Abstract
The marine environment presents a high biodiversity and a valuable source of bioactive compounds with therapeutic and biotechnological potential. Among the organisms present in marine environment, the endophytic fungi isolated from seaweed stand out. These microorganisms have aroused interest in the scientific community regarding its various activities such as antiviral, antimicrobial, antioxidant, photoprotective, cytotoxic, genotoxic, anti-inflammatory, and anticancer, besides establishing important ecological relations with its hosts. Anticancer molecules derived from marine natural sources are a promising target against different types of cancer. The disease’s high rates of morbidity and mortality affect millions of people world wild and the search for new therapeutic alternatives is needed. Thus, this review partially summarizes the methodologies for the isolation of seaweed-derived endophytic fungi, as well as describes the anticancer compounds isolated from such microorganisms, reported in the literature from 2009 to the present. In addition, it describes how some biotechnological processes can help in the discovery of bioactive compounds, especially with anticancer activity.
1. Introduction
Cancer cells can develop from any tissue in the human body, they multiply and grow uncontrolled. Some types of cancers can form tumors and spread throughout the body, forming metastases [1]. Talking about cancer brings a wealth of information and discussions about the disease and major challenges that still loom in the 21st century. It is a disorder with remarkable growth and high rates of morbidity and mortality. Only in 2018, it was reported about 18 million cancer cases and 10 million deaths. The most common types of cancer are lung, breast (women) and colorectal, this one with 1.8 million new cases [1,2,3]. An estimate of the International Agency for Research on Cancer (IARC), World Health Organization is that in 2020 the number will increase to approximately 1 million [4]. Despite these scary numbers, not all cancers are malignant, with reports of some patients with a survival rate of over 90% [1]. Different treatment approaches may be used for cancer, such as surgical removal, radiotherapy, immunotherapy, and chemotherapy. Chemotherapy drugs can often be ineffective, where drug resistance occurs, undesirable side effects, reducing the patient’s quality of life. Recently, new drugs have been introduced to the market to increase the treatment potential of chemotherapeutic agents [1,3]. These drugs can be derived from natural sources and, in its original structure, semi-synthetics or analogous structure [5,6,7].
Plants, microorganisms (marine and terrestrial) and marine organisms are sources of natural products with new and unique chemical structures. In addition, many of these molecules are potential anticancer agents [6,8]. The recent approaches for prospecting new compounds with bioactivity, includes extracts dereplication, in silico strategies, mathematical and statistical modeling that can predict structure-activity relationships, and provide information about the physicochemical properties of moleculesto optimize the search/isolation process for the target drug [8].
In recent years, the search for new compounds from the marine environment has constantly increased due to the discovery of distinct chemical structures and their bioactivity. There are four clinical approved drugs from natural marine products that are in use for the treatment of cancer: cytarabine (Cytosar-U®), it is a derivative from a marine sponge, used for leukemia; trabectidine (Yondelis®), isolated from a tunicate, medicinally in use for ovarian cancer and soft tissue sarcoma; eribulin mesylate (Halaven®, for metastatic breast cancer), a derivative from a sponge and, brentuximab vedotin (Adcetris®, a conjugated antibody used in Hodgkin’s lymphoma and anaplastic large T-cell malignant lymphoma), from a mollusk source. Marine-derived substances with antitumoral activities are continuously being isolated and tested. For example, lurbinectedin, plitidepsin and plinabulin (isolated from marine fungus) are currently in phases I, II, and III of clinical trials [5,7,9].
The microorganisms’ classes that will be discussed in this article are the endophytic fungi derived from seaweed. In the marine environment, they can also be hosted in sponges for example; however, there are reports that their presence is prominent in macroalgae. Since the discovery of new substances in the marine environment, new sources are being investigated all the time, isolated fungal metabolites have shown to belong to different chemical classes, for instance, polyketides, lactones, steroids, alkaloids, terpenoids, isocoumarins, and phenols. Isolated fungal metabolites have various biological activities, such as antiviral, antimicrobial, antioxidant, photoprotective, cytotoxic, genotoxic, anti-inflammatory, anticancer and kinase inhibitors (drug targets for a number of therapeutic areas) [10,11,12,13,14].
Cytotoxic metabolites from endophytic fungi are well represented for the dimeric diketopirerazines, as an example we can cite the leptosins, which are inhibitors of the DNA topoisomerases (I and II) and, great candidates for anticancer drugs. They were isolated from the fungus Leptoshaeria sp., an endophyte of the macroalgae Sargassum tortile. Terpenoids, lactones, and alkaloids isolated from marine fungi also have cytotoxic activity against leukemia, HCT-116 colon carcinoma, A549 lung cancer cells and others [13,15].
The aim of this review is to report the findings about anticancer compounds isolated from seaweed derived-endophytic fungi in the last ten years (2009-2019) which have shown bioactivity against different types of cancer cell lines: NCI-H460 and NCI-H446 (human lung carcinoma), A-549 (adenocarcinomic human alveolar basal epithelial), MDA-MB-231 (human breast cancer), HCT-116 (human colon carcinoma), etc. Herein, we report the compounds from a variety species of macroalgae including Laurencia, Sargassum and Pterocladiella and their endophytes, especially, Aspergillus, Cladosporium and Penicillium. The secondary metabolites that are shown in the current paper present in vitro and/or in vivo studies tested in different cancer cells lines. We also summarize some mechanisms of action and biotechnological processes to obtain anticancer natural products. Due to the high incidence rate of cancer, resistance to many chemotherapeutic drugs and their adverse effects on the patient, as mentioned above, the relentless search for new natural products leads becomes necessary, in order to alleviate these unpleasant symptoms that occur during treatment and increase the chances of cure of the patient.
2. Endophytic Fungi
Endophytic fungi are a polyphyletic group comprising mainly Ascomycetous fungi [16], which have been on the focus of research interest of mycologists, chemists and pharmacists regarding to its ecological importance and biotechnological applications. In the past decades, much work has been done regarding the study of these microorganisms and many definitions for this ecological group has been proposed.
Petrini [17] defined endophytes as all microorganisms inhabiting plant organs that, at some time of their life, can colonize internal plant tissues without causing apparent harm to the host. Wilson [18] described the term “endophyte” as any organism living within plant tissues (Gr. Endon, within; phyton, plant) without manifestation of disease symptoms [19]. For Schulz and Boyle [20] fungal endophytes are colonizers of plant tissues that do not cause any visible disease symptoms to the host at any specific moment. Despite the definitions for the term and the word’s etymology reference to these microorganisms as plant associated only, currently the term endophyte has been used as synonym of mutualistic [21]. Fungal endophytes can be found in all climatic conditions in obligate or facultative relations. The lack of precise information points the need to understand the coexisting behavior between endophytes and their hosts and why host defenses does not work to eliminate the colonizing endophytes [19]. This might be related with the latent stage endophytes assume once they enter the host organism. However, endophytes can be influenced by environmental conditions or ontogenetic state of the host to become pathogenic.
All plants in natural ecosystems studied to date appear to be symbiotic with fungal endophytes [22,23]. Regarding to the marine environment, endophytes can be found in associations with seaweed and represent an ecologically defined group of marine-derived organisms denominated Marine Algicolous Fungi (MAFs) [24]. These organisms benefit from this ecological relation usually getting nutrition and protection from the host organism, in return, endophytes play important roles in the ecological adaptation of their host, such as increasing environmental stress tolerance, improving plant vigor and decreasing herbivore attack by producing certain secondary metabolites [25,26].
2.1. Ecological Role of Endophytic Fungi
Fungal associations with land plants date back from early evolutionary times [27]. Symbionts can affect host ecology, capacity of adaptation, and evolution [28], which includes the ability to form host communities, community structure and diversity of associated microorganisms [23].
Macroalgae are a prolific source of natural products with industrial interest and can have the secondary metabolism influenced by associated microorganisms such as endophytic fungi and vice-versa [29]. As it occurs in land plants, marine derived endophytic fungi were found to have physiological and ecological roles for the fungal-host interaction, which comprise nutritional enhancement, stabilization of host skeleton, and secondary metabolite production [30]. For example, Zuccaro et al. [31] reported a new species of Acremonium spp., Acremonium fuci which the conidia were only capable to germinate in the presence of Fucus serratus tissue or its aqueous tissue homogenates, and not in seawater alone. The production of these metabolites provides chemical adaptation to environmental conditions, substrate competitionand, also it helps to protect the host against pathogens attacks [19]. A recent study also reported a possible beneficial association of endophytic fungi and a marine brown alga, where the metabolites pyrenocines were able to protect the algae Pyropia yezoensis against the infection by protistan pathogens of marine algae, highlighting the importance of this symbiosis [32].
Many macroalgal species have been studied worldwide regarding their associated fungal communities, which includes the genera Ascophyllum, Ballia, Caulerpa, Ceramium, Ceratiodictyon, Cladophora, Chondrus, Dictyota, Dilsea, Egregia, Enteromorpha, Fucus, Gelidiella, Gracilaria, Grateloupia, Halimeda, Halymenia, Hypnea, Laminaria, Lobophora, Padina, Porphyra, Portieria, Saccorhiza, Sargassum, Stoechospermum, Turbinaria, and Ulva [33].
This review provides information concerning nine macroalgae genera, being Sargassum and Laurencia the most studied to date, followed by Enteromorpha, Ulva, Codium, Grateloupia, Leathesia, Pterocladiella, and Undaria. Associated to these genera, we can correlate 10 genera of endophytic fungi, which includes Aspergillus, Cladosporium, Paecilomyces, Chaetomium, Penicillium, Guignardia, Phoma, Talaromyces, Gibberella, Coniothyrium, being Aspergillus and Penicillium the prevalent ones (Table 1). Despite the number of studies addressing fungal endophytes from macroalgae, these works focus mainly in the secondary metabolite production of these organisms, which highlights the need for studies regarding the ecological roles of these microorganisms in macroalgae hosts.

Table 1.
Antitumor compounds isolated from seaweed derived-endophytic fungi literature from 2009 to the present. (B) = Brown macroalgae, (G) = Green macroalgae, (R) = Red macroalgae and Rf. = Reference.
2.2. Isolation of Endophytic Fungi
Since fungal endophytes live within host tissues, the isolation of these microorganisms is a method-dependent process [34]. The sterilization method has much influence in the microorganism’s isolation and, the techniques are diverse for each host organism being studied. Distinct hosts require different sterilization times, for example, thicker leaves require longer times and extreme sterilization conditions than thin leaves [22]. Epiphytic contaminants may interfere in the study if the sterilization method is not efficient, on the other hand the method cannot be very aggressive, and otherwise the number of isolates will be reduced, since it caused damage to the host tissues at the beginning of the process.
A control is also needed to make sure the fungi growing on the fragments are truly endophytes. Schulz et al. [35] developed a control method for discard epiphytic fungi. The method consists on making leaves imprints on the agar plate surface. The absence of fungi growing out on the imprinted agar plate indicates that the sterilization method can be considered effective. This review combined 24 studies regarding the marine fungi endophytes isolation, among them, we found out three variations of sterilization methodologies (Table 1), differing in time and sterility application.
Our research group developed a protocol for fungi endophytes isolation from tropical and polar macroalgae, from adaptations of the study of methodologies published by Erbert and co-workers [36], based on the publication of Kjer and co-workers [37]. The protocol consists in the utilization of three different sterilization methods, which begins with the macroalgae rinsing in distilled seawater followed by: (i) 15 seconds in ethanol (70% v/v), washing off macroalgae fragments in three different distilled water recipients to remove sterilizers residue; (ii) 5 seconds in ethanol 70% (v/v) followed by 5 seconds in sodium hypochlorite (2.5% v/v); and (iii) 5 seconds in ethanol 70% (v/v) followed by 10 seconds in sodium hypochlorite (2.5% v/v), after the sterilization procedure macroalgae segments are used to make imprints on agar surface (negative control 1). Macroalgae is then aseptically cut into fragments (0.5 mm to 1 cm) and placed in agar plates. The third distilled water of the washing procedure is also inoculated in agar plates, aiming to check no microorganism’s growth (negative control 2). To the selective isolation of fungi, the culture medium utilized is supplemented with chloramphenicol (200 mg·L−1) [38].
3. Cytotoxic Secondary Metabolites Produced by Endophytic Fungi
Fungi as well as bacteria, are versatile organisms that can be found in diverse habitats, occupying even inhospitable ecological niches, of all ecosystems around the planet. The ecological role of these organisms goes far beyond interactions between living beings, as they can adapt to virtually any environment and promote new biological interactions from chemical communications. The association between fungi and marine algae promotes the biosynthesis of metabolites with therapeutic potential by these microorganisms [62,63,64]. Currently, a large number of unique chemical structures with biological and pharmacological activities have been isolated of fungi from the marine environment and despite the absence of metabolites derived from theses microorganisms in the clinical pipeline, dozens of them have been classified as potential chemotherapy candidates [65]. Among these compounds, the chemical classes that have been seen are: polyketides, alkaloids, peptides, lactones, terpenes, and sterols. Distinct substances can be mentioned: asperpyrone A-D [49], cladosporols F−I [51], phomaketides A-E [60], cytoglobosins C-D [50], variloid A-B [56], cyclo-(Tyr-Leu), cyclo-(Phe-Pro) [54], insulicolide A [40], asperolide A-C, wentilactone A-B [44], penicisteroids A-B [57,58] and several others that were isolated from different species of fungi and can beseen on Table 1. The antitumor activity of these compounds was evaluated against several tumor cell lines, such as HeLa (human epithelial carcinoma), A-549 (adenocarcinomic human alveolar basal epithelial), HepG2 (human hepatocellular carcinoma), NCI-H460 and NCI-H446 (human lung carcinoma), SMMC-7721 (human hepatocarcinoma), SW1990 (human pancreatic cancer), MCF-7 (human breast adenocarcinoma), MDA-MB-231 (human breast cancer, HCT-116 (human colon carcinoma), PANC-1 (human pancreatic cancer), Caco-2 (human colorectal adenocarcinoma), Huh-7 (human hepatocarcinoma), DU145 (human prostate cancer), HL-60 (human leukemia), and others (Table 1).
The chemical and biological potential of fungi of marine origin in the search for new structures with promising antitumor activities led to the identification of several relevant compounds. Over the past few years, several metabolites produced by seaweed derived-endophytic fungi or other organisms have shown potent antitumor effects, which have been evaluated by different mechanisms, such as the ability to kill cancer cells with low or no toxicity to healthy host cells, blocking key enzymes, stimulating the pathways of death, or promoting growth arrest [65,66]. The current review, which covers the period 2009 to 2019, emphasizes the isolated metabolites from seaweed derived-endophytic fungi, that were able to inhibit growth different cancer cells.
3.1. Alkaloids and Nitrogen-containing Heterocycles
Cytochalasans are fungal alkaloids with biological activities cytoskeletal processes, including cytotoxicity against tumor cell lines. Cytoglobosins C and D (1-2) (Figure 1), cytochalasan derivatives, isolated from the cultures of Chaetominum globosum QEN-14, an endophytic fungus derived from the marine green algae Ulva pertusa showed moderate cytotoxic activity against adenocarcinomic human alveolar basal epithelial cell A-549 (IC50 2.26 and 2.55 µM, respectively) [50].
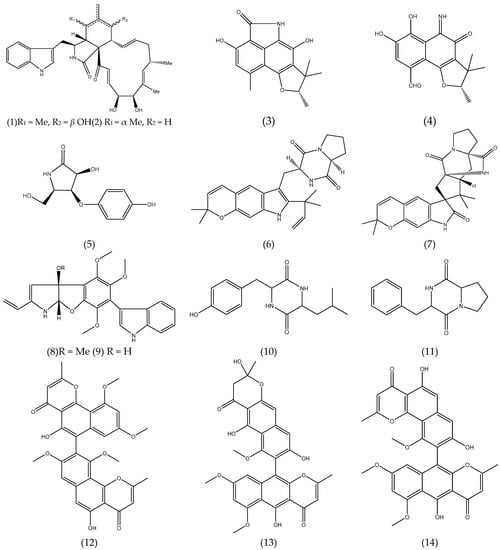
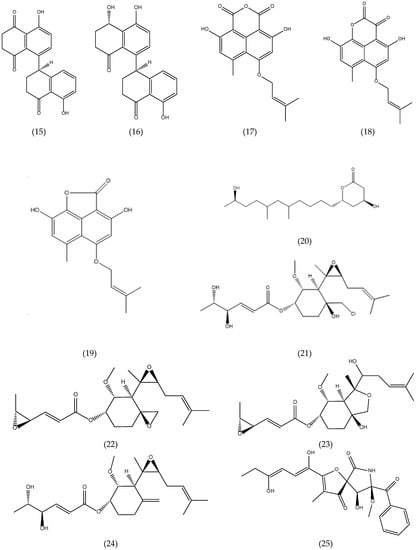
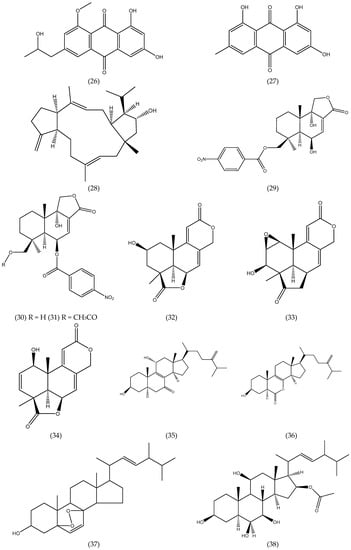
Figure 1.
Chemical structures of natural products isolated from seaweed derived-endophytic fungi with antitumor potential published from 2009 to 2019.
Polyketides-type alkaloids (−)−cereolactam (3) and (−)−cereoaldomine (4) (Figure 1) are phenalenone derivates that selectively inhibit the human leukocyte elastase (HLE) with IC50 values of 9.28 and 3.01 µM, respectively. These metabolites are as unprecedented structural types and may be formed by the biosynthetic degradation of phenalenone-type precursors. Compounds 3 and 4 were isolated from the Coniothyrium cereale, an endophytic fungus derived from marine green algae Enteromorpha sp. [67].
A pyrrolidine derivative,3-hydroxy-5-(hydroxymethyl)-4-(4’-hydroxyphenoxy)pyrrolidin-2-one (5) (Figure 1) was isolated from the cultures of Gibberella zeae, an endophytic fungus isolated from the marine green alga Codium fragile. Compound 5 showed that it possessed 61.80% and 17.60% inhibitory rates against A-549 and BEL-7402 tumor cell lines at 10 µM, respectively [68].
Prenylated indole alkaloids are a class of secondary metabolites commonly found in filamentous fungi, especially in the genus Penicillium and Aspergillus. Some of these compounds have insecticidal, cytotoxic, anthelmintic and antibacterial activities. Two prenylated indole alkaloids, dihydrocarneamide A (6) and iso-notoamide B (7) (Figure 1) were isolated from marine-derived endophytic fungus Paecilomyces variotii EN-291. These compounds were assayed for their cytotoxic activities against human large cell lung carcinoma cell line (NCI-H460) and showed weak activity with IC50 values of 69.30 and 55.90 µmol L−1, respectively [69]. The indole alkaloids, varioloid A (8) and B (9) (Figure 1) were also isolated from the marine alga-derived endophytic fungus Paecilomyces variotii EN-291. Compound 8 showed potent cytotoxicity against A-549, HCT116, and HepG2 cell lines, with IC50 values of 3.50, 6.40, and 2.50 μgmL−1, respectively, while compound 9 also showed considerable activities, with IC50 values of 4.60, 8.20, and 6.60 μg mL−1, respectively [56].
From the culture of the endophytic fungus Guignardia sp. isolated from brown algae Undaria pinnatifida (Harv.) Sur. collected in Changdao sea area, China, two of the five peptides that were isolated, cyclo-(Tyr-Leu) (10), cyclo-(Phe-Pro) (11) (Figure 1) presented cytotoxic activity. Both compounds exhibited activity against KB cell line with IC50 of 10.00 μg mL−1, comparable to that of 5-fluorouracil (2.50 μg mL−1) co-assayed as a positive reference [54].
3.2. Polyketides
As an example of polyketides with anticancer potential, natural naphthopyrones isolated from the endophytic fungus Aspergillus sp. XNM-4 derived from brown algae Leathesia nana are highlighted. Compounds asperpyrone B (12), aurasperone F (13) and especially asperpyrone A (14) (Figure 1) exhibited potent cytotoxicity on PANC-1, A-549, MDA-MB-231, Caco-2, SK-OV-3 and Hl-7702 cells. Compound (14) possessed the greatest inhibitory effects against PANC-1, with an IC50 value of 8.25 ± 2.20 µM [49].
Four new cladosporol derivatives, clodosporols F-I, the know clodosporol C and its new epimer, cladosporol J were isolated and identified from the marine algal-derived endophytic fungus Cladosporium cladosporioides EN-399. All compounds were assayed for cytotoxicity activity. Cladodosporol H (15) (Figure 1) exhibited significant cytotoxicity against A-549, Huh7 and LM3 cell lines with IC50 values of 5.00, 1.00, and 4.10 µM, respectively, clodosporol C (16) (Figure 1) showed activity against H446 cell line with IC50 value of 4.00 µM [51]. Cladosporols are known to be unique to the fungal genus Cladosporium and have attracted the attention of researchers because of their ability to stimulate cell cycle G1-phase arrest in human colon carcinoma HT-29 cells, thus demonstrating their promising antitumor activity [51,70,71,72].
From the culture of the marine-derived fungus Coniothyrium cereale isolated from the green alga Enteromorpha sp. collected from Fehmarn, Baltic Sea, seven new phenalenone derivatives as well as known compounds were isolated and assayed for cytotoxicity activity against human urinary bladder carcinoma cells 5637 (HTB-9) and HLE. The isolated compounds, coniosclerodin (17), conioscleroderolide (18) and coniolactone (19) (Figure 1) showed potent inhibition against HLE cell line with IC50 values of 7.20, 13.30 and 10.90 µM, respectively [52].
Penicitide A (20) (Figure 1) was isolated from the Penicillium chrysogenum QEN-24S, an endophytic fungus isolated from an unidentified marine red algal species of the genus Laurencia. Compound 20 exhibited moderate cytotoxic activity against the human hepatocellular liver carcinoma (HepG2) cell line with IC50 value of 32.00 µg mL−1 [57].
Seven new polyketides, phomaketides A-E, pseurotins A3 and G, besides 11 known compounds were purified from endophytic fungal strain Phoma sp. NTOU4195 isolated from the marine red alga Pterocladiella capillacea and evaluated for their antiangiogenic activity. Angiogenesis is defined as the formation of blood vessels from an existing vascular network towards a tumor, is crucial for the progression of the disease in tumor types. Thus, the antitumor activity of compounds isolated from Phoma sp. was assessed by the ability to prevent or not the capillary-like tube formation, which is one of the most important steps in angiogenesis. Compound phomaketide A (21) (Figure 1) exhibited the most potent antiangiogenic activity by suppressing the tube formation of endothelial progenitor cells (EPCs) with an IC50 of 8.10 µM. The others compound phomaketide C (22), D (23), E (24) and pseurotin G (25) (Figure 1) showed weak activity with IC50 of 17.80, 16.20, 19.20 and 16.70 µM, respectively [60].
3.3. Quinones
A new metabolite named isorhodoptilometrin-1-methy ether (26) (Figure 1) along with the known compounds emodin, 1-methyl emodin, evariquinone, 7-hydroxyemodin 6,8-methyl ether, siderin, arugosin C and variculanol were isolated from endophytic fungal strain Aspergillus versicolor, reported as endophyte from the inner tissue of the green alga Halimeda opuntia. Compound 26 demonstrated mild solid tumor selectivity HepG2 compared to the human normal cells (CFU-GM) when 3 µg of the pure compound was applied to the filter disk, while compounds emodin (27) and variculanol (28) (Figure 1) showed weak activity against HCT-116 and HepG2, respectively [43].
Five new polyhydroxylated hydroanthraquinone derivatives were isolated and identified from the culture extract of Talaromycesis landicus EN-501, an endophytic fungus obtained from the inner tissue of the marine red alga Laurencia okamurai. These compounds were assayed for cytotoxicity against sensitive and cisplatin-resistant human ovarian cancer cell lines A2780 and A2780 CisR, respectively. However, none of the compounds were active (IC50 < 10 µM) [61].
3.4. Terpenoids and Sterols
3.4.1. Terpenoids
The chemical investigation of the endophytic fungus Aspergillus ochraceus Jcma1F17, which was isolated from a marine alga Coelarthrum sp. resulted in the isolation of a new nitrobenzoyl sesquiterpenoid, 6β, 9α-dihydroxy-14-p-nitrobenzoylcinnamolide (29), and a known analogue, insulicolide A (30) (Figure 1). The compounds were evaluated for their cytotoxic activity against H1975, U937, K562, BGC-823, Molt-4, MCF-7, A-549, Hela, HL60 and Huh-7. Compounds 29 and 30 displayed significant cytotoxicity against 10 human cancer cell lines, with IC50 values of 1.95 to 6.35 µM [40]. There are few nitrobenzoyl sesquiterpenoids reported in the literature, most of them being obtained from fungi of the genus Aspergillus of marine origin that were isolated from sediment [73], and from marine organisms such as seaweed [40,41,72] and sponges [73].
Two new nitrobenzoyl sesquiterpenoids, insulicolide B and C were also isolated from culture extracts of the marine-derived fungus A. ochraceus Jcma1F17, together with three known nitrobenzoyl sesquiterpenoids and a derivative sesquiterpenoid. All compounds were evaluated for their cytotoxicity against three renal carcinoma cell lines, ACHN, OS-RC-2, and 786-O cells. Compounds 14-O-acetylinsulicolide A (31), 6β,9α-dihydroxy-14-p-nitrobenzoylcinnamolide (29) and insulicolide A (30) (Figure 1) displayed activities with IC50 values of 0.89 to 8.20 µM. Further studies indicated that compound 31 arrested the cell cycle at the G0/G1 phase at a concentration of 1 μM and induced late apoptosis at a concentration of 2.00 μM after a 72-h treatment of 786-O cells [41]. Therefore, the nitrobenzoyl sesquiterpenoids have attracted attention of researchers, because of their good antitumor potential.
Three new tetranorlabdane diterpenoids, asperolides A–C and five related derivatives (a tetranorditerpenoid derivative, wentilactones A and B, botryosphaerin B and LL-Z1271-β) were obtained of the culture extract of Aspergillus wentii EN-48, an endophytic fungus isolated from an unidentified marine brown alga species of the genus Sargassum. All compounds were assayed for their cytotoxic activities against HeLa, HepG2, MCF-7, MDA-MB-231, NCI-H460, SMMC-7721 and SW1990 tumor cell lines, with fluorouracil and adriamycin as positive controls. None of these compounds had significant activity (IC50 ≤ 10 µM). Wentilactone B (32) (Figure 1) was the most potent among the tested compounds with IC50 value of 17.00 µM against SMMC-7721 cell line [44].
Other researchers have explored the promising antitumor activity of compound 32. Zhang et al. [46] demonstrated that wentilactone B (32) could efficiently induce SMMC-7721 cells apoptosis, but not normal hepatic cells and inhibit the metastasis of this cell line. In addition, further studies have shown that wentilactone B (32) can significantly induce cell cycle arrest at G2 phase and mitochondrial-related apoptoses, accompanying the accumulation of reactive oxygen species (ROS). Therefore, this agent may be a potentially useful compound for developing anticancer agents for hepatocellular carcinoma [45].
Further studies carried out with wentilactone A (33), isolated from endophytic fungus A. wentii EN-48 [44], demonstrated that compound 33 triggered G2/M phase arrest and mitochondrial-related apoptosis in human lung carcinoma cells (NCI-H460 and NCI-H446), accompanying the ROS accumulation. In vivo studies, wentilactone A (33) (Figure 1) suppresses tumor growth without adverse toxicity and presented the same mechanism as that in vitro [47]. Asperolide A (34) (Figure 1) isolated from the endophytic fungus A. wentii EN-48 as previously mentioned [44] was also evaluated for its antitumor activity in NCI-H460 cells. Compound 34 leads to the inhibition of NCI-H460 lung carcinoma cell proliferation by G2/M arrest with the activation of the Ras/Raf/MEK/ERK signaling and p53-dependent p21 pathway. An in vivo study with asperolide A (34) illustrated a marked inhibition of tumor growth, and little toxicity compared to cisplatin therapy, which proves its potential antitumor activity [48].
3.4.2. Sterols
Two new steroid derivatives, 3β, 11α-dihydroxyergosta-8,24(28)-dien-7-one (35) and 3β-hydroxyergosta-8,24(28)-dien-7-one, and a rare 7-norsteroid with an un usual pentalactone B-ring system, the 7-Nor-esgosterolide (36) (Figure 1) were characterized from the culture extract of Aspergillus ochraceus EN-31, an endophytic fungus isolated from the marine brown alga Sargassum kjellmanianum. Others nine known related steroids were isolated and identified. The steroids cited were assayed for their cytotoxic activities against NCI-H460, SMMC-7721, SW1990, DU145, HepG2, HeLa and MCF-7 tumor cell lines. Compound 35 displayed selective cytotoxic activity against the SMMC-7721 cell line with an IC50 value of 28.00 μg mL−1, while compound 36 exhibited selective cytotoxic activity against NCI-H460, SMMC-7721 and SW1990 cell lines with IC50 values of 5.00, 7.00 and 28.00 μg mL−1, respectively [39].
From the culture of the endophytic fungus Guignardia sp. isolated from brown algae Undaria pinnatifida (Harv.) Sur. collected in Changdao sea area, China, ergosterol peroxide, 6,22-diene-5,8-epidioxyergosta-3-ol (37) (Figure 1) and ergosterol were isolated and assayed for cytotoxic activity against KB cell line. Compound 37 exhibited activity with IC50 of 20.00 μg mL−1 [54].
Penicisteroids A (38) and B (39), two new polyoxygenated steroids, together with seven known steroids were obtained from the culture extract of Penicillium chrysogenum QEN-24S, an endophytic fungus isolated from unidentified marine red algal species of the genus Laurencia. The cytotoxicity against seven tumor cells was determined and compound 38 displayed selective activity against the tumor cells line HeLa, SW1990 and NCI-H460 with the IC50 of 15.00, 31.00 and 40.00 μg mL−1, respectively, while the other compounds displayed weak or no appreciable activity. The hydroxyl group at C-6 in B-ring seems essential for their cytotoxicity, which is likelythe reason for that penicisteroid A (38) (Figure 1) showed cytotoxic activityagainst the cell lines HeLa, SW1990, and NCI-H460, while penicisteroid B (39) (Figure 1) showed no activity [58].
3.5. Others
From the culture of the fungus Aspergillus tennesseensis, a marine algal-derived endophytic fungus isolated from the fresh tissue of an unidentified marine alga, were isolated two new compounds with a prenylated diphenyl ether structure, diorcinol L and (R)-diorcinol B, along seven known compounds. All compounds were evaluated for cytotoxicity against six tumor cells lines (A-549, Du145, HeLa, MCF-7, MDA-MB-231 and THP-1) in vitro. Compound 3-(2-(1-hydroxy-1-methyl-ethyl)-6-methyl-2,3-dihydrobenzofuran-4-yloxy)-5-methylphenol (40) (Figure 1) selectively exhibited cytotoxicity against the THP-1 cell line with the IC50 value of 7.00 μg mL−1, while others displayed weak or no inhibitory activity (IC50> 50 μg mL−1) [42].
A new chromone derivatives, 2-(hydroxymethyl)-8-methoxy-3-methyl-4H-chromen-4-one (chromanone A) (41) (Figure 1), was obtained from de Penicillium sp., an endophytic fungus isolated from seaweed Ulva sp. The researchers tested the modulatory effect of compound 41 on carcinogen metabolizing enzymes. Carcinogens is activated by cytochrome P-450 1A (CYP1A) and detoxified by glutathione S-transferases (GST), quinine reductase (QR) and epoxide hydrolase (mEH). The researchers demonstrated in their studies that chromanone A is a promising inhibitor of CYP1A activity up to 60% of the stimulated-CYP1A in Hepa1c1c7 cells, and it significantly induced GST but not total thiols at low concentrations. In addition, chromanone A had influence on QR activity, while it resulted in a significant dose-dependent echancement mEH activity in Hepa1c1c7 cells. Therefore, chromanone A may act as an active tumoranti-initiating via modulation of carcinogen metabolizing enzymes and protection from DNA damage [59].
4. Biotechnology of Marine Endophytic Fungi
As previously reported endophytic fungi of marine origin have a promising therapeutic and biotechnological potential. Bioprospecting and the development of these products require sustainable processes that substantially increase the biomass of such microorganisms. Thus, marine biotechnology can contribute significantly to the production of these bioactive metabolites at various levels of the process, including obtaining, production, processing and development. However, biotechnological protocols applied to obtain bioactive metabolites of marine fungi are still quite deficient [74].
The methods and technologies applied in marine fungal biotechnology derive largely from terrestrial fungi processes and rarely reflect the specific demands of fungi of marine origin [74]. The most famous example of biotechnology applied to endophytic fungi is taxol, a multibillion-dollar anticancer compound produced in yew plant Taxus brevifolia by the terrestrial endophytic fungus Taxomyces andrenae [75,76]. The fascinating discoveries from studies with terrestrial endophytic fungi motive the studies of theses microorganisms of marine origin, mainly those isolated from seaweed. However, the ability to produce metabolites of therapeutic and/or biotechnological interest by endophytic fungi from marine algae has been underestimated, since many genes related to the biosynthesis of these substances are silenced in artificial laboratory culture conditions. Some strategies were applied to activate these silent gene clusters in filamentous endophytic fungi, for instance, the co-culture of microorganisms, mimetizing the original ecosystem is a possibility to stilted the production of natural products. Besides that, the epigenetic manipulation as well as allow the possibility to obtain new compounds and, through gene inactivation (“knock outs”), transcription factors, activation by the over expression and deletion of genes [77,78].
Biotechnological Processes to Obtain Bioactive Metabolites of Endophytic Fungi
In general, for the discovery of a new product with therapeutic, alimentary, cosmetic or biotechnological potential, a species of endophytic fungus is selected, its metabolites are extracted, it is carried out the screening for several activities (bioactivity-guided), process of isolation and the natural product is obtained in its pure form. This strategy of discovery is slow, tedious, intensely laborious and sometimes inefficient [79]. Dereplication and analytical methods are great strategies that are used to preview and to explore new secondary metabolites. Advances in this area of research have provided new strategies this discovery. It is possible to recognize by platforms (SMURF, antiSMASH, Fun-Gene Clusters) the gene clusters, which are responsible to synthesize individual groups of compounds, a disadvantage of this technique, is the inactivation of genes in the laboratory conditions as mentioned above. Starting with crude extracts and fractions it is possible to develop a sequence to dereplicate natural products. After the extraction and fractionation, the samples are analyzed indifferent equipments: LC-MS, and LC-MS/MS, UV, IR and NMR spectra, etc. The results are compared with known compounds present in databases (MarinLit, MassBank, MetLin, AntiBase, DNP, DMNP, PubChem) [80,81]. Kildgaard et al. [82] used the dereplication technique to analyze compounds from bioactive marine-derived fungi. The authors showed an integrated approach using ultra-high-performance liquid chromatography-diode array detection-quadrupole time off light mass spectrometry (UHPLC-DAD-QTOFMS) and identified polyketides, non-ribosomal peptides, terpenes, meroterpenoids and four novel isomers of the known anticancer compound asperphenamate, from marine-derived strains of Aspergillus, Penicillium and Emericellopsis. Another example of using the dereplication technique can be observed in the work of El-Elimat et al. [83], who analyzed fungal secondary metabolites in culture extracts using LC/DAD/MS with MS/MS spectral database for to search novel potent anticancer compounds before engaging in isolation process. An excellent tool for known and unknown compounds is the GNPS (Global Natural Products Social molecular networking), where MS/MS spectra are uploaded and, through the MS/MS are grouped forming chemically similar clusters. Thereafter, the known compounds are recognized by GNPS and nodes of unknown compounds can be selected as the target of possible new metabolites [80,81,84,85,86].
Biotechnology can help to accelerate the discovery of natural products of endophytic fungi of marine origin [74]. Silber et al. [74] describe some strategies for the application of marine biotechnology to the discovery of new compounds with antimicrobial activity and a large part of these strategies can be applied to other therapeutic activities or biotechnological processes. For example: (i) controlled miniaturization to increase screening efficiency, that is, the small-scale culture in fermentation systems using microtiter plates [87,88,89] or specialized miniaturized fermentation systems (System Duetz or BioLector) [90,91,92]; (ii) directed stimulus of strains to expand the chemodiversity, because the proper understanding of the endophytic fungi that produce the bioactive substances and their ecological role help to find the ideal conditions of cultivation and production and can extend the chemical diversity [74,93]; (iii) metabolomic, proteomic, and transcriptomic studies in order to elucidate the metabolic state of the cells and indicating regulatory sites at the levels of DNA, RNA and protein; from this knowledge it is possible to induce and direct the biosynthetic process and later to control it [94,95]. A comparative proteomic study of a fungus of marine origin Microascus brevicaulis revealed how the biotechnological fermentation process should be controlled in order to increase the production of anticancer compounds, scopularides A and B [96].
One of the major challenges for those working with natural products, regardless of source is obtaining sufficient amounts of the metabolite of interest for drug development. Biotechnological approaches such as the production using full fermentative process can assist in obtaining these products. That is, large-scale cultivation (bioreactors) using different approaches to optimize culture media and induce increased production of target molecules are an alternative to this problem [79]. However, the research addressing the optimization of marine fungal fermentation in bioreactors is insufficient [97]. The use of bioreactors allows the control of crucial factors such as aeration, dissolved oxygen, carbon dioxide, pH, salinity, temperature, foaming and growth under agitation or in static mode, among others. The control and understanding of such parameters is the basis for successful strategies on a larger scale [98]. Xu et al. [99] optimized the static culture conditions of Aspergillus wentii EM-48, a fungus isolated from brown seaweed in order to increase the production of asperid A. Factors such as salinity, initial pH, temperature, culture time and addition of plant growth regulators were evaluated in the study cited. Zhou et al. [100] increased biosynthesis of compound 1403C (also called SZ-6858) synthesized by mangrove endophytic fungus Halorosellinia sp. in bioreactor fermentation. Medium, culture pH, agitation speed, impeller type, inoculum level and dissolved oxygen were considered and systematically optimized, and an integrated nutrition and bioprocess strategy was established. The resulting 1403C production reached 2.07 g·L−1, which was 143.5% higher than the original production. Another study showed that the distribution of anticancer 1403C in the fermentation broth of the fungus Halorosellinia sp. was closely related to pH variations. The 1403C levels in the supernatant decreased while in mycelium increased with increasing pH. Thus, pH regulation was proposed and applied in order to accumulate the compound in the mycelium prior to broth extraction [101].
In addition to the physical-chemical parameters that must be controlled, the morphology also influences the biosynthesis of the target product in filamentous fungi of marine origin. Filamentous fungi have the ability to grow in different morphological appearances, ranging from dispersed filaments to highly dense networks of mycelia (pellets). Therefore, depending on the morphology presented by the fungus under study, effects such as oxygenation and nutrient levels may alter the biosynthesis of these microorganisms. For example, it was observed that in higher speeds for the fungus Penicillium chysogenum showed some consequences, like as, a decreasing in penicillin productions (1250 and 1500 rpm) and elevation in cell growth (100–900 rpm), besides restriction of oxygen in 700 rpm, however, this does not mean that it diminishes the production of other substances for the fungus P. chysogenum [102]. These and other parameters should be studied and explored in transferring crops to larger scales as in bioreactors.
When the use of bioreactors cannot be carried out in an economically feasible manner and there is a structural chemical complexity limiting the synthesis of the product of interest, semi-synthetic processes offer an alternative pathway in the development of natural products derived from marine fungi [74]. In the semi-synthesis, precursor molecules obtained by fermentation process are processed by synthesis, or a synthetic product is modified by bioconversion using enzymes, whole cells or even fermentative processes [103]. Jesus et al. [104] report the bio-oxidation of rac-camphor using whole cells of marine-derived fungus Botryosphaeria sp. isolated from marine alga Bostrychia radicans obtaining mainly (49%) 6-endo-hydroxycamphor and other products. These products are important for use in pharmaceutical formulations and these chemical modifications usually cannot be done by conventional organic synthesis.
In addition to the biotechnological applications already described (bioreactors and semi-synthesis) for the production of bioactive compounds from marine fungi, there isalso the use of heterologous systems and genetic and metabolic engineering [74]. For example, transfer of DNA from the environment to a host strain may allow the production of novel compounds [105]. The use of molecular techniques provides an alternative for heterologous expression of "silent" gene aggregates and directed manipulation of biosynthetic genes, optimizing the production process of the target compound. However, these techniques are still limited because of the complexity and size of the genetic groups encoding natural products. An example is the reprogramming of the biosynthetic pathway leading to the over expression of ennantianins, isolated from Halosarpheia sp. [74]. A review considering silent biosynthetic gene clusters activation brings several compounds isolated from fungi, as terraquinone A from Aspergillus terreus, emodin from A. nidulans, new cladochrome congener from Cladosporium cladosporides and nygerone A from A. niger [106]. Another study performed with Glarea lozoyensis, led to the isolation of pneumocandin derivatives, such as caspofungin from the pneumocandin B0 pathway. The new substances obtained show more pharmacological properties which are important for drug discovery [107]. In relation to genetic and metabolic engineering, the improvement of high yield lineages and the cloning of genes involved in the biosynthetic pathway allow, for example, to increase the flow of primary metabolites in the production of secondary metabolites. The greatest success was the optimization of the fermentation process and breeding of Acremonium chrysogenum strains in the production of cephalosporin C [108].
After the production process of the metabolites, for example in bioreactors, the next step in the development of a product of therapeutic interest by biotechnological procedures is “downstream processing” (DSP). This process comprises the steps of separating, disrupting, capturing and concentrating the cells, as well as extracting, purifying, refining, and obtaining the final product. Few authors report these DSP processes in fungal biotechnology, a classic example is the enzymatic treatment as part of the purification of cephalosporin [74,108]. The most effective process with cost, energy and space reduction is one that applies continuous “upstream and downstream processing”, but this procedure seems to be empirical and there are still no standards that are suitable for all chemical classes to be obtained [109,110]. From the purified final product, other processes related to medicinal chemistry can be performed, such as structure-activity relationship (SAR) studies and mechanism of action. These works help to improve bioavailability and to understand the therapeutic application of the compound [74].
All procedures cited here to advancethe performance in the discovery, production, and procurement of a natural product from marine endophytic fungi need to be improved taking into account the ideal developmental conditions and particular characteristics of these microorganisms. Therefore, further studies related to fungal biotechnology should be carried out in order to obtain more information and improve the procedures for obtaining a quality final product.
5. Conclusions
Marine fungi can grow on a wide variety of substrates such as wood, sediment, sand, mangroves, corals, shellfish, and marine invertebrates, on the surface and interior of algae. Among the fungi that live associated with macroalgae, endophytic fungi have a diversity of species and stand out as an extraordinary source of new bioactive compounds, as well as compounds known from other sources. Microorganisms have advantages such as the short growth cycle, easy preservation and control of cultivation conditions, besides perform strain through mutation breeding, genetic engineering, and other means. Therefore, it can significantly increase productivity and achieve industrial production. Consequently, studying seaweed-derived endophytic fungi to produce secondary metabolites has a very broad prospect on medical research and development.
The incidence rate of cancer has been growing, due to the influence of the living environment and living habits and the increasing aging of the population. High costs, serious side-effects, and multidrug-resistance tumors accelerate the complexity and difficulty of cancer treatment. Thus, the increasing demand for anticancer drugs in the international market, the search for novel and effective molecules from endophytic fungi has become urgent. This review summarizes a total of 41 (Figure 1) compounds, which belongs to alkaloids, terpenes, steroids, polyketides, and quinones. All of them showing cytotoxic activities, produced by seaweed derived-endophytic fungi described from 2009 to 2019. In addition, the isolation techniques of the microorganisms discussed here must be well defined, as well as the procedures to obtain these metabolites. In this review, it was possible to note that few studies have detailed the forms of isolation of endophytic fungi, as well as the scarce number of papers describing the fungal biotechnology application to obtaining bioactive products from fungi of marine origin, mainly anticancer compounds.
Author Contributions
T.R.T. and G.S.d.S., and L.A. in Pharmaceutical Sciences, wrote the manuscript with the support from the idea of H.M.D. and P.C., H.M.D. encouraged T.R.T., G.S.d.S. and L.A. to investigate and supervised the work, helped in data interpretation and manuscript evaluation and acted as the corresponding author. All authors discussed the results and contributed to the final manuscript.
Funding
This study had financial and logistic support from the Brazilian Antarctic Program (PROANTAR/MCT/CNPq N64/2013) and Brazilian Marine Force, National Institute of Science and Technology (INCT: BioNat), Grant # 465637/2014–0, and the State of São Paulo Research Foundation (FAPESP), Grants # 2014/50926–0 and # 2016/06931-4.
Acknowledgments
The authors are thankful to University of São Paulo for providing access to necessary resources, the financial and Fellowship Support from the Brazilian research funding agencies Coordination of Improvement of Higher Level Personnel (CAPES), National Council for Scientific and Technological Development (CNPq). The department of Physics and Chemistry are acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nivetha, X.R.; Soundari, M.J.J.; Nadar, M.S.A.M.; Premnath, D.; Selvakumar, P.M.; Chang, R. An insight into cancer and anticancer drugs. Acta Sci. Med. Sci. 2019, 3, 32–43. [Google Scholar]
- Worldwide Cancer Data: Global Cancer Statistics for the Most Common Cancers. Available online: https://www.wcrf.org/dietandcancer/cancer-trends/worldwide-cancer-data (accessed on 31 July 2019).
- Rocha, D.H.A.; Seca, A.M.; Pinto, D.C.G.A. Seaweed secondary metabolites in vitro and in vivo anticancer activity. Mar. Drugs 2018, 16, 410. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. World Health Organization. Available online: http://gco.iarc.fr/tomorrow/graphic-isotype?type=0&population=900&mode=population&sex=0&cancer=39&age_group=value&apc_male=0&apc_female=0 (accessed on 31 July 2019).
- Gerwick, W.H.; Moore, B.S. Lessons from the past and charting the future of Marine Natural Products drug discovery and chemical biology. Chem. Biol. 2012, 19, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Newman, D.J. Natural products: Current Approaches and perspectives. In Natural Products as Source of Molecules with Therapeutic Potential; Cechinel, F.V., Ed.; Springer: Cham, Switzerland, 2018; pp. 309–331. [Google Scholar] [CrossRef]
- Jimenez, P.C.; Wilke, D.V.; Costa-Lotufo, L.V. Marine drugs for cancer: Surfacing biotechnological innovations from the oceans. Clinics 2018, 73, e482s. [Google Scholar] [CrossRef]
- Rayan, A.; Raiyn, J.; Falah, M. Nature is the best source of anticancer drugs: Indexing natural products for their anticancer bioactivity. PLoS ONE 2017, 12, e0187925. [Google Scholar] [CrossRef]
- Dyshlovoy, S.A.; Honecker, F. Marine compounds and cancer: 2017 updates. Mar. Drugs 2018, 16, 41. [Google Scholar] [CrossRef]
- Pavão, G.B.; Venâncio, V.P.; Oliveira, A.L.L.; Hernandes, L.C.; Almeida, M.R.; Antunes, L.M.G.; Debonsi, H.M. Differential genotoxicity and cytotoxicity of phomoxanthone A isolated from the fungus Phomopsis longicolla in HL60 cells and peripheral blood lymphocytes. Toxicol. In Vitro 2016, 37, 211–217. [Google Scholar] [CrossRef]
- Maciel, O.M.C.; Tavares, R.S.N.; Caluz, D.R.E.; Gaspar, L.R.; Debonsi, H.M. Photoprotective potential of metabolites isolated from algae-associated fungi Annulohypoxylon stygium. J. Photochem. Photobiol. B 2018, 178, 316–322. [Google Scholar] [CrossRef]
- Deshmukh, S.K.; Prakash, V.; Ranjan, N. Marine fungi: A source of potential anticancer compounds. Front. Microbiol. 2017, 8, 2536. [Google Scholar] [CrossRef]
- Jeewon, R.; Luckhun, A.B.; Bhoyroo, V.; Sadeer, N.B.; Mahomoodally, F.M.; Rampadarath, S.; Puchooa, D.; Sarma, V.; Kumar Durairajan, S.S.; Hyde, K.D. Pharmaceutical Potential of Marine Fungal Endophytes. In Endophytes and Secondary Metabolites; Jha, S., Ed.; Reference Series in Phytochemistry; Springer: Cham, Switzerland, 2019; pp. 1–23. [Google Scholar] [CrossRef]
- Li, T.; Wang, N.; Zhang, T.; Zhang, B.; Sajeevan, T.P.; Joseph, V.; Armstrong, L.; He, S.; Yan, X.; Naman, B. A Systematic Review of Recently Reported Marine Derived Natural Product Kinase Inhibitors. Mar. Drugs 2019, 17, 493. [Google Scholar] [CrossRef]
- Yanagihara, M.; Sasaki-Takahashi, N.; Sugahara, T.; Yamamoto, S.; Shinomi, M.; Yamashita, I.; Hayashida, M.; Yamanoha, B.; Numata, A.; Yamori, T.; et al. Leptosins isolated from marine fungus Leptoshaeria species inhibit DNA topoisomerases I and/or II and induce apoptosis by inactivation of Akt/protein kinase B. Cancer Sci. 2005, 96, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Aly, A.H.; Debbab, A.; Kjer, J.; Proksch, P. Fungal endophytes from higher plants: A prolific source of phytochemicals and other bioactive natural products. Fungal Divers. 2010, 41, 1–16. [Google Scholar] [CrossRef]
- Petrini, O. Fungal Endophytes of Tree Leaves. In Microbial Ecology of Leaves; Andrews, J.H., Hirano, S.S., Eds.; Brock/Springer: New York, NY, USA, 1991; pp. 179–197. ISBN 978-1-4612-3168-4. [Google Scholar]
- Wilson, D. Endophyte: The Evolution of a Term, and Clarification of Its Use and Definition. Oikos 1995, 73, 274–276. [Google Scholar] [CrossRef]
- Singh, R.P.; Kumaru, P.; Reddy, C.R.K. Antimicrobial compounds from seaweeds-associated bacteria and fungi. Appl. Microbiol. Biotechnol. 2015, 99, 1571–1586. [Google Scholar] [CrossRef]
- Schulz, B.; Boyle, C. The endophytic continuum. Mycol. Res. 2005, 109, 661–686. [Google Scholar] [CrossRef]
- Mani, V.M.; Soundari, A.P.G.; Karthiyaini, D.; Preeth, K. Bioprospecting endophytic fungi and their metabolites from medicinal tree Aegle marmelosin Western Ghats, India. Mycobiology 2015, 43, 303–310. [Google Scholar] [CrossRef]
- Hyde, K.D.; Soytong, K. The fungal endophyte dilemma. Fungal Divers. 2008, 33, 163–173. [Google Scholar]
- Rodriguez, R.J.; White, J.F., Jr.; Arnold, A.E.; Redman, A.R.A. Fungal endophytes: Diversity and functional roles. New Phytol. 2009, 182, 314–330. [Google Scholar] [CrossRef]
- Ji, N.Y.; Wang, B.G. Mycochemistry of marine algicolous fungi. Fungal Divers. 2016, 80, 301–342. [Google Scholar] [CrossRef]
- Yan, J.F.; Broughton, S.J.; Yang, S.L.; Gange, A.C. Do endophytic fungi grow through their hosts systemically? Fungal Ecol. 2015, 13, 53–59. [Google Scholar] [CrossRef]
- Tan, R.X.; Zou, W.X. Endophytes: A rich source of functional metabolites. Nat. Prod. Rep. 2001, 18, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Debbab, A.; Aly, A.H.; Proksch, P. Endophytes and associated marine derived fungi—Ecological and chemical perspectives. Fungal Divers. 2012, 57, 45–83. [Google Scholar] [CrossRef]
- Brundrett, M.C. Understanding the roles of multifunctional mycorrhizal and endophytic fungi. In Microbial Root Endophytes; Schulz, B.J.E., Boyle, C.J.C., Sieber, T.N., Eds.; Springer: Berlin, Germany, 2006; pp. 281–293. [Google Scholar] [CrossRef]
- Felício, R.; Pavão, G.B.; Oliveira, A.L.; Erbert, C.; Conti, R.; Pupo, M.T.; Furtado, N.A.J.C.; Ferreira, E.G.; Costa-Lotufo, L.V.; Young, M.C.M.; et al. Antibacterial, antifungal and cytotoxic activities exhibited by endophytic fungi from the Brazilian marine red alga Bostrychia tenella (Ceramiales). Braz. J. Pharmacog. 2015, 25, 641–650. [Google Scholar] [CrossRef]
- Zhou, K.; Zhang, X.; Zhang, F.; Li, Z. Phylogenetically diverse cultivable fungal community and polyketide synthase (PKS), nonribosomal peptide synthase (NRPS) genes associated with the South China Sea sponges. Microb. Ecol. 2011, 62, 644–654. [Google Scholar] [CrossRef]
- Zuccaro, A.; Mitchell, J.I. Fungal communities of seaweeds. In The Fungal Community, 3rd ed.; Dighton, J., White, J.F., Eds.; CRC Press: Boca Raton, FL, USA, 2005; pp. 533–579. [Google Scholar] [CrossRef]
- Vallet, M.; Strittmatter, M.; Murúa, P.; Lacoste, S.; Dupont, J.; Hubas, C.; Prado, S. Chemically-mediated interactions between macroalgae, their fungal endophytes, and protistan pathogens. Front. Microbiol. 2018, 9, 3161. [Google Scholar] [CrossRef] [PubMed]
- Godinho, V.M.; Furbino, L.E.; Santiago, I.F.; Pellizzari, F.M.; Yokoya, N.; Pupo, D.; Cantrell, C.L. Diversity and bioprospecting of fungal communities associated with endemic and cold-adapted macroalgae in Antarctica. ISME J. 2013, 7, 14–34. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.D.; Hyde, K.D.; Liew, E.C. Detection and taxonomic placement of endophytic fungi within frond tissues of Livistona chinensis based on rDNA sequences. Mol. Phylogenet. Evol. 2001, 20, 1–13. [Google Scholar] [CrossRef]
- Schulz, B.; Guske, S.; Dammann, U.; Boyle, C. Endophyte-host interactions. II. Defining symbiosis of the endophyte-host interaction. Symbiosis 1998, 25, 213–227. [Google Scholar]
- Erbert, C.; Lopes, A.A.; Yokoya, N.S.; Furtado, N.A.J.C.; Conti, R.; Pupo, M.T.; Lopes, J.L.C.; Debonsi, H.M. Antibacterial compound from the endophytic fungus Phomopsis longicolla isolated from the tropical red seaweed Bostrychia radicans. Bot. Mar. 2012, 55, 435–440. [Google Scholar] [CrossRef]
- Kjer, J.; Debbab, A.; Aly, A.H.; Proksch, P. Methods for isolation of marine-derived endophytic fungi and their bioactive secondary products. Nat. Protoc. 2010, 5, 479–490. [Google Scholar] [CrossRef]
- Teixeira, T.R.; Santos, G.S.; Turatti, I.C.C.; Paziani, M.H.; Kress, M.R.Z.; Colepicolo, P.; Debonsi, H.M. Characterization of the lipid profile of Antarctic brown seaweeds and their endophytic fungi by gas chromatography–mass spectrometry (GC–MS). Polar Biol. 2019, 42, 1431–1444. [Google Scholar] [CrossRef]
- Cui, C.M.; Li, X.M.; Meng, L.; Li, C.S.; Huang, C.G.; Wang, B.G. 7-Nor-ergosterolide, a Pentalactone Containing Norsteroid and Related Steroids from the Marine-Derived Endophytic Aspergillus ochraceus EN-31. J. Nat. Prod. 2010, 73, 1780–1784. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Lin, X.; Zhou, X.; Wan, J.; Lu, X.; Yang, B.; Ai, W.; Lin, J.; Zhang, T.; Tu, Z.; et al. Cytotoxic and antiviral nitrobenzoyl sesquiterpenoids from the marine-derived fungus Aspergillus ochraceus Jcma1F17. Med. Chem. Commun. 2014, 5, 701–705. [Google Scholar] [CrossRef]
- Tan, Y.; Yang, B.; Lin, X.; Luo, X.; Pang, X.; Tang, L.; Liu, Y.; Li, X.; Zhou, X. Nitrobenzoyl Sesquiterpenoids with Cytotoxic Activities from a Marine-Derived Aspergillus ochraceus Fungus. J. Nat. Prod. 2018, 81, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.X.; Wang, X.F.; Ren, G.W.; Yuan, X.L.; Deng, N.; Ji, G.X.; Li, W.; Zhang, P. Prenylated Diphenyl Ethers from the Marine Algal-Derived Endophytic Fungus Aspergillus tennesseensis. Molecules 2018, 23, 2368. [Google Scholar] [CrossRef]
- Hawas, U.W.; El-Beih, A.A.; El-Halawany, A.M. Bioactive Anthraquinones from Endophytic Fungus Aspergillus versicolor Isolated from Red Sea Algae. Arch. Pharm. Res. 2012, 35, 1749–1756. [Google Scholar] [CrossRef]
- Sun, H.F.; Li, X.M.; Meng, L.; Cui, C.M.; Gao, S.S.; Li, C.S.; Huang, C.G.; Wang, B.G. Asperolides A−C, Tetranorlabdane Diterpenoids from the Marine Alga-Derived Endophytic Fungus Aspergillus wentii EN-48. J. Nat. Prod. 2012, 75, 148–152. [Google Scholar] [CrossRef]
- Zhang, Z.; Miao, L.; Sun, H.; Wei, S.; Wang, B.; Huang, C.; Jiao, B. Wentilactone B induces G2/M phase arrest and apoptosis via the Ras/Raf/MAPK signaling pathway inhuman hepatoma SMMC-7721 cells. Cell Death Dis. 2013, 4, e675. [Google Scholar] [CrossRef]
- Zhang, Z.; Miao, L.; Sun, W.; Jiao, B.; Wang, B.; Yao, L.; Huang, C. Wentilactone B from Aspergillus wentii Induces Apoptosis and Inhibits Proliferation and Migration of Human Hepatoma SMMC-7721 Cells. Biol. Pharm. Bull. 2012, 35, 1964–1971. [Google Scholar] [CrossRef]
- Lv, C.; Hong, Y.; Miao, L.; Li, C.; Xu, G.; Wei, S.; Wang, B.; Huang, C.; Jiao, B. Wentilactone A as a novel potential antitumor agent induces apoptosis and G2/M arrest of human lung carcinoma cells, and is mediated by HRas-GTP accumulation to excessively activate the Ras/Raf/ERK/p53-p21 pathway. Cell Death Dis. 2013, 4, e952. [Google Scholar] [CrossRef]
- Lv, C.; Sun, W.; Sun, H.; Wei, S.; Chen, R.; Wang, B.; Huang, C. Asperolide A, a Marine-Derived Tetranorditerpenoid, Induces G2/M Arrest in Human NCI-H460 Lung Carcinoma Cells, Is Mediated by p53-p21 Stabilization and Modulated by Ras/Raf/MEK/ERK Signaling Pathway. Mar. Drugs 2013, 11, 316–331. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Guo, C.; Meng, J.; Tian, H.; Guo, S.; Shi, D. Discovery of Natural Dimeric Naphthopyrones as Potential Cytotoxic Agents through ROS-Mediated Apoptotic Pathway. Mar. Drugs 2019, 17, 207. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.M.; Li, X.M.; Li, C.S.; Proksch, P.; Wang, B.G. Cytoglobosins A-G, Cytochalasans from a Marine-Derived Endophytic Fungus, Chaetomium globosum QEN-14. J. Nat. Prod. 2010, 73, 729–733. [Google Scholar] [CrossRef]
- Li, H.L.; Li, X.M.; Mándi, A.; Antus, S.; Li, X.; Zhang, P.; Liu, Y.; Kurtán, T.; Wang, B.G. Characterization of Cladosporols from the Marine Algal-Derived Endophytic Fungus Cladosporium cladosporioides EN-399 and Configurational Revision of the Previously Reported Cladosporol Derivatives. J. Org. Chem. 2017, 82, 9946–9954. [Google Scholar] [CrossRef] [PubMed]
- Elsebai, M.F.; Kehraus, S.; Lindequist, U.; Sasse, F.; Shaaban, S.; Gutschow, M.; Josten, M.; Sahl, H.G.; Konig, G.M. Antimicrobial phenalenone derivatives from the marine-derived fungus Coniothyrium cereale. Org. Biomol. Chem. 2011, 9, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Tang, X.Z.; Miao, F.P.; Ji, N.Y. A New Pyrrolidine Derivative and Steroids from an Algicolous Gibberella zeae Strain. Nat. Prod. Commun. 2011, 6, 1243–1246. [Google Scholar] [CrossRef]
- Wang, F.W. Bioactive metabolites from Guignardia sp., an endophytic fungus residing in Undaria pinnatifida . Chin. J. Nat. Med. 2012, 10, 72–76. [Google Scholar] [CrossRef]
- Zhang, P.; Li, X.M.; Wang, J.N.; Li, X.; Wang, B.-G. Prenylated indole alkaloids from the marine-derived fungus Paecilomyces variotii. Chin. Chem. Lett. 2015, 26, 313–316. [Google Scholar] [CrossRef]
- Zhang, P.; Li, X.M.; Mao, X.X.; Mándi, A.; Kurtàn, T.; Wang, B.G. Varioloid A, a new indolyl-6,10b-dihydro-5aH-[1]benzofuro[2 –b] indole derivative from the marine alga-derived endophytic fungus Paecilomyces variotii EN-291. Beilstein J. Org. Chem. 2016, 12, 2012–2018. [Google Scholar] [CrossRef]
- Gao, S.S.; Li, X.M.; Du, F.Y.; Li, C.S.; Proksch, P.; Wang, B.G. Secondary Metabolites from a Marine-Derived Endophytic Fungus Penicillium chrysogenum QEN-24S. Mar. Drugs. 2011, 9, 59–70. [Google Scholar] [CrossRef]
- Gao, S.S.; Li, X.M.; Li, C.S.; Proksch, P.; Wang, B.G. Penicisteroids A and B, antifungal and cytotoxic polyoxygenated steroids from the marine alga-derived endophytic fungus Penicillium chrysogenum QEN-24S. Bioorg. Med. Chem. Lett. 2011, 21, 2894–2897. [Google Scholar] [CrossRef] [PubMed]
- Gamal-Eldeen, A.M.; Abdel-Lateff, A.; Okino, T. Modulation of carcinogen metabolizing enzymes bychromanone A; a newchromone derivative from algicolous marine fungus Penicillium sp. Environ. Toxicol. Pharmacol. 2009, 28, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Wang, S.W.; Wang, G.J.; Pang, K.L.; Lee, C.K.; Kuo, Y.H.; Cha, H.J.; Lin, R.K.; Lee, T.H. Angiogenesis Inhibitors and Anti-Inflammatory Agents from Phoma sp. NTOU4195. J. Nat. Prod. 2016, 79, 2983–2990. [Google Scholar] [CrossRef] [PubMed]
- Li, H.L.; Li, X.M.; Li, X.; Wang, C.Y.; Liu, H.; Kassack, M.U.; Meng, L.H.; Wang, B.G. Antioxidant Hydroanthraquinones from the Marine Algal-Derived Endophytic Fungus Talaromyces islandicus EN-501. J. Nat. Prod. 2017, 80, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Gunatilaka, A.A.L. Natural products from plant-associated microorganisms: Distribution, structural diversity, bioactivity, and implications of their occurrence. J. Nat. Prod. 2006, 69, 509–526. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.S. New aspects of natural products in drug discovery. Trends Microbiol. 2007, 15, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.T.; Fischbach, M.A. Natural products version: 2.0: Connecting genes to molecules. J. Am. Chem. Soc. 2010, 132, 2469–2493. [Google Scholar] [CrossRef]
- Gomes, N.G.M.; Lefranc, F.; Kijjoa, A.; Kiss, R. Can Some Marine-Derived Fungal Metabolites Become Actual Anticancer Agents? Mar. Drugs 2015, 13, 3950–3991. [Google Scholar] [CrossRef]
- Evidente, A.; Kornienko, A.; Cimmino, A.; Andolfi, A.; Lefranc, F.; Mathieu, V.; Kiss, R. Fungal metabolites with anticancer activity. Nat. Prod. Rep. 2014, 31, 617–627. [Google Scholar] [CrossRef]
- Elsebai, M.F.; Natesan, L.; Kehraus, S.; Mohamed, I.E.; Schnakenburg, G.; Sasse, F.; Shaaban, S.; Gutschow, M.; Koning, G.M. HLE-Inhibitory Alkaloids with a Polyketide Skeleton from the Marine-Derived Fungus Coniothyrium cereale. J. Nat. Prod. 2011, 74, 2282–2285. [Google Scholar] [CrossRef]
- Zurlo, D.; Leone, C.; Assante, G.; Salzano, S.; Renzone, G.; Scaloni, A.; Foresta, C.; Colantuoni, V.; Lupo, A. Cladosporol a stimulates G1-phase arrest of the cell cycle by up-regulation of p21(waf1/cip1) expression in human colon carcinoma HT-29 cells. Mol. Carcinog. 2013, 52, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Zurlo, D.; Assante, G.; Moricca, S.; Colantuoni, V.; Lupo, A. Cladosporol A, a new peroxisome proliferator-activated receptor γ (PPARγ) ligand, inhibits colorectal cancer cells proliferation through β-catenin/TCF pathway inactivation. Biophy. Acta Gen. Subj. 2014, 1840, 2361–2372. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Qiu, X.; Wang, R.; Duan, L.; Chen, S.; Luo, J.; Kong, L. Inhibition of Human Gastric Carcinoma Cell Growth in Vitro and in Vivo by Cladosporol Isolated from the Paclitaxel-Producing Strain Alternaria alternata var. monosporus. Biol. Pharm. Bull. 2009, 32, 2072–2074. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.X.; Jin, X.J.; Draskovic, M.; Crews, M.S.; Tenney, K.; Valeriote, F.A.; Yao, X.J.; Crews, P. Unraveling the Numerous Biosynthetic Products of the Marine Sediment-Derived Fungus, Aspergillus insulicola. Phytochem. Lett. 2012, 5, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Belofsky, G.N.; Jensen, P.R.; Renner, M.K.; Fenical, W. New cytotoxic sesquiterpenoid nitrobenzoyl esters from a marine isolate of the fungus Aspergillus versicolor. Tetrahedron Lett. 1998, 54, 1715–1724. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Anbuchezhian, R.; Sun, W.; Shao, C.L.; Zhang, F.L.; Yin, Y.; Yu, Z.S.; Li, Z.Y.; Wang, C.Y. Cytotoxic Nitrobenzoyloxy-substituted Sesquiterpenes from Sponge derived Endozoic Fungus Aspergillus insulicola MD10-2. Curr. Pharm. Biotechnol. 2016, 17, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Silber, J.; Kramer, A.; Labes, A.; Tasdemir, D. From Discovery to Production: Biotechnology of Marine Fungi for the Production of New Antibiotics. Mar. Drugs 2016, 14, 137–157. [Google Scholar] [CrossRef]
- Strobel, G.A. Rainforest endophytes and bioactive products. Crit. Rev. Biotechnol. 2002, 22, 315–333. [Google Scholar] [CrossRef]
- Raghukumar, C. Marine fungal biotechnology: An ecological perspective. Fungal Divers. 2008, 31, 19–35. [Google Scholar]
- Chambergo, F.S.; Valencia, S.Y. Fungal biodiversity to biotechnology. Appl. Microbiol. Biotechnol. 2016, 100, 2567–2577. [Google Scholar] [CrossRef]
- Brakhage, A.A.; Schroeckh, V. Fungal secondary metabolites-Strategies to activate silent gene clusters. Fungal Genet. Biol. 2011, 48, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.B.; Cheung, R.C.; Wong, J.H.; Bekhit, A.A.; BekhitAel, D. Antibacterial products of marine organisms. Appl. Microbiol. Biotechnol. 2015, 99, 4145–4173. [Google Scholar] [CrossRef] [PubMed]
- Greco, K.; Keller, N.P.; Rokas, A. Unearthing fungal chemodiversity and prospects for drug discovery. Curr. Opin. Microbiol. 2019, 51, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Pereira, F.; Aires-de-Sousa, J. Computational Methodologies in the Exploration of Marine Natural Product Leads. Mar. Drugs 2018, 16, 236–266. [Google Scholar] [CrossRef] [PubMed]
- Kildgaard, S.; Mansson, M.; Dosen, I.; Klitgaard, A.; Frisvad, J.C.; Larsen, T.O.; Nielsen, K.F. Accurate Dereplication of Bioactive Secondary Metabolites from Marine-Derived Fungi by UHPLC-DAD QTOFMS and a MS/HRMS Library. Mar. Drugs 2014, 12, 3681–3705. [Google Scholar] [CrossRef] [PubMed]
- El-Elimat, T.; Figueroa, M.; Ehrmann, B.M.; Cech, N.B.; Pearce, C.J.; Oberlies, N.H. High-Resolution MS, MS/MS, and UV Database of Fungal Secondary Metabolites as a Dereplication Protocol for Bioactive Natural Products. J. Nat. Prod. 2013, 76, 1709–1716. [Google Scholar] [CrossRef]
- Naman, C.B.; Rattan, R.; Nikoulina, S.E.; Lee, J.; Miller, B.W.; Moss, N.A.; Armstrong, L.; Boudreau, P.D.; Debonsi, H.M.; Valeriote, F.A.; et al. Integrating Molecular Networking and Biological Assays To Target the Isolation of a Cytotoxic Cyclic Octapeptide, Samoamide A, from an American Samoan Marine Cyanobacterium. J. Nat. Prod. 2017, 80, 625–633. [Google Scholar] [CrossRef]
- Naman, C.B.; Almaliti, J.; Armstrong, L.; Caro-Díaz, E.J.; Pierce, M.L.; Glukhov, E.; Fenner, A.; Spadafora, C.; Debonsi, H.M.; Dorrestein, P.C.; et al. Discovery and Synthesis of Caracolamide A, an Ion Channel Modulating Dichlorovinylidene Containing Phenethylamide from a Panamanian Marine Cyanobacterium cf. Symploca Species. J. Nat. Prod. 2017, 80, 2328–2334. [Google Scholar] [CrossRef]
- Philippus, A.C.; Zatelli, G.A.; Wanke, T.; Barros, M.G.A.; Kami, S.A.; Lhullier, C.; Armstrong, L.; Sandjo, L.P.; Falkenberg, M. Molecular networking prospection and characterization of terpenoids and C 15-acetogenins in Brazilian seaweed extracts. RSC Adv. 2018, 8, 29654–29661. [Google Scholar] [CrossRef]
- Linde, T.; Hansen, N.B.; Lübeck, M.; Lübeck, P.S. Fermentation in 24-well plates is an efficient screening platform for filamentous fungi. Lett. Appl. Microbiol. 2014, 59, 224–230. [Google Scholar] [CrossRef]
- Girarda, P.; Jordana, M.; Tsaob, M.; Wurma, F.M. Small-scale bioreactor system for process development and optimization. Biochem. Eng. J. 2001, 7, 117–119. [Google Scholar] [CrossRef]
- Kramer, A.; Paun, L.; Imhoff, J.F.; Kempken, F.; Labes, A. Development and validation of a fast and optimized screening method for enhanced production of secondary metabolites using the marine Scopulariopsis brevicaulis strain LF580 producing anti-cancer active scopularide A and B. PLoS ONE 2014, 9, e103320. [Google Scholar] [CrossRef] [PubMed]
- Duetz, W.A.; Ruedi, L.; Hermann, R.; O’Connor, K.; Buchs, J.; Witholt, B. Methods for intense aeration, growth, storage, and replication of bacterial strains in microtiter plates. Appl. Environ. Microbiol. 2000, 66, 2641–2646. [Google Scholar] [CrossRef]
- Samorski, M.; Müller-Newen, G.; Buchs, J. Quasi-continuous combined scattered light and fluorescence measurements: A novel measurement technique for shaken microtiter plates. Biotechnol. Bioeng. 2005, 92, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Kensy, F.; Zang, E.; Faulhammer, C.; Tan, R.K.; Buchs, J. Validation of a high-throughput fermentation system based on online monitoring of biomass and fluorescence in continuously shaken microtiter plates. Microb. Cell. Fact. 2009, 4, 1–17. [Google Scholar] [CrossRef]
- Joint, I.; Muhling, M.; Querellou, J. Culturing marine bacteria—An essential prerequisite for biodiscovery. Microb. Biotechnol. 2010, 3, 564–575. [Google Scholar] [CrossRef]
- Monaghan, R.L.; Barrett, J.F. Antibacterial drug discovery-Then, now and the genomics future. Biochem. Pharmacol. 2006, 71, 901–909. [Google Scholar] [CrossRef]
- Kniemeyer, O. Proteomics of eukaryotic microorganisms: The medically and biotechnologically important fungal genus Aspergillus. Proteomics 2011, 11, 3232–3243. [Google Scholar] [CrossRef]
- Kramer, A.; Beck, H.C.; Kumar, A.; Kristensen, L.P.; Imhoff, J.F.; Labes, A. Proteomic analysis of anti-cancerous scopularide production by a marine Microascus brevicaulis strain and its UV mutant. PLoS ONE 2015, 10, e0140047. [Google Scholar] [CrossRef]
- Bhadury, P.; Mohammad, B.T.; Wright, P.C. The current status of natural products from marine fungi and their potential as anti-infective agents. J. Ind. Microbiol. Biotechnol. 2006, 33, 325–337. [Google Scholar] [CrossRef]
- Lang, S.; Hüners, M.; Lurtz, V. Bioprocess engineering data on the cultivation of marine prokaryotes and fungi. Adv. Biochem. Eng. Biotechnol. 2005, 97, 29–62. [Google Scholar] [CrossRef]
- Xu, R.; Li, X.; Xu, G.; Wang, B. Optimizing production of asperolide A, a potential anti-tumor tetranorditerpenoid originally produced by the algal-derived endophytic fungus Aspergillus wentii EN-48*. Chin. J. Oceanol. Limn. 2017, 35, 658–663. [Google Scholar] [CrossRef]
- Zhou, W.; Cai, M.; Zhou, J.; Jiang, T.; Zhou, J.; Wang, M.; Zhou, X.; Zhang, Y. Nutrition and bioprocess development for efficient biosynthesis of an antitumor compound from marine-derived fungus. J. Ind. Microbiol. Biotechnol. 2013, 40, 1131–1142. [Google Scholar] [CrossRef]
- Zhou, W.; Cai, M.; Na, K.; Shen, C.; Zhang, X.; Zhou, X.; Zhao, W.; Zhang, Y. pH-Dependent accumulation of anticancer compound on mycelia in fermentation of marine fungus. J. Ind. Microbiol. Biotechnol. 2014, 41, 1169–1173. [Google Scholar] [CrossRef]
- Papagianni, M. Advances in citric acid fermentation by Aspergillus niger: Biochemical aspects, membrane transport and modeling. Biotechnol. Adv. 2007, 25, 244–263. [Google Scholar] [CrossRef]
- Schneider, P.; Misiek, M.; Hoffmeister, D. In vivo and in vitro production options for fungal secondary metabolites. Mol. Pharm. 2008, 5, 234–242. [Google Scholar] [CrossRef]
- Jesus, H.C.R.; Jeller, A.H.; Debonsi, H.M.; Alves, P.B.; Porto, A.L.M. Multiple Monohydroxylation Products from rac-Camphor by Marine Fungus Botryosphaeria sp. Isolated from Marine Alga Bostrychia radicans. J. Braz. Chem. Soc. 2017, 28, 498–504. [Google Scholar] [CrossRef]
- Xiong, Z.Q.; Wang, J.F.; Hao, Y.Y.; Wang, Y. Recent advances in the discovery and development of marine microbial natural products. Mar. Drugs 2013, 11, 700–717. [Google Scholar] [CrossRef]
- Rutledge, P.J.; Challis, G.L. Discovery of microbial natural products by activation of silent biosynthetic gene clusters. Nat. Rev. Microbiol. 2015, 13, 509–523. [Google Scholar] [CrossRef]
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R. The re-emergence of natural products for drug Discovery in the genomics era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef]
- Elander, R.P. Industrial production of β-lactam antibiotics. Appl. Microbiol. Biotechnol. 2003, 61, 385–392. [Google Scholar] [CrossRef]
- Jungbauer, A. Continuous downstream processing of biopharmaceuticals. Trends Biotechnol. 2013, 31, 479–492. [Google Scholar] [CrossRef]
- Zydney, A.L. Continuous downstream processing for high value biological products: A review. Biotechnol. Bioeng. 2016, 113, 465–475. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).