Proteomic and Membrane Lipid Correlates of Re-duced Host Defense Peptide Susceptibility in a snoD Mutant of Staphylococcus aureus
Abstract
1. Introduction
2. Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Proteomic Analyses
2.3. Fatty Acid Extraction and Gas Chromatographic Analysis of Fatty Acid Methyl Esters (GC-FAME)
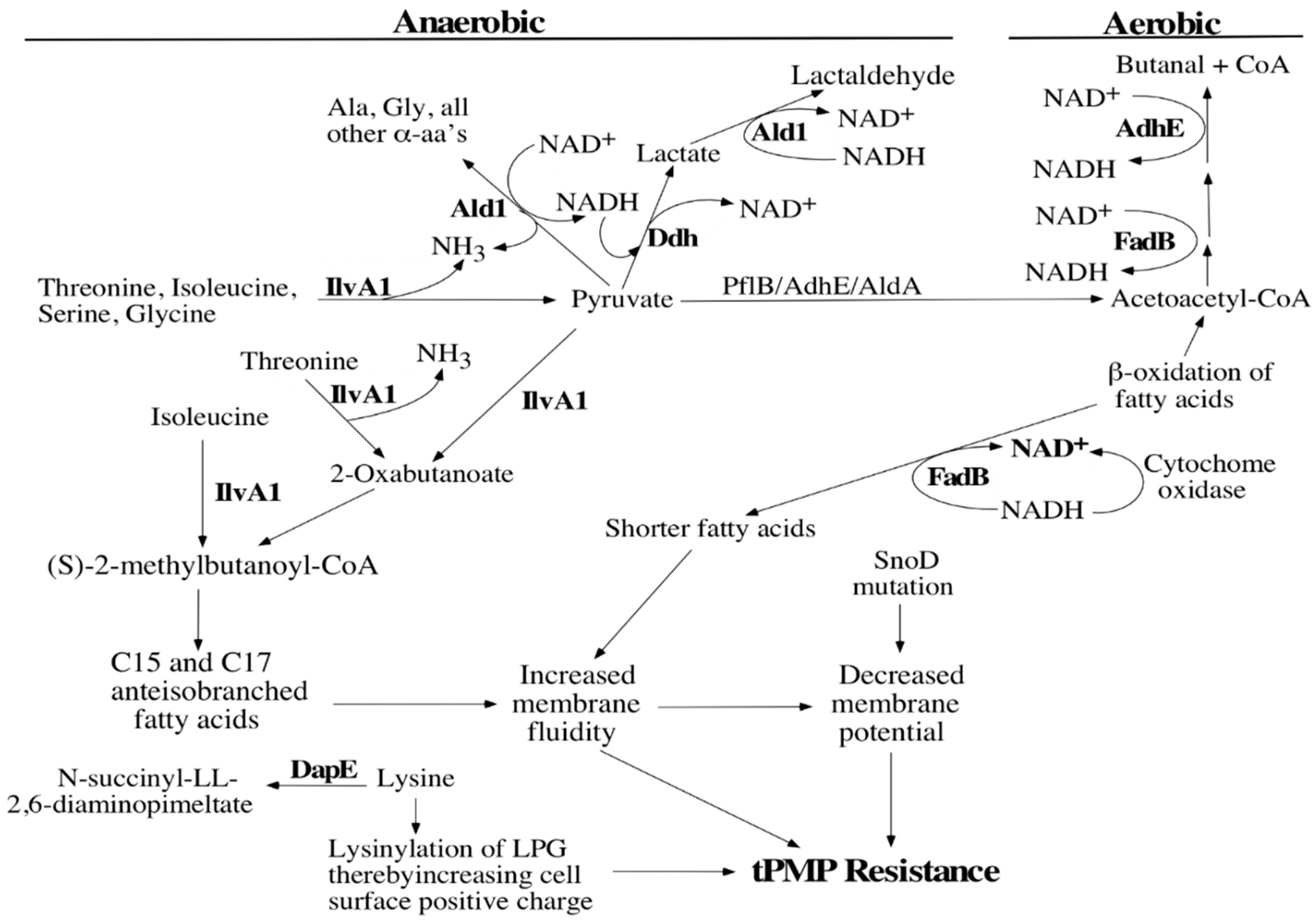
2.4. Pathway Analysis and Bioinformatics
3. Results
3.1. Proteomics Analyses
3.2. Fatty Acid Analyses
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yeaman, M.R.; Bayer, A.S. Antimicrobial peptides from platelets. Drug Resist. Updates 1999, 2, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Fowler, V.G.J.; Sakoulas, G.; McIntyre, L.M.; Meka, V.G.; Arbeit, R.D.; Cabell, C.H.; Stryjewski, M.E.; Eliopoulos, G.M.; Reller, L.B.; Corey, G.R.; et al. Persistent bacteremia due to methicillin-resistant Staphylococcus aureus infection is associated with agr dysfunction and low-level in vitro resistance to thrombin-induced platelet microbicidal protein. J. Infect. Dis. 2004, 190, 1140–1149. [Google Scholar] [CrossRef] [PubMed]
- Moise, P.A.; Forrest, A.; Bayer, A.S.; Xiong, Y.Q.; Yeaman, M.R.; Sakoulas, G. Factors influencing time to vancomycin-induced clearance of nonendocarditis methicillin-resistant Staphylococcus aureus bacteremia: Role of platelet microbicidal protein killing and agr genotypes. J. Infect. Dis. 2010, 201, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, V.; Yeaman, M.R.; Kim, E.; Cheung, A.L.; Sullam, P.M.; Bayer, A.S. Phenotypic resistance to thrombin-induced platelet microbicidal protein in vitro correlates with enhanced virulence in experimental endocarditis due to Staphylococcus aureus. Infect. Immun. 1997, 65, 3293–3299. [Google Scholar] [PubMed]
- Kupferwasser, L.I.; Yeaman, M.R.; Shapiro, S.M.; Nast, C.C.; Bayer, A.S. In vitro susceptibility to thrombin-induced platelet microbicidal protein is associated with reduced disease progression and complication rates in experimental Staphylococcus aureus endocarditis: Microbiological, histopathologic, and echocardiographic analyses. Circulation 2002, 105, 746–752. [Google Scholar] [PubMed]
- Mercier, R.C.; Dietz, R.M.; Mazzola, J.L.; Bayer, A.S.; Yeaman, M.R. Beneficial influence of platelets on antibiotic efficacy in an in vitro model of Staphylococcus aureus-induced endocarditis. Antimicrob. Agents Chemother. 2004, 48, 2551–2557. [Google Scholar] [CrossRef] [PubMed]
- Koo, S.P.; Bayer, A.S.; Sahl, H.G.; Proctor, R.A.; Yeaman, M.R. Staphylocidal action of thrombin-induced platelet microbicidal protein is not solely dependent on transmembrane potential. Infect. Immun. 1996, 64, 1070–1074. [Google Scholar] [PubMed]
- Weidenmaier, C.; Peschel, A.; Kempf, V.A.; Lucindo, N.; Yeaman, M.R.; Bayer, A.S. DltABCD-and MprF-mediated cell envelope modifications of Staphylococcus aureus confer resistance to platelet microbicidal proteins and contribute to virulence in a rabbit endocarditis model. Infect. Immun. 2005, 73, 8033–8038. [Google Scholar] [CrossRef] [PubMed]
- Mishra, N.N.; Liu, G.Y.; Yeaman, M.R.; Nast, C.C.; Proctor, R.A.; McKinnell, J.; Bayer, A.S. Carotenoid-related alteration of cell membrane fluidity impacts Staphylococcus aureus susceptibility to host defense peptides. Antimicrob. Agents Chemother. 2011, 55, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Bayer, A.S.; Prasad, R.; Chandra, J.; Koul, A.; Mishra, S.; Varma, A.; Skurray, R.A.; Firth, N.; Brown, M.H.; Koo, S.P.; et al. In vitro resistance of Staphylococcus aureus to thrombin-induced platelet microbicidal protein is associated with alterations in cytoplasmic membrane fluidity. Infect. Immun. 2000, 68, 3548–3553. [Google Scholar] [CrossRef] [PubMed]
- Bayer, A.S.; Kupferwasser, L.I.; Brown, M.H.; Skurray, R.A.; Grkovic, S.; Jones, T.; Mukhopadhay, K.; Yeaman, M.R. Low-level resistance of Staphylococcus aureus to thrombin-induced platelet microbicidal protein-1 in vitro associated with qacA gene carriage is independent of multidrug efflux pump activity. Antimicrob. Agents Chemother. 2006, 50, 2448–2454. [Google Scholar] [CrossRef] [PubMed]
- Bayer, A.S.; McNamara, P.; Yeaman, M.R.; Lucindo, N.; Jones, T.; Cheung, A.L.; Sahl, H.G.; Proctor, R.A. Transposon disruption of the complex I NADH oxidoreductase gene (snoD) in Staphylococcus aureus is associated with reduced susceptibility to the microbicidal activity of thrombin-induced platelet microbicidal protein-1. J. Bacteriol. 2006, 188, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Guffanti, A.A.; Oudegam, B.; Krulwich, T.A. Mrp, a multigene, multifunctional locus in Bacillus subtilis with roles in resistance to cholate and to Na+ and in pH homeostasis. J. Bacteriol. 1999, 181, 2394–2402. [Google Scholar] [PubMed]
- Hiramatsu, T.; Kodama, K.; Kuroda, T.; Mizushima, T.; Tsuchiya, T. A putative multisubunit Na+/H+ antiporter from Staphylococcus aureus. J. Bacteriol. 1998, 180, 6642–6648. [Google Scholar] [PubMed]
- Yeaman, M.R.; Puentes, S.M.; Norman, D.C.; Bayer, A.S. Partial purification and staphylocidal activity of thrombin-induced platelet microbicidal protein. Infect. Immun. 1992, 60, 1202–1209. [Google Scholar] [PubMed]
- Fuchs, S.; Pané-Farré, J.; Kohler, C.; Hecker, M.; Engelmann, S. Anaerobic gene expression in Staphylococcus aureus. J. Bacteriol. 2007, 189, 4275–4289. [Google Scholar] [CrossRef] [PubMed]
- Kohler, C.; von Eiff, C.; Liebeke, M.; McNamara, P.J.; Lalk, M.; Proctor, R.A.; Hecker, M.; Engelmann, S. A defect in menadione biosynthesis induces global changes in gene expression in Staphylococcus aureus. J. Bacteriol. 2008, 190, 6351–6364. [Google Scholar] [CrossRef] [PubMed]
- Büttner, K.; Bernhardt, J.; Scharf, C. A comprehensive two-dimensional map of cytosolic proteins of Bacillus subtilis. Electrophoresis 2001, 22, 2908–2935. [Google Scholar] [CrossRef]
- Engelmann, S.; Hecker, M. Proteomic analysis to investigate regulatory networks in Staphylococcus aureus. Methods Mol. Biol. 2008, 431, 25–45. [Google Scholar]
- Candiano, G.; Bruschi, M.; Musante, L.; Santucci, L.; Ghiggeri, G.M.; Carnemolla, B.; Orecchia, P.; Zardi, L.; Righetti, P.G. Blue silver: A very sensitive colloidal coomassie G-250 staining for proteome analysis. Electrophoresis 2004, 25, 1327–1333. [Google Scholar] [CrossRef]
- Eymann, C.; Dreisbach, A.; Albrecht, D.; Gentner, S.; Tam, L.T.; Büttner, K.; Buurman, G.; Scharf, C.; Venz, S.; Völker, U.; et al. A comprehensive proteome map of growing Bacillus subtilis cells. Proteomics 2004, 4, 2849–2876. [Google Scholar] [CrossRef]
- University of Nebraska Transposon Mutant Library, University of Nebraska Medical Center, Department of Pathology & Microbiology Center for Staphylococcal Research [CSR]. 2019. Available online: www.unmc.edu/pathology/csr/research/library.html (accessed on 10 June 2019).
- Kegg Pathway Maps. Kanehisa Laboratories, Japan. 2019. Available online: www.genome.jp/kegg/kegg3a.html (accessed on 10 June 2019).
- Beranová, J.; Mansilla, M.C.; de Mendoza, D.; Elhottová, D.; Konopásek, I. Differences in cold adaptation of Bacillus subtilis under anaerobic and aerobic conditions. J. Bacteriol. 2010, 192, 4164–4171. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, K.; Whitmire, W.; Xiong, Y.Q.; Molden, J.; Jones, T.; Peschel, A.; Staubitz, P.; Adler-Moore, J.; McNamara, P.J.; Proctor, R.A.; et al. In vitro susceptibility of Staphylococcus aureus to thrombin-induced platelet microbicidal protein-1 (tPMP-1) is influenced by cell membrane phospholipid composition and asymmetry. Microbiology 2007, 153, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Sharma-Kuinkel, B.K.; Mongodin, E.F.; Myers, J.R.; Vore, K.L.; Canfield, G.S.; Fraser, C.M.; Rude, T.H.; Fowler, V.G., Jr.; Gill, S.R. Potential Influence of Staphylococcus aureus clonal complex 30 genotype and transcriptome on hematogenous infections. Open Forum Infect. Dis. 2015, 2, ofv093. [Google Scholar] [CrossRef] [PubMed]
- Mayer, S.; Steffen, W.; Steuber, J.; Götz, F. The Staphylococcus aureus Nuo-like protein MpsA, contributes to the generation of membrane potential. J. Bacteriol. 2015, 197, 794–806. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Boyle-Vavra, S.; de Jonge, B.L.; Ebert, C.C.; Daum, R.S. Cloning of the Staphylococcus aureus ddh gene encoding NAD+-dependent D-lactate dehydrogenase and insertional inactivation in a glycopeptide-resistant isolate. J. Bacteriol. 1997, 179, 6756–6763. [Google Scholar] [CrossRef] [PubMed]
- Kohler, C.; von Eiff, C.; Peters, G.; Proctor, R.A.; Hecker, M.; Engelmann, S. Physiological characterization of a heme- deficient mutant of Staphylococcus aureus by a proteomic approach. J. Bacteriol. 2003, 185, 6928–6937. [Google Scholar] [CrossRef]
- Klein, W.; Weber, M.H.; Marahiel, M.A. Cold shock response of Bacillus subtilis: Isoleucine-dependent switch in the fatty acid branching pattern for membrane adaptation to low temperatures. J. Bacteriol. 1999, 181, 5341–5349. [Google Scholar] [PubMed]
- Kaneda, T. Iso-and anteiso-fatty acids in bacteria: Biosynthesis, function, and taxonomic significance. Microbiol. Rev. 1991, 55, 288–302. [Google Scholar]
- Yount, N.Y.; Cohen, S.E.; Kupferwasser, D.; Waring, A.J.; Ruchala, P.; Sharma, S.; Wasserman, K.; Jung, C.-L.; Yeaman, M.R. Context mediates antimicrobial efficacy of kinocidin congener peptide RP-1. PLoS ONE 2011, 6, e26727. [Google Scholar] [CrossRef]
- Haines, T.H. Do sterols reduce proton and sodium leaks through lipid bilayers? Prog. Lipid Res. 2001, 40, 299–324. [Google Scholar] [CrossRef]
- Xiong, Y.Q.; Bayer, A.S.; Elazegui, L.; Yeaman, M.R. A synthetic congener modeled on a microbicidal domain of thrombin-induced platelet microbicidal protein-1 recapitulates staphylocidal mechanisms of the native molecule. Antimicrob. Agents Chemother. 2006, 50, 3786–3792. [Google Scholar] [CrossRef] [PubMed][Green Version]
| Protein (Annotation From 315) | Function | Ratio log2 (SH1000/snoD) |
|---|---|---|
| Increased in parent | ||
| AdhE (SA0143) | Acetaldehyde DH | 1.46 |
| (SA2367) | Theoretical protein | 1.044 |
| Decreased in mutant | ||
| DapE (SA1814) | Lysine metabolism | 1.021 |
| (SA2366) | Theoretical protein | 1.051 |
| AgrA (SA1844) | Virulence regulator | 1.287 |
| Increased in mutant | ||
| IlvA1 (SA1271) | Threonine/serine dehydrase | −4.17 |
| Ald1 (SA1272) | α-amino acid dehydrogenase | −1.667 |
| Ddh (SA2312) | D-lactate dehydrogenase | −1.006 |
| Fatty Acid Species | SH1000-98 | * p value | SH1000-WT | * p value | ||
|---|---|---|---|---|---|---|
| Aerobic | Anaerobic | Aerobic | Anaerobic | |||
| Anteiso (C13–17) | 64.49 ± 0.11 | 39.07 ± 0.49 | 0.006 | 61.16 ± 1.16 | 35.85 ± 1.10 | 0.002 |
| Unsaturated Polyunsaturated subset (C20) | 3.58 ± 0.14 0.42 ± 0.01 | 12.81 ± 0.16 0.49 ± 0.01 | 0.0003 0.045 | 3.565 ± 0.40 0.315 ± 0.01 | 12.10 ± 0.09 0.42 ± 0.01 | 0.016 0.027 |
| Short Isos (C13–15) | 5.97 ± 0.08 | 14.69 ± 0.25 | 0.007 | 6.895 ± 0.13 | 16.60 ± 0.41 | 0.015 |
| Long Isos (C16–20) | 6.03 ± 0.05 | 5.205 ± 0.05 | 0.003 | 7.195 ± 0.08 | 5.30 ± 0.03 | 0.009 |
| Fatty Acid Species | Aerobic | Anaerobic | ||||
|---|---|---|---|---|---|---|
| SH1000-WT | SH1000-98 | p-value * | SH1000-WT | SH1000-98 | p-value ** | |
| 13:0 iso | - | 0.325 ± 0.01 | 0.265 ± 0.02 | |||
| 13:0 anteiso | - | 0.105 ± 0.01 | - | - | ||
| 14:0 iso | 1.18 ± 0.03 | 0.78 ± 0.01 | 0.01 | 1.985 ± 0.02 | 1.455 ± 0.04 | 0.007 |
| 14:0 | 0.215 ± 0.02 | 0.195 ± 0.01 | 1.07 ± 0.08 | 0.765 ± 0.011 | ||
| 15:1 iso G | 0.165 ± 0.01 | 0.15 ± 0.00 | 0.325 ± 0.02 | - | ||
| 15:0 iso | 5.75 ± 0.11 | 5.185 ± 0.06 | 14.29 ± 0.42 | 12.97 ± 0.20 | ||
| 15:0anteiso | 43.96 ± 0.72 | 46.15 ± 0.04 | 31.025 ± 0.93 | 33.495 ± 0.40 | ||
| 15:0 | - | - | 0.795 ± 0.05 | 0.31 ± 0.01 | 0.03 | |
| 16:0 iso | 3.05 ± 0.09 | 1.965 ± 0.06 | 0.009 | 1.17 ± 0.01 | 1.11 ± 0.01 | |
| 16:0 | 1.75 ± 0.10 | 1.81 ± 0.00 | 3.94 ± 0.16 | 3.98 ± 0.06 | ||
| 15:0 2OH | - | - | 0.23 ± 0.01 | 0.205 ± 0.01 | ||
| 17:0 iso | 3.93 ± 0.07 | 3.55 ± 0.06 | 3.835 ± 0.01 | 3.695 ± 0.05 | ||
| 17:0 anteiso | 17.2 ± 0.44 | 18.24 ± 0.16 | 4.82 ± 0.17 | 5.57 ± 0.08 | 0.06 | |
| 17:0 | 0.215 ± 0.04 | 0.2 ± 0.01 | 2.075 ± 0.011 | 0.96 ± 0.04 | 0.02 | |
| 18:0 iso | 1.555 ± 0.01 | 1.02 ± 0.01 | 0.0025 | 0.395 ± 0.04 | 0.375 ± 0.04 | |
| 18:1w9c | 2.775 ± 0.04 | 3 ± 0.13 | 5.91 ± 0.03 | 6.13 ± 0.08 | ||
| 18:1w7C | 0.525 ± 0.01 | 0.58 ± 0.01 | 1.35 ± 0.03 | 1.515 ± 0.04 | ||
| 18:0 | 6.75±0.014 | 6.93 ± 0.08 | 10.4 ± 0.17 | 10.58 ± 0.00 | ||
| 19:0 iso | 1.335±0.01 | 1.185 ± 0.01 | 1.07 ± 0.01 | 1.135 ± 0.04 | ||
| 19:0 anteiso | 3.61 ± 0.04 | 3.68 ± 0.03 | 0.97 ± 0.04 | 1.225 ± 0.02 | 0.04 | |
| 19:0 | 0.245±0.04 | 0.235 ± 0.01 | 2.25 ± 0.18 | 1.145 ± 0.06 | ||
| 20:4 w6,9,12,15c | 0.315± 0.01 | 0.415 ± 0.01 | 0.005 | 0.42 ± 0.01 | 0.49 ± 0.01 | 0.04 |
| 20:1 w9c | 2.49 | - | 4.835 ± 0.04 | 5.165 ± 0.04 | ||
| 20:0 | 2.5 ± 0.056 | 2.615 ± 0.063 | 3.495 ± 0.08 | 4.055 ± 0.06 | 0.02 | |
| 20:0 iso | 0.375 ± 0.01 | 0.31 ± 0.014 | - | - | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kohler, C.; Proctor, R.A.; Bayer, A.S.; Yeaman, M.R.; Lalk, M.; Engelmann, S.; Mishra, N.N. Proteomic and Membrane Lipid Correlates of Re-duced Host Defense Peptide Susceptibility in a snoD Mutant of Staphylococcus aureus. Antibiotics 2019, 8, 169. https://doi.org/10.3390/antibiotics8040169
Kohler C, Proctor RA, Bayer AS, Yeaman MR, Lalk M, Engelmann S, Mishra NN. Proteomic and Membrane Lipid Correlates of Re-duced Host Defense Peptide Susceptibility in a snoD Mutant of Staphylococcus aureus. Antibiotics. 2019; 8(4):169. https://doi.org/10.3390/antibiotics8040169
Chicago/Turabian StyleKohler, Christian, Richard A. Proctor, Arnold S. Bayer, Michael R. Yeaman, Michael Lalk, Susanne Engelmann, and Nagendra N. Mishra. 2019. "Proteomic and Membrane Lipid Correlates of Re-duced Host Defense Peptide Susceptibility in a snoD Mutant of Staphylococcus aureus" Antibiotics 8, no. 4: 169. https://doi.org/10.3390/antibiotics8040169
APA StyleKohler, C., Proctor, R. A., Bayer, A. S., Yeaman, M. R., Lalk, M., Engelmann, S., & Mishra, N. N. (2019). Proteomic and Membrane Lipid Correlates of Re-duced Host Defense Peptide Susceptibility in a snoD Mutant of Staphylococcus aureus. Antibiotics, 8(4), 169. https://doi.org/10.3390/antibiotics8040169







