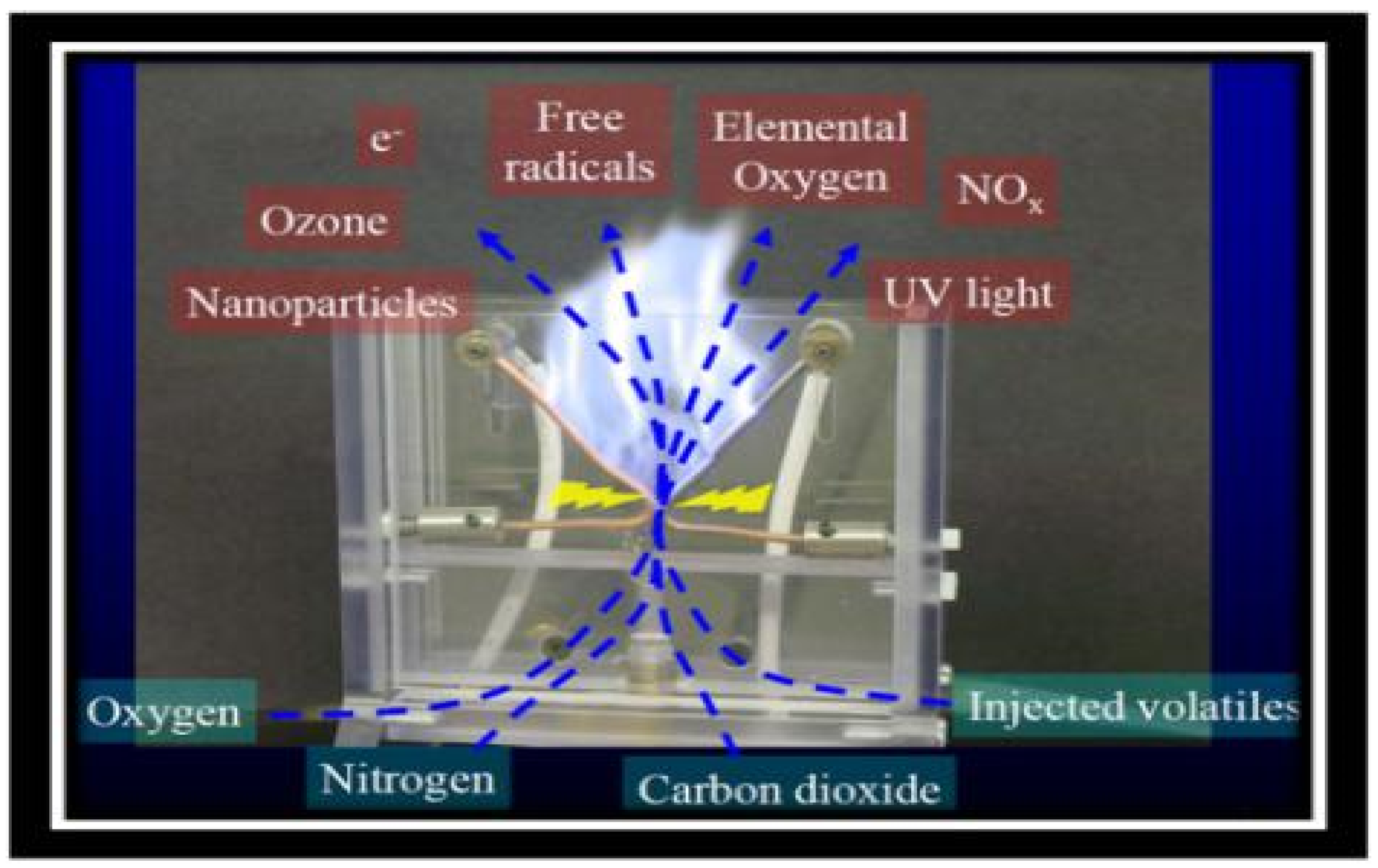
2.3. High Voltage Electric Field Cold Plasma Treatment (HVCP)
2.4. Residual Enzyme Activity
2.4.1. Peroxidase Enzyme Residual Activity
2.4.2. Polyphenol Oxidase Enzyme Residual Activity
2.4.3. Pectin Methylesterase Enzyme Residual Activity
2.4.4. Determination of Lipoxygenase Residual Activity
2.5. Determination of Coloring Compounds
2.5.1. Determination of Total Carotenoids
2.5.2. Determination of Lycopene Contents
2.5.3. Determination of β-carotene and Lutein
2.6. Determination of Chlorogenic Acid
2.7. Determination of Sugar Content in Carrot Juice
2.8. Phytochemicals Analysis
2.8.1. Determination of Mineral Contents
2.8.2. Total Phenolic Content
2.8.3. Total Flavonoid Contents
2.8.4. Total Tannin Contents
2.8.5. Determination of Brix
2.8.6. Determination of pH in Carrot Juice
2.8.7. Determination of Titratable Acidity
2.8.8. Determination of Color Changes in Carrot Juice
2.8.9. Determination of Ascorbic Acid
2.9. Microbiological Analysis
2.10. Statistical Analysis
3. Results and Discussion
3.1. Effect of HVCP on Enzyme Inactivation
3.2. Estimation of Coloring Compound
3.2.1. Determination of Chlorogenic Acid
3.2.2. Determination of Total Carotenoids and β-Carotene
3.2.3. Effect of HVCP on Lycopene and Lutein Contents
3.3. Determination of Sugars Content in Processed Carrot Juice
3.4. Impact of HVCP on the Mineral Profile of Carrot Juice
3.5. Effect of HVCP on Ascorbic Acid of Carrot Juice
3.6. Effect of HVCP Treatment on Phenolic Compounds
3.7. Effect of HVCP Treatment on pH, °Brix, Acidity, and Color Index
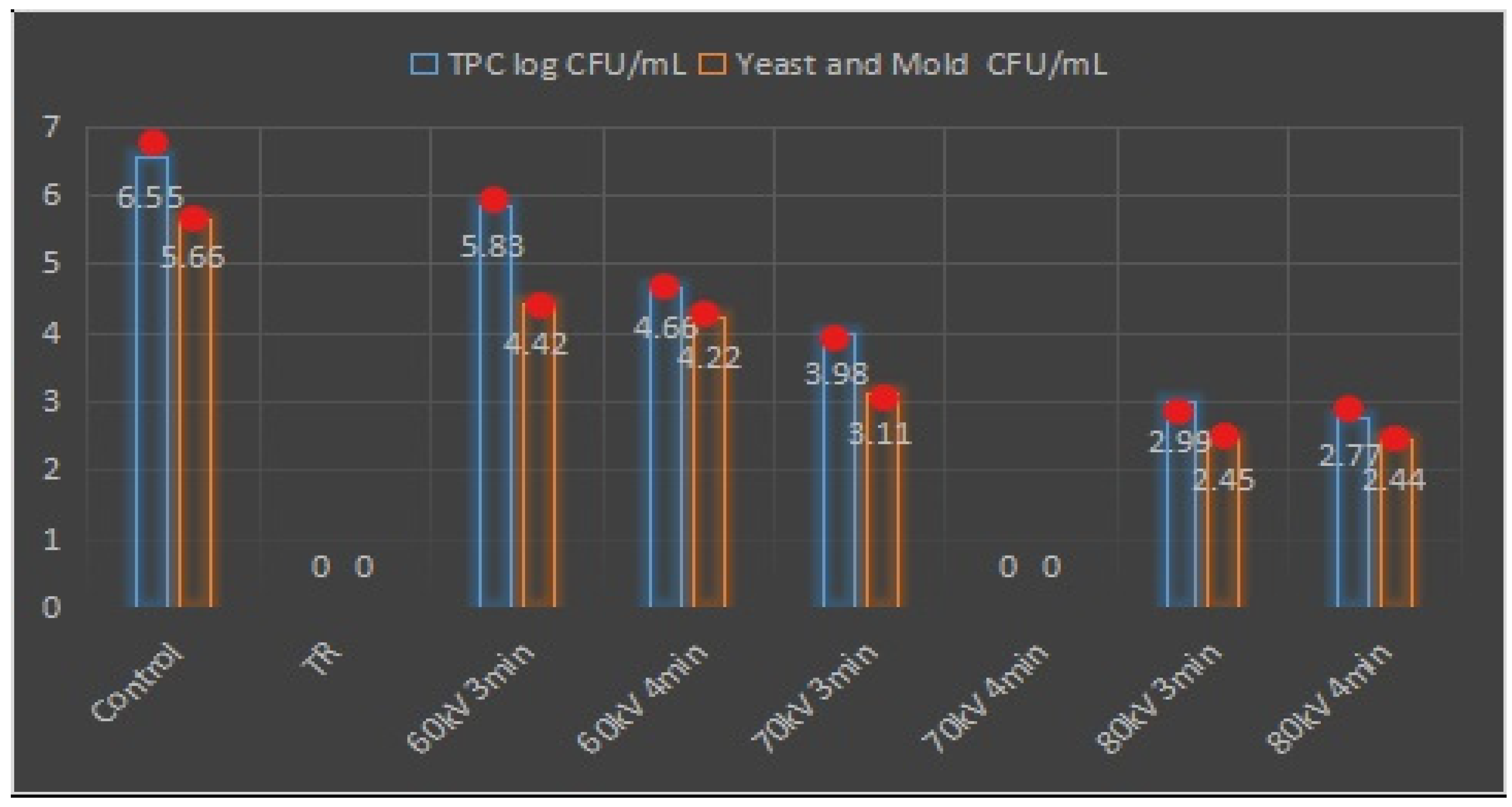
3.8. Antimicrobial Activity Assay
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shivhare, U.S.; Gupta, M.; Basu, S.; Raghavan, G.S.V. Optimization of blanching process for carrots. J. Food Process. Eng. 2009, 32, 587–605. [Google Scholar] [CrossRef]
- Sharma, K.D.; Karki, S.; Thakur, N.S.; Attri, S. Chemical composition, functional properties, and processing of carrot—A review. J. Food Sci. Technol. 2012, 49, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Talcott, S.T.; Howard, L.R. Phenolic autoxidation is responsible for color degradation in processed carrot puree. J. Agric. Food Chem. 1999, 47, 2109–2115. [Google Scholar] [CrossRef] [PubMed]
- Fellows, P. Tecnología de Alimentos Procesados: Principios y Practices; Acribia: Zaragoza, Spain, 1994; p. 48. [Google Scholar]
- Tournas, V.H.; Heeres, J.; Burgess, L. Moulds and yeasts in fruit salads and fruit juices. Food Microbiol. 2006, 23, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.S.; Shaw, P.E.; Parish, M.E. Orange and tangerine juices. In Fruit Juice Processing Technology; Nagy, S., Chen, C.S., Shaw, P.E., Eds.; Ag Science: Auburndale, FL, USA, 1993; pp. 110–165. [Google Scholar]
- Espachs-Barroso, A.; Barbosa-Cánovas, G.V.; Martín-Belloso, O. Microbial and enzymatic changes in fruit juice induced by high intensity pulse electric fields. Food Res. Int. 2003, 19, 253–273. [Google Scholar] [CrossRef]
- Gomez, P.L.; Welti-Chanes, J.; Alzamora, S.M. Hurdle technology in fruit processing. Annu. Rev. Food Sci. Technol. 2011, 2, 447–465. [Google Scholar] [CrossRef]
- Urvi, S.; Pietro, R.; Yuyuan, Z.; Caroline, L.; Schauer, V.M.; Gregory, F.; Jasreen, K.S. Effects of cold plasma treatments on spot-inoculated Escherichia coli O157: H7 and quality of baby kale (Brassica oleracea) leaves. Innov. Food Sci. Emerg. 2018, 57, 102104. [Google Scholar]
- Afshari, R.; Hosseini, H. Atmospheric pressure plasma technology: A new tool for food preservation. Int. Conf. Environ. Energy Biotechnol. 2012, 33, 275–278. [Google Scholar]
- Klockow, P.A.; Keener, K.M. Safety and quality assessment of packaged spinach treated with a novel ozone-generation system. LWT-Food Sci.Technol. 2009, 42, 1047–1053. [Google Scholar] [CrossRef]
- Smet, C.; Baka, M.; Steen, L.; Fraeye, I.; Walsh, J.L.; Valdramidis, V.P.; Van Impe, J.F. Combined effect of cold atmospheric plasma, intrinsic and extrinsic factors on the microbial behavior in/on (food) model systems during storage. Innov. Food Sci. Emerg. 2019, 53, 3–17. [Google Scholar] [CrossRef]
- Tolouie, H.; Mohammad-Ifar, M.A.; Ghomi, H.; Yaghoubi, A.S.; Hashemi, M. The impact of atmospheric cold plasma treatment on inactivation of lipase and lipoxygenase of wheat germs. Innov. Food Sci. Emerg. 2018, 47, 346–352. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Misra, N.N.; Cullen, P.J. Kinetics of tomato peroxidase inactivation by atmospheric pressure cold plasma based on dielectric barrier discharge. Innov. Food Sci. Emerg. 2013, 19, 153–157. [Google Scholar] [CrossRef]
- Ekezie, F.G.C.; Sun, D.W.; Cheng, J.H. A-review on recent advances in cold plasma technology for the food industry: Current applications and future trends. Trends Food Sci. Tech. 2017, 69, 46–58. [Google Scholar] [CrossRef]
- Nikfardjam, M.P.; Maier, D. Development of a headspace trap HRGC/MS method for the assessment of the relevance of certain aroma compounds on the sensorial characteristics of commercial apple juice. Food Chem. 2011, 126, 1926–1933. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Liu, X.; Li, J.; Liu, S.; Zhang, H.; Bai, Y. Effects of dielectric barrier discharge plasma on the inactivation of Zygosaccharomycesrouxii and quality of apple juice. Food Chem. 2018, 254, 201–207. [Google Scholar] [CrossRef] [PubMed]
- BursaćKovačević, D.; Putnik, P.; Dragović-Uzelac, V.; Pedisić, S.; RežekJambrak, A.; Herceg, Z. Effects of cold atmospheric gas-phase plasma on anthocyanins and color in pomegranate juice. Food Chem. 2016, 190, 317–323. [Google Scholar] [CrossRef]
- Kwak, S.S.; Kim, S.K.; Lee, M.S.; Jung, K.H.; Park, I.H.; Liu, J.R. Acidic peroxidases from suspension-cultures of sweet potato. Phytochemistry 1995, 39, 981–984. [Google Scholar] [CrossRef]
- Augustin, M.A.; Ghazali, H.M.; Hashim, H. Polyphenol-oxidase from guava (Psidium guajava L.). J. Sci. Food Agric. 1985, 36, 1259–1265. [Google Scholar] [CrossRef]
- De-Assis, S.A.; Martins, A.B.G.; Guaglianoni, D.G.; de-Faria Oliveira, O.M.M. Purification and characterization of pectin methylesterase from acerola (Malpighia glabra L.). J. Sci. Food Agric. 2002, 87, 1845–1849. [Google Scholar] [CrossRef]
- Kim, Y.; Park, K.; Lee, C. Purification and thermal inactivation of two lipoxygenases iso-enzymes from potato tubers. Korean J. Food Sci. Technol. 1987, 19, 397–402. [Google Scholar]
- Liao, H.; Sun, Y.; Ni, Y.; Liao, X.; Hu, X.; Wu, J.; Chen, F. The effect of enzymatic mash treatment, pressing, centrifugation, homogenization, deaeration, sterilization, and storage on carrot juice. J. Food Process. Eng. 2007, 30, 421–435. [Google Scholar] [CrossRef]
- Oliu, G.O.; Serrano, I.O.; Fortuny, R.S.; Belloso, O.M. Effects of high intensity pulsed electric field processing conditions on lycopene, vitamin C and antioxidant capacity of watermelon juice. Food Chem. 2009, 115, 1312–1319. [Google Scholar] [CrossRef]
- Saqib, J.; Muhammad, A.; Bing, H.; Tao, W.; Malik, M.H.; Lei, S.; Zhu, X.; Zeng, X. Quality of carrot juice as influenced by blanching and sonication treatments. LWT-Food Sci. Technol. 2014, 55, 16–21. [Google Scholar]
- Kahle, K.; Kraus, M.; Richling, E. Polyphenol profiles of apple juices. Mol. Nutr. Food Res. 2005, 49, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Hurst, W.J.; Martin, R.A.; Zoumas, B.L. Application of HPLC to the characterization of individual carbohydrates in foods. J. Food Sci. 1979, 44, 892–895. [Google Scholar] [CrossRef]
- American Public Health Association. Standard Method for the Examination of Water and Waste Water, 20th ed.; Water Environment Federation: New York, NY, USA, 1998. [Google Scholar]
- Saqib, J.; Abid, M.; Hu, B.; Muhammad, H.M.; Saeed, U.M.; Lei, S.; Wu, T.; Zeng, X. Influence of sonication and high hydrostatic pressure on the quality of carrot juice. Int. J. Food Sci. Tech. 2014, 49, 2449–2457. [Google Scholar]
- Broadhurst, R.B.; Jones, W.T. Analysis of condensed tannins using acidified vanillin. J. Sci. Food Agric. 1978, 29, 788–794. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis; AOAC International: Gaithersburg, MD, USA, 1999. [Google Scholar]
- Martínez-Hernández, G.B.; Amodio, M.L.; Colelli, G. Carvacrol-loaded chitosan nanoparticles maintain quality of fresh-cut carrots. Innov. Food Sci. Emerg. 2017, 41, 56–63. [Google Scholar] [CrossRef]
- Albertos; Martin-Diana, A.B.; Cullen, P.J.; Tiwari, B.K.; Shikha Ojha, K.; Bourke, P.; Rico, D. Shelf-life extension of herring (Clupea harengus) using in-package atmospheric plasma technology. Innov. Food Sci. Emerg. 2019, 53, 85–91. [Google Scholar] [CrossRef]
- Lee, H.; Jung, E.K.; Myong-Soo, C.; Sea, C.M. Cold plasma treatment for the microbiological safety of cabbage, lettuce, and dried figs. Food Microbiol. 2015, 51, 74–80. [Google Scholar] [CrossRef]
- Ismail, A.; Marjan, Z.M.; Foong, C.W. Total antioxidant activity, and phenolic content in selected vegetables. Food Chem. 2004, 87, 581–586. [Google Scholar] [CrossRef]
- Abid, M.; Jabbar, S.; Wu, T.; Hashim, M.M.; Hu, B.; Lei, S.; Zhang, X.; Zeng, X. Effect of ultrasound on different quality parameters of apple juice. Ultrason. Sonochem. 2013, 20, 1182–1187. [Google Scholar] [CrossRef] [PubMed]
- Aadil, R.M.; Zeng, X.N.; Sun, D.W.; Wang, M.S.; Liu, Z.W.; Zhang, Z.H. Combined effects of sonication and pulsed electric field on selected quality parameters of grapefruit juice. LWT-Food Sci. Technol. 2015, 62, 890–893. [Google Scholar] [CrossRef]
- Huang, W.; Bi, X.; Zhang, X.; Liao, X.; Hu, X.; Wu, J. Comparative study of enzymes, phenolics, carotenoids, and color of apricot nectars treated by high hydrostatic pressure and high-temperature short time. Innov. Food Sci. Emerg. 2013, 18, 74–82. [Google Scholar] [CrossRef]
- Shi, J.; Maguer, M.L. Lycopene in tomatoes: Chemical and physical properties affected by food processing. Crit. Rev. Food Sci. 2000, 40, 1–42. [Google Scholar] [CrossRef]
- Garcia, E.; Barrett, D.M. Assessing lycopene content in California processing tomatoes. J. Food Process. Pres. 2006, 30, 56–70. [Google Scholar] [CrossRef][Green Version]
- Rodrıguez-Sevilla, M.D.; Villanueva-Suárez, M.J.; Redondo-Cuenca, A. Effects of processing conditions on soluble sugars content of carrot, beetroot, and turnip. Food Chem. 1999, 66, 81–85. [Google Scholar] [CrossRef]
- Sert, D.; Aygun, A.; Demir, M.K. Effects of ultrasonic treatment and storage temperature on egg quality. Poultry Sci. 2011, 90, 869–875. [Google Scholar] [CrossRef]
- Barba, F.J.; Esteve, M.J.; Frigola, A. Physicochemical and nutritional characteristics of blueberry juice after high-pressure processing. Food Res. Int. 2013, 50, 545–549. [Google Scholar] [CrossRef]
- Schlüter, O.; Ehlbeck, J.; Hertel, C.; Habermeyer, M.; Roth, A.; Engel, K.H.; Eisenbrand, G. Opinion on the use of plasma processes for treatment of foods. Mol. Nutr. Food Res. 2013, 57, 920–927. [Google Scholar] [CrossRef]
- Alves-Filho, E.G.; Almeida, F.D.L.; Cavalcante, R.S.; de Brito, E.S.; Cullen, P.J.; Frias, J.M.; Rodrigues, S. 1H NMR spectroscopy and chemometrics evaluation of non-thermal processing of orange juice. Food Chem. 2016, 204, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.; Almeida, D.P.F.; Pintado, M. Changes in phenolic compounds during storage of pasteurized strawberry. Food Bioprocess. Tech. 2014, 7, 1840–1846. [Google Scholar] [CrossRef]
- Odriozola-Serrano, I.; Soliva-Fortuny, R.; Martin-Belloso, O. Phenolic acids, flavonoids, vitamin C and antioxidant capacity of strawberry juices processed by high-intensity pulsed electric fields or heat treatments. Eur. Food Res. Technol. 2008, 228, 239–248. [Google Scholar] [CrossRef]
- Rodríguez, Ó.; Gomes, W.F.; Rodrigues, S.; Fernandes, F.A.N. Effect of indirect cold plasma treatment on cashew apple juice (Anacardiumoccidentale L.). LWT-Food Sci. Technol. 2017, 84, 457–463. [Google Scholar] [CrossRef]
- Zoran, H.; Kovačević, D.B.; Kljusurić, J.G.; Jambrak, A.R.; Zorić, Z.; Dragović-Uzelac, V. Gas phase plasma impact on phenolic compounds in pomegranate juice. Food Chem. 2016, 190, 665–672. [Google Scholar]
- Almeida, F.D.L.; Cavalcante, R.S.; Cullen, P.J.; Frias, J.M.; Bourke, P.; Fernandes, F.A.N.; Rodrigues, S. Effects of atmospheric cold plasma and ozone on prebiotic orange juice. Innov. Food Sci. Emerg. 2015, 32, 127–135. [Google Scholar] [CrossRef]
- Brandenburg, R.; Ehlbeck, J.; Stieber, M.; Zeymer, J.; Schlüter, O.; Weltmann, K.D. Antimicrobial treatment of heat-sensitive materials by means of atmospheric pressure Rf-driven plasma jet. Contrib. Plasma Phys. 2007, 47, 72–79. [Google Scholar] [CrossRef]
- Stevens, C.; Wilson, C.; Lu, J.; Khan, V.; Chalutz, E.; Droby, S.; Pusey, L. Plant hormesis induced by ultraviolet light-C for controlling postharvest diseases of tree fruits. Crop. Prot. 1996, 15, 129–134. [Google Scholar] [CrossRef]
- Sarangapani, C.; Thirumdas, R.; Devi, Y.; Trimukhe, A.; Deshmukh, R.R.; Annapurna, U.S. Effect of low-pressure plasma on physicochemical and functional properties of parboiled rice flour. LWT-Food Sci. Technol. 2016, 69, 482–489. [Google Scholar] [CrossRef]
- Khoddami, A.; Wilkes, M.A.; Roberts, T.H. Techniques for analysis of plant phenolic compounds. Molecules 2013, 18, 2328–2375. [Google Scholar] [CrossRef]
- Grzegorzewski, F.; Ehlbeck, J.; Schlüter, O.; Kroh, L.W.; Rohn, S. Treating lamb’s lettuce with a cold plasma—Influence of atmospheric pressure Ar plasma immanent species on the phenolic profile of Valerianellalocusta. LWT-Food Sci. Technol. 2011, 44, 2285–2289. [Google Scholar] [CrossRef]
- Makris, D.P.; Rossiter, J.T. Hydroxyl free radical-mediated oxidative degradation of quercetin and morin: A preliminary investigation. J. Food Compos. Anal. 2002, 15, 103–113. [Google Scholar] [CrossRef]
- Fareez, I.M.; Lim, S.M.; Zulkefli, N.A.A.; Mishra, R.K.; Ramasamy, K. Cellulose Derivatives Enhanced Stability of Alginate-Based Beads Loaded with Lactobacillus Plantarum LAB12 against Low pH, High Temperature and Prolonged Storage. Probiotics Antimicro. 2018, 10, 543. [Google Scholar] [CrossRef] [PubMed]
- Ying, W.; Zirong, W.; Yahong, Y.; Zhenpeng, G.; Kangquan, G.; Tianli, Y. Application of gas-phase surface discharge plasma with a spray reactor for Zygosaccharomycesrouxii LB inactivation in apple juice. Innov. Food Sci. Emerg. 2019, 52, 450–456. [Google Scholar]



| Working Conditions | Flow Rate/Units |
|---|---|
| Elements (Na, P, K, and Mg) | 589.5, 213.6, 766.5, and 285.2 nm |
| Nebulized gas discharge | 0.85 L/Min |
| Plasma gas discharge | 16.5 L/Min |
| Auxiliary gas discharge | 0.21 L/Min |
| Plasma gas discharge | 15 L/Min |
| Sample flow rate | 1.8 mL/Min |
| Operating power | 1450 W |
| View Axial | Interface shear gas |
| Sample uptake rate | 1.25 mL/mint |
| Spray chamber | cyclonic |
| Nebuliser type | Meinhard |
| Nebuliser set up | Instant |
| Replicates | 3 times |
| Treatment | PPO Residual Activity (%) | POD Residual Activity (%) | PME Residual Activity (%) | LOX Residual Activity (%) |
|---|---|---|---|---|
| Control | 100 ± 0.00 a | 100 ± 0.00 a | 100 ± 0.00 a | 100 ± 0.00 a |
| TP-100-5 | 11.10 ± 0.01 f | 15.23 ± 0.25 g | 10.09 ± 0.11 f | 13.12 ± 0.32 g |
| HVCP 60 kV 3 min | 77.90 ± 0.20 b | 74.40 ± 0.52 b | 79.12 ± 0.20 b | 81.47 ± 0.12 b |
| HVCP 60 kV 4 min | 59.63 ± 0.43 c | 60.22 ± 0.20 c | 77.22 ± 0.31 c | 72.19 ± 0.12 c |
| HVCP 70 kV 3 min | 30.09 ± 0.53 e | 21.19 ± 0.14 f | 27.44 ± 0.80 h | 31.73 ± 0.13 f |
| HVCP 70 kV 4 min | 11.20 ± 0.09 f | 15.73 ± 0.35 g | 10.21 ± 0.19 f | 13.42 ± 0.21 g |
| HVCP 80 kV 3 min | 30.45 ± 0.40 e | 40.50 ± 0.14 e | 39.78 ± 0.41 e | 43.16 ± 0.65 e |
| HVCP 80 kV 4 min | 40.32 ± 0.72 d | 51.34 ± 0.27 d | 51.41 ± 0.55 d | 63.12 ± 0.22 d |
| Treatment | β-Carotene µg/100 mL | Chlorogenic Acid µg/mL | Carotenoids (µg/mL) | Lycopene (µg/mL) | Lutein (µg/mL) |
|---|---|---|---|---|---|
| Control | 24.11 ± 0.10 d | 22.30 ± 09 e | 8.22 ± 02 e | 0.52 ± 01 f | 1.22 ± 06 g |
| TP-100-5 | 20.63 ± 0.25 e | 18.67 ± 08 g | 7.81 ± 03 f | 0.61 ± 09 e | 1.35 ± 05 f |
| HVCP 60 kV 3 min | 24.12 ± 0.90 d | 21.93 ± 01 f | 9.01 ± 09 d | 0.91 ± 03 d | 1.43 ± 07 e |
| HVCP 60 kV 4 min | 24.21 ± 0.27 d | 23.16 ± 02 d | 10.03 ± 08 c | 1.83 ± 05 b | 1.56 ± 09 d |
| HVCP 70 kV 3 min | 25.23 ± 0.69 c | 24.04 ± 05 c | 9.19 ± 07 d | 1.08 ± 06 c | 1.51 ± 01 d |
| HVCP 70 kV 4 min | 26.54 ± 0.11 a | 27.31 ± 06 a | 12.06 ± 05 a | 1.93 ± 04 a | 2.03 ± 23 a |
| HVCP 80 kV 3 min | 25.19 ± 0.21 c | 24.31 ± 06 c | 11.23 ± 05 b | 1.80 ± 04 b | 1.63 ± 04 c |
| HVCP 80 kV 4 min | 25.84 ± 0.12 b | 25.31 ± 06 b | 11.03 ± 05 b | 1.81 ± 04 b | 1.76 ± 12 b |
| Treatment | Sucrose g/L | Fructose g/L | Glucose g/L |
|---|---|---|---|
| Control | 41.04 ± 01 e | 18.65 ± 01 b | 20.52 ± 06 c |
| TP-100-5 | 36.13 ± 03 f | 14.11 ± 02 e | 16.11 ± 02 e |
| HVCP 60 kV 3 min | 41.32 ± 02 c | 18.01 ± 04 b | 20.91 ± 03 b |
| HVCP 60 kV 4 min | 41.88 ± 06 d | 18.03 ± 03 b | 20.83 ± 04 b |
| HVCP 70 kV 3 min | 42.36 ± 06 b | 18.11 ± 02 b | 21.99 ± 06 a |
| HVCP 70 kV 4 min | 43.16 ± 03 a | 19.31 ± 01 a | 21.78 ± 02 a |
| HVCP 80 kV 3 min | 42.92 ± 04 b | 16.19 ± 07 c | 17.08 ± 04 d |
| HVCP 80 kV 4 min | 41.33 ± 01 c | 15.22 ± 02 d | 16.23 ± 02 e |
| Treatment | Ascorbic Acid mg/100mL | Total Phenols GAE (µg/g) | Total Flavonoid CE (µg/g) | Tannin CE mg/100mL |
|---|---|---|---|---|
| Control | 24.11 ± 0.10 e | 9.77 ± 0.20 e | 0.65 ± 0.88 f | 14.65 ± 0.13 g |
| TP-100-5 | 22.63 ± 0.25 f | 8.44 ± 0.11 f | 0.66 ± 0.34 e | 11.09 ± 0.11 h |
| HVCP 60 kV 3 min | 25.12 ± 0.90 c | 10.03 ± 0.9 c | 0.86 ± 0.51 c | 16.81 ± 0.09 d |
| HVCP 60 kV 4 min | 24.21 ± 0.27 d | 9.80 ± 0.17 d | 0.65 ± 0.09 f | 15.65 ± 020 f |
| HVCP 70 kV 3 min | 24.23 ± 0.69 d | 9.83 ± 0.12 d | 0.65 ± 0.23 f | 15.73 ± 0.86 e |
| HVCP 70 kV 4 min | 24.54 ± 0.08 d | 9.87 ± 0.04 d | 1.26 ± 0.55 a | 18.96 ± 0.50 a |
| HVCP 80 kV 3 min | 25.24 ± 0.67 b | 10.32 ± 0.2 b | 0.83 ± 0.43 d | 17.03 ± 0.99 c |
| HVCP 80 kV 4 min | 25.50 ± 0.84 a | 10.45 ± 0.13 a | 1.01 ± 0.22 b | 17.81 ± 0.21 b |
| Treatment | Brix | Acidity | pH | Color Index | ||
|---|---|---|---|---|---|---|
| L* | a* | b* | ||||
| Control | 7.77 ± 0.20 a | 0.10 ± 0.01 a | 6.08 ± 0.01 a | 35.31 ± 0.24 c | 19.53 ± 0.22 c | 27.06 ± 0.10 e |
| TP-100-5 | 7.77 ± 0.20 a | 0.11 ± 0.01 a | 6.08 ± 0.02 a | 38.83 ± 0.22 a | 20.83 ± 0.21 b | 34.59 ± 0.11 a |
| HVCP 60 kV 3 min | 7.77 ± 0.21 a | 0.10 ± 0.01 a | 6.08 ± 0.01 a | 36.72 ± 0.34 b | 18.58 ± 0.23 d | 30.95 ± 0.88 b |
| HVCP 60 kV 4 min | 7.77 ± 0.21 a | 0.11 ± 0.01 a | 6.08 ± 0.02 a | 33.76 ± 0.33 e | 17.80 ± 0.24 e | 26.35 ± 0.22 f |
| HVCP 70 kV 3 min | 7.77 ± 0.20 a | 0.11 ± 0.01 a | 6.08 ± 0.02 a | 34.51 ± 0.32 d | 18.21 ± 0.26 d | 29.12 ± 0.12 c |
| HVCP 70 kV 4 min | 7.77 ± 0.22 a | 0.11 ± 0.01 a | 6.08 ± 0.01 a | 38.41 ± 0.25 a | 21.04 ± 0.19 a | 33.96 ± 0.88 a |
| HVCP 80 kV 3 min | 7.77 ± 0.21 a | 0.11 ± 0.01 a | 6.08 ± 0.01 a | 36.50 ± 0.13 b | 16.49 ± 0.22 f | 29.19 ± 0.09 c |
| HVCP 80 kV 4 min | 7.77 ± 0.21 a | 0.11 ± 0.01 a | 6.08 ± 0.02 a | 35.51 ± 0.32 c | 19.21 ± 0.26 c | 28.12 ± 0.12 d |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umair, M.; Jabbar, S.; Nasiru, M.M.; Sultana, T.; Senan, A.M.; Awad, F.N.; Hong, Z.; Zhang, J. Exploring the Potential of High-Voltage Electric Field Cold Plasma (HVCP) Using a Dielectric Barrier Discharge (DBD) as a Plasma Source on the Quality Parameters of Carrot Juice. Antibiotics 2019, 8, 235. https://doi.org/10.3390/antibiotics8040235
Umair M, Jabbar S, Nasiru MM, Sultana T, Senan AM, Awad FN, Hong Z, Zhang J. Exploring the Potential of High-Voltage Electric Field Cold Plasma (HVCP) Using a Dielectric Barrier Discharge (DBD) as a Plasma Source on the Quality Parameters of Carrot Juice. Antibiotics. 2019; 8(4):235. https://doi.org/10.3390/antibiotics8040235
Chicago/Turabian StyleUmair, Muhammad, Saqib Jabbar, Mustapha Muhammad Nasiru, Tayyaba Sultana, Ahmed M. Senan, Faisal Nureldin Awad, Zhuang Hong, and Jianhao Zhang. 2019. "Exploring the Potential of High-Voltage Electric Field Cold Plasma (HVCP) Using a Dielectric Barrier Discharge (DBD) as a Plasma Source on the Quality Parameters of Carrot Juice" Antibiotics 8, no. 4: 235. https://doi.org/10.3390/antibiotics8040235
APA StyleUmair, M., Jabbar, S., Nasiru, M. M., Sultana, T., Senan, A. M., Awad, F. N., Hong, Z., & Zhang, J. (2019). Exploring the Potential of High-Voltage Electric Field Cold Plasma (HVCP) Using a Dielectric Barrier Discharge (DBD) as a Plasma Source on the Quality Parameters of Carrot Juice. Antibiotics, 8(4), 235. https://doi.org/10.3390/antibiotics8040235








