Evaluation of Immunocompetent Urinary Tract Infected Balb/C Mouse Model For the Study of Antibiotic Resistance Development Using Escherichia Coli CFT073 Infection
Abstract
1. Introduction
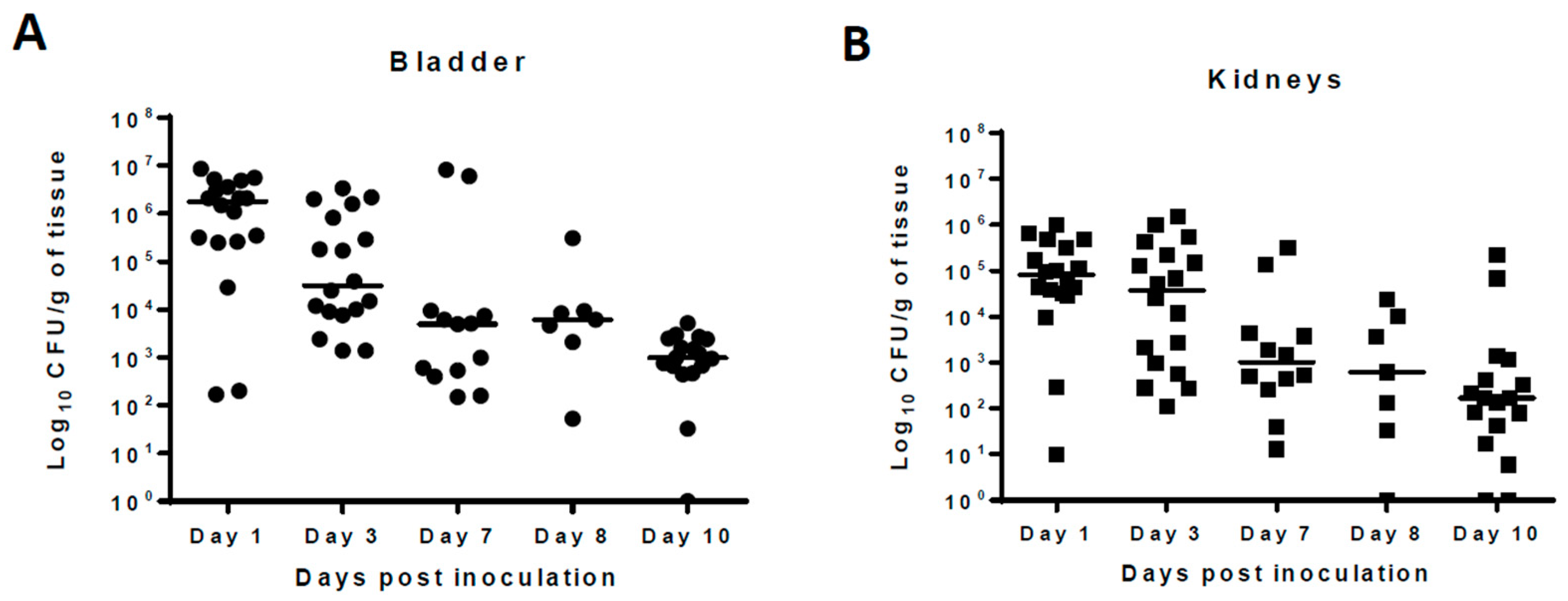
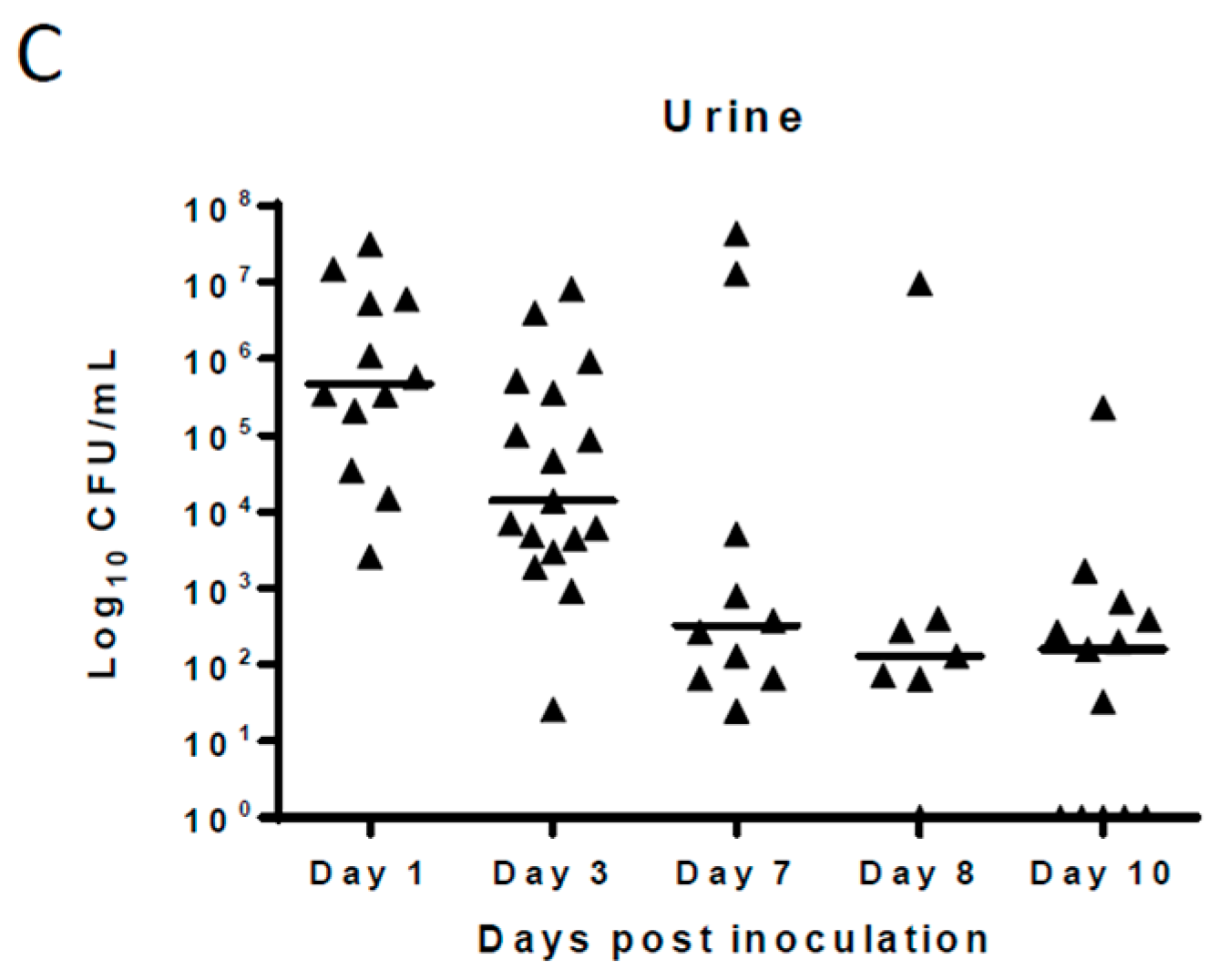
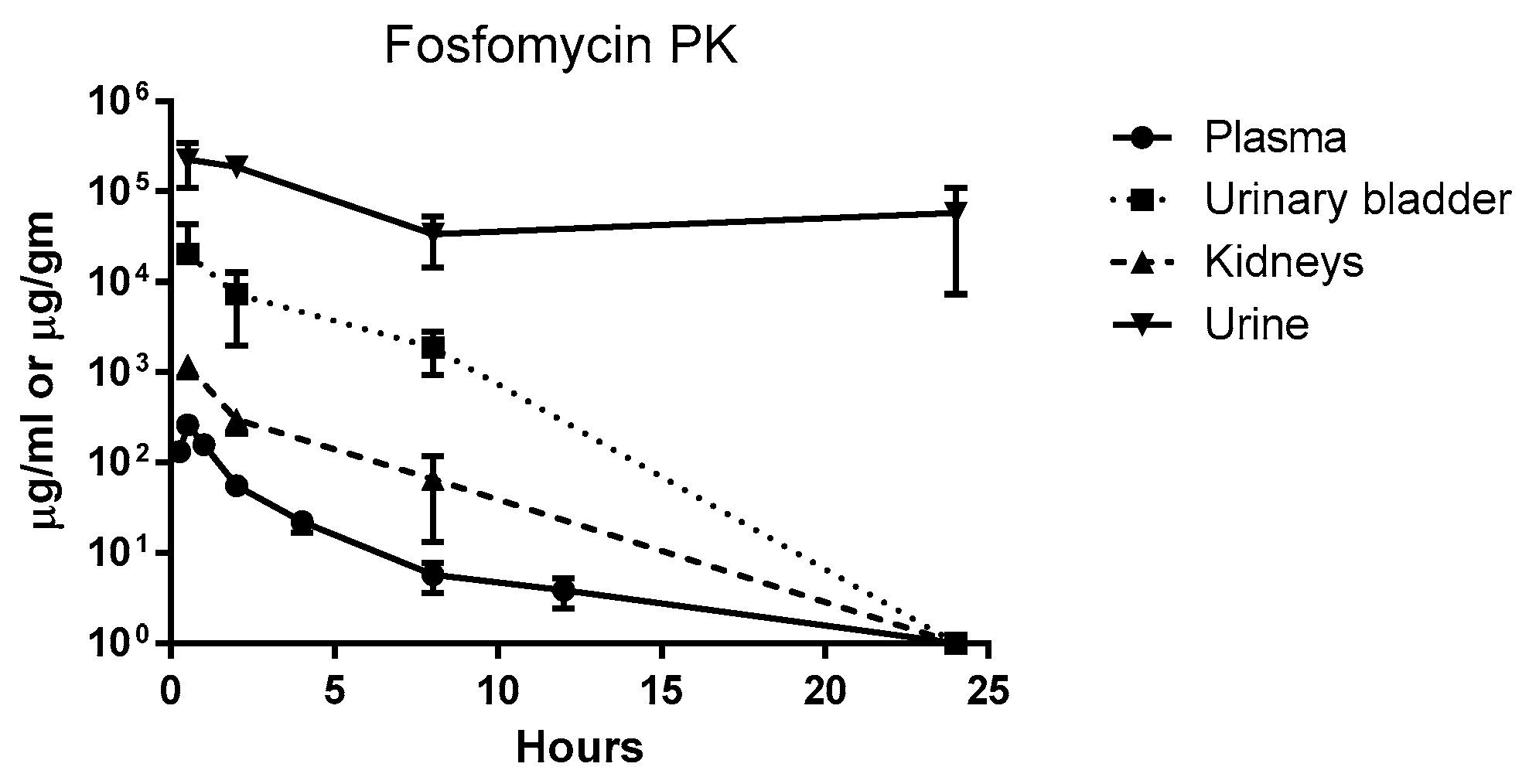
2. Results
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains, Media, and Chemicals
4.2. Animals
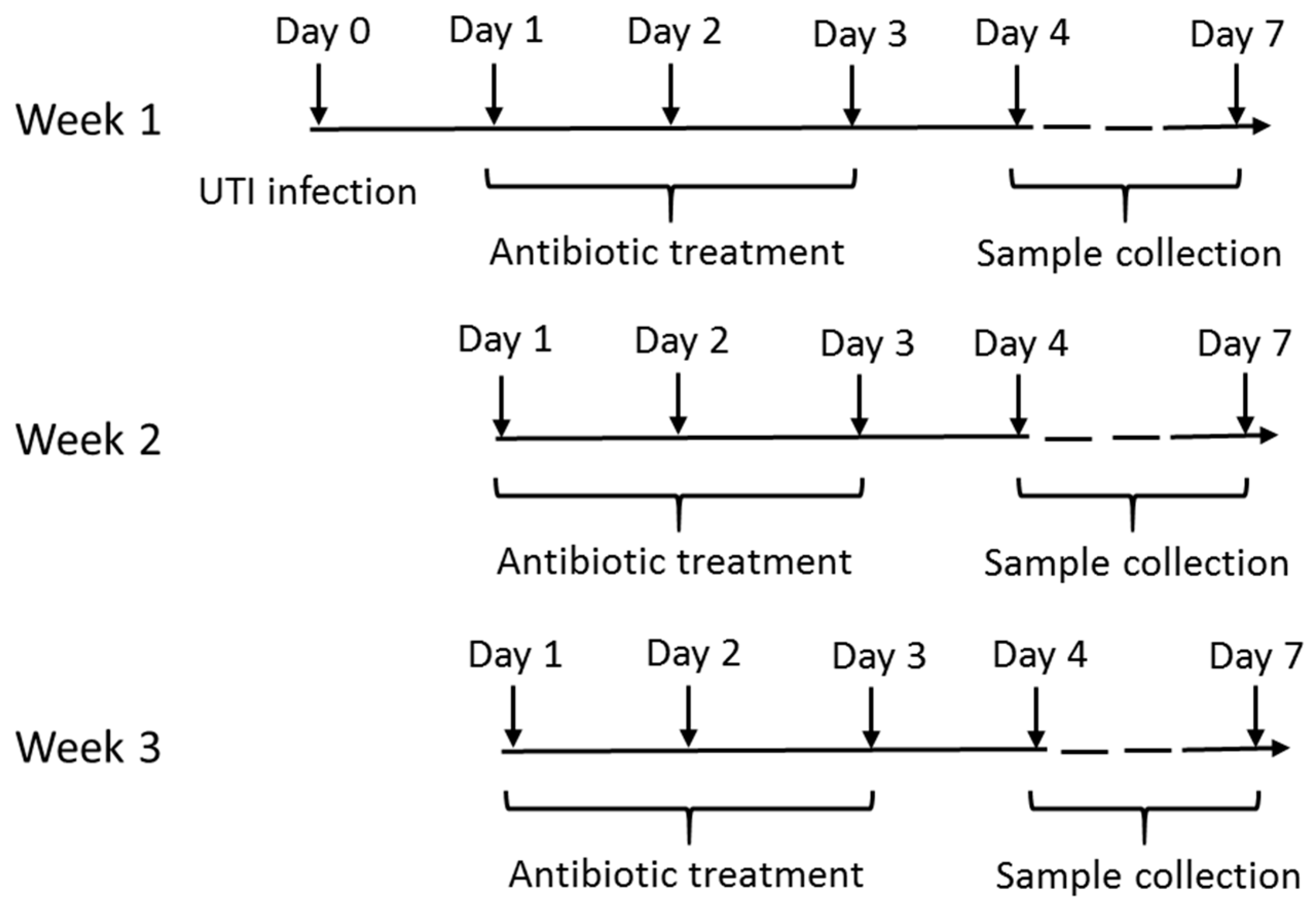
4.3. Experimental Design
4.4. Murine Ascending Urinary Tract Infection
4.5. Drug Administration
4.6. Sample Collection and Preparation
4.7. Estimation of Total and Resistant UPEC Population
4.8. Estimation of Antibiotics in Urinary Tract Tissues, Urine, and Plasma
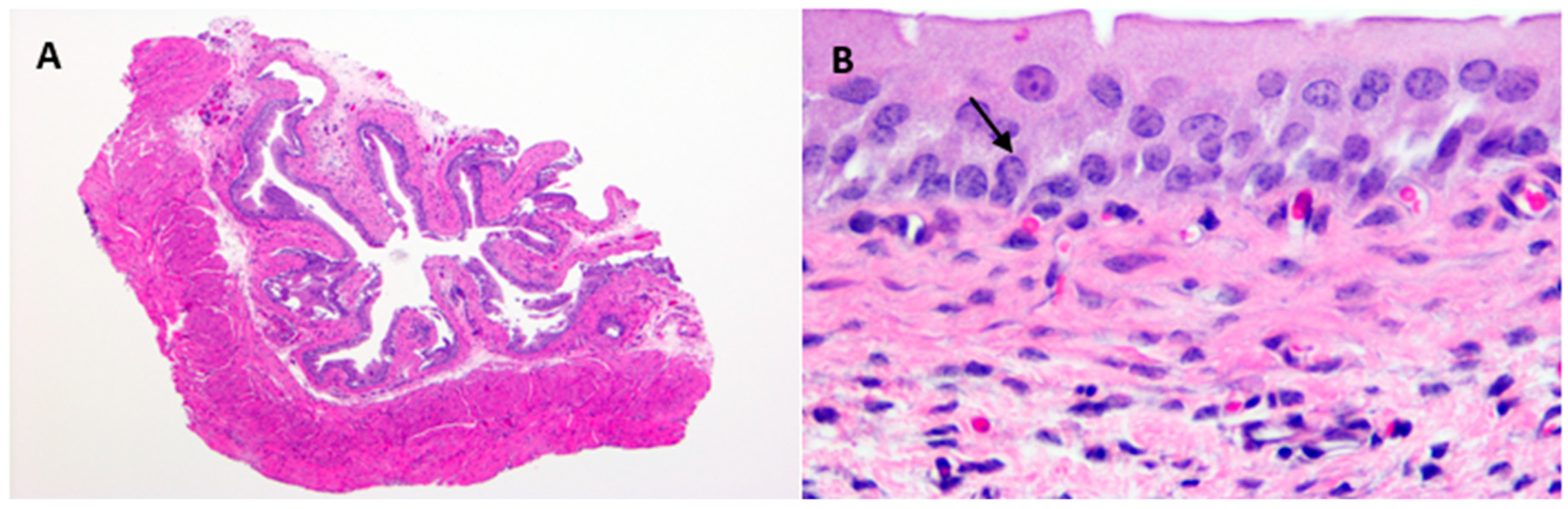
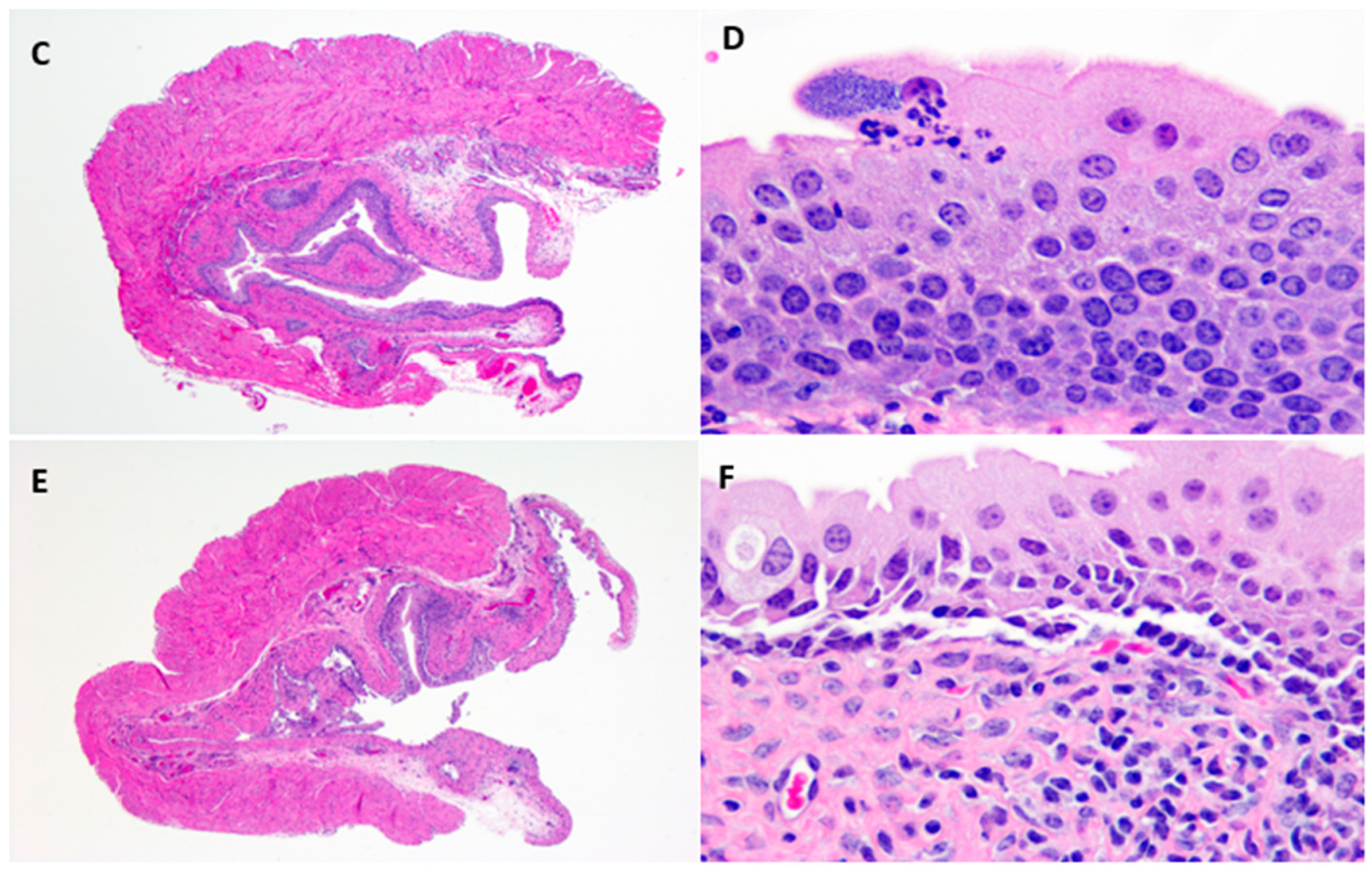
4.9. Histopathology
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Carey, A.J.; Tan, C.K.; Ipe, D.S.; Sullivan, M.J.; Cripps, A.W.; Schembri, M.A.; Ulett, G.C. Urinary tract infection of mice to model human disease: Practicalities, implications and limitations. Crit. Rev. Microbiol. 2016, 42, 780–799. [Google Scholar] [CrossRef] [PubMed]
- Ronald, A. The etiology of urinary tract infection: Traditional and emerging pathogens. Am. J. Med. 2002, 113, 14–19. [Google Scholar] [CrossRef]
- Sanchez, G.V.; Master, R.N.; Karlowsky, J.A.; Bordon, J.M. In vitro antimicrobial resistance of urinary Escherichia coli isolates among U.S. outpatients from 2000 to 2010. Antimicrob. Agents Chemother. 2012, 56, 2181–2183. [Google Scholar] [CrossRef] [PubMed]
- Sastry, S.; Clarke, L.G.; Alrowais, H.; Querry, A.M.; Shutt, K.A.; Doi, Y. Clinical Appraisal of Fosfomycin in the Era of Antimicrobial Resistance. Antimicrob. Agents Chemother. 2015, 59, 7355–7361. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Vouloumanou, E.K.; Samonis, G.; Vardakas, K.Z. Fosfomycin. Clin. Microbiol. Rev. 2016, 29, 321–347. [Google Scholar] [CrossRef] [PubMed]
- Vardakas, K.Z.; Legakis, N.J.; Triarides, N.; Falagas, M.E. Susceptibility of contemporary isolates to fosfomycin: A systematic review of the literature. Int. J. Antimicrob. Agents 2016, 47, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Karageorgopoulos, D.E.; Wang, R.; Yu, X.H.; Falagas, M.E. Fosfomycin: Evaluation of the published evidence on the emergence of antimicrobial resistance in Gram-negative pathogens. J. Antimicrob. Chemother. 2012, 67, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef]
- Davis, S.D. Dissociation between results of in vitro and in vivo antibiotic susceptibility tests for some strains of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 1974, 5, 281–288. [Google Scholar] [CrossRef]
- Jenkins, S.G.; Schuetz, A.N. Current concepts in laboratory testing to guide antimicrobial therapy. Mayo Clin. Proc. 2012, 87, 290–308. [Google Scholar] [CrossRef]
- Lefort, A.; Chau, F.; Lepeule, R.; Dubee, V.; Kitzis, M.D.; Dion, S.; Fantin, B. Activity of fosfomycin alone or combined with cefoxitin in vitro and in vivo in a murine model of urinary tract infection due to Escherichia coli harbouring CTX-M-15-type extended-spectrum beta-lactamase. Int. J. Antimicrob. Agents 2014, 43, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Prasad, V.; Ho, N. In vitro versus in vivo culture sensitivities: An unchecked assumption? Southwest J. Pulm. Crit. Care 2013, 6, 125–127. [Google Scholar]
- Thulin, E.; Sundqvist, M.; Andersson, D.I. Amdinocillin (Mecillinam) resistance mutations in clinical isolates and laboratory-selected mutants of Escherichia coli. Antimicrob. Agents Chemother. 2015, 59, 1718–1727. [Google Scholar] [CrossRef] [PubMed]
- Washington, J.A. Discrepancies between in vitro activity of and in vivo response to antimicrobial agents. Diagn. Microbiol. Infect. Dis. 1983, 1, 25–31. [Google Scholar] [CrossRef]
- Jones, R.N.; Dudley, M.N. Microbiologic and pharmacodynamic principals applied to the antimicrobial susceptibility testing of ampicillin/sulbactam: Analysis of the correlations between in vitro test results and clinical response. Diagn. Microbiol. Infect. Dis. 1997, 28, 5–18. [Google Scholar] [CrossRef]
- Bantar, C.; Alcazar, G.; Franco, D.; Salamone, F.; Vesco, E.; Stieben, T.; Obaid, F.; Fiorillo, A.; Izaguirre, M.; Oliva, M.E. Are laboratory-based antibiograms reliable to guide the selection of empirical antimicrobial treatment in patients with hospital-acquired infections? J. Antimicrob. Chemother. 2007, 59, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Doern, G.V.; Brecher, S.M. The clinical predictive value (or lack thereof) of the results of in vitro antimicrobial susceptibility tests. J Clin. Microbiol. 2011, 49, 11–14. [Google Scholar] [CrossRef]
- Zhao, M.; Lepak, A.J.; Andes, D.R. Animal models in the pharmacokinetic/pharmacodynamic evaluation of antimicrobial agents. Bioorg. Med. Chem. 2016, 24, 6390–6400. [Google Scholar] [CrossRef] [PubMed]
- Inglis, G.D.; Zaytsoff, S.J.M.; Selinger, L.B.; Taboada, E.N.; Uwiera, R.R.E. Therapeutic administration of enrofloxacin in mice does not select for fluoroquinolone resistance in Campylobacter jejuni. Can. J. Microbiol. 2018, 64, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Pultz, M.J.; Nerandzic, M.M.; Stiefel, U.; Donskey, C.J. Emergence and acquisition of fluoroquinolone-resistant gram-negative bacilli in the intestinal tracts of mice treated with fluoroquinolone antimicrobial agents. Antimicrob. Agents Chemother. 2008, 52, 3457–3460. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.D. Activity of gentamicin, tobramycin, polymyxin B, and colistimethate in mouse protection tests with Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 1975, 8, 50–53. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Allou, N.; Cambau, E.; Massias, L.; Chau, F.; Fantin, B. Impact of low-level resistance to fluoroquinolones due to qnrA1 and qnrS1 genes or a gyrA mutation on ciprofloxacin bactericidal activity in a murine model of Escherichia coli urinary tract infection. Antimicrob. Agents Chemother. 2009, 53, 4292–4297. [Google Scholar] [CrossRef] [PubMed]
- Rossi, B.; Soubirou, J.F.; Chau, F.; Massias, L.; Dion, S.; Lepeule, R.; Fantin, B.; Lefort, A. Cefotaxime and Amoxicillin-Clavulanate Synergism against Extended-Spectrum-beta-Lactamase-Producing Escherichia coli in a Murine Model of Urinary Tract Infection. Antimicrob. Agents Chemother. 2016, 60, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Monogue, M.L.; Nicolau, D.P. Translational Efficacy of Humanized Exposures of Cefepime, Ertapenem, and Levofloxacin against Extended-Spectrum-beta-Lactamase-Producing Escherichia coli in a Murine Model of Complicated Urinary Tract Infection. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Blango, M.G.; Mulvey, M.A. Persistence of uropathogenic Escherichia coli in the face of multiple antibiotics. Antimicrob. Agents Chemother. 2010, 54, 1855–1863. [Google Scholar] [CrossRef] [PubMed]
- Heatley, N.G. An arrangement for obtaining successive urine and blood samples from the unanaesthetized mouse: serum levels and excretion of penicillin G and p-aminobenzyl penicillin. Q. J. Exp. Physiol. Cogn. Med. Sci. 1959, 44, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Michalopoulos, A.S.; Livaditis, I.G.; Gougoutas, V. The revival of fosfomycin. Int. J. Infect. Dis. 2011, 15, 732–739. [Google Scholar] [CrossRef]
- Jakobsen, L.; Cattoir, V.; Jensen, K.S.; Hammerum, A.M.; Nordmann, P.; Frimodt-Moller, N. Impact of low-level fluoroquinolone resistance genes qnrA1, qnrB19 and qnrS1 on ciprofloxacin treatment of isogenic Escherichia coli strains in a murine urinary tract infection model. J. Antimicrob. Chemother. 2012, 67, 2438–2444. [Google Scholar] [CrossRef]
- Hung, C.S.; Dodson, K.W.; Hultgren, S.J. A murine model of urinary tract infection. Nat. Protoc. 2009, 4, 1230–1243. [Google Scholar] [CrossRef]
- Matta, M.K.; Chockalingam, A.; Gandhi, A.; Stewart, S.; Xu, L.; Shea, K.; Patel, V.; Rouse, R. LC-MS/MS based quantitation of ciprofloxacin and its application to antimicrobial resistance study in Balb/c mouse plasma, urine, bladder and kidneys. Anal. Methods 2018, 1237–1246. [Google Scholar] [CrossRef]
- Gandhi, A.; Matta, M.; Garimella, N.; Zere, T.; Weaver, J. Development and validation of a LC-MS/MS method for quantitation of fosfomycin—Application to in vitro antimicrobial resistance study using hollow-fiber infection model. Biomed. Chromatogr. BMC 2018, 32, 4214. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, A.; Matta, M.; Zere, T.; Weaver, J. Combining LC-MS/MS and hollow-fiber infection model for real-time quantitation of ampicillin to antimicrobial resistance. Future Sci. OA 2019, 5, FSO349. [Google Scholar] [CrossRef] [PubMed]
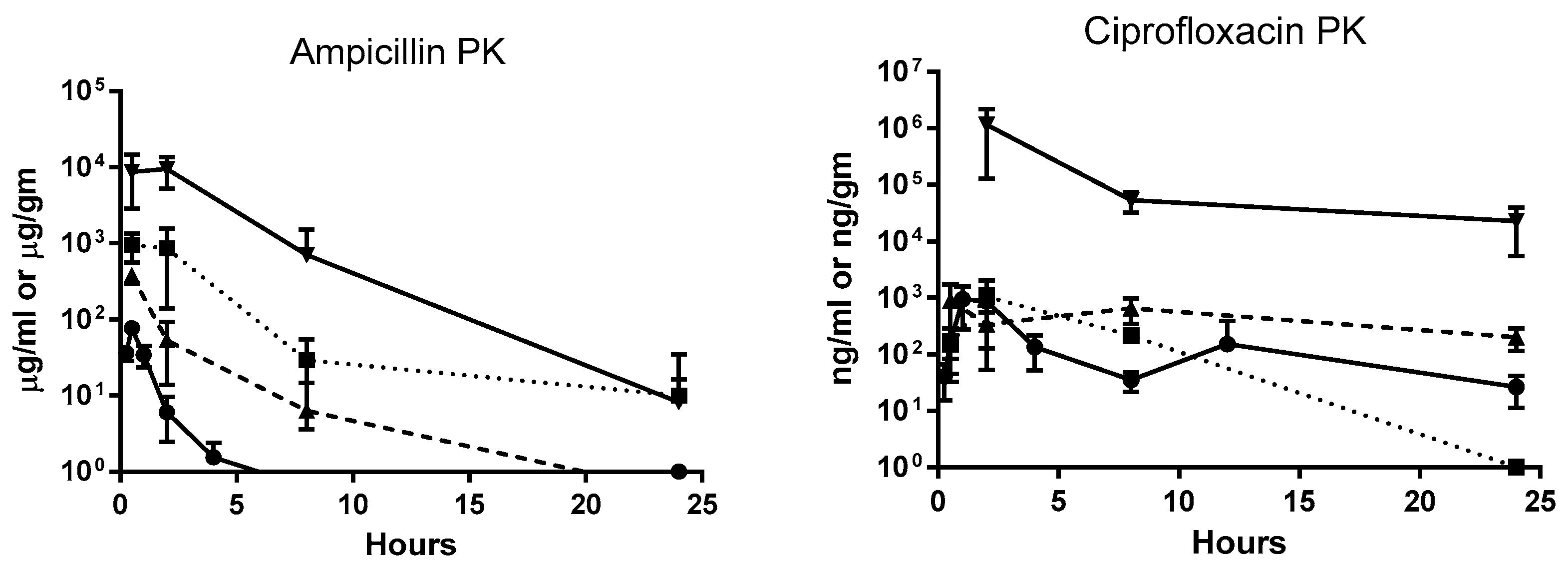
| Ampicillin Concentrations (µg/mL) and Bacterial Counts (cfu/gm or mL) @ | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time (h) | Plasma | Bladder | Kidneys | Urine # | ||||||
| Amp(µg/mL) High Dose | Amp (µg/mL) High Dose | Median Log10 cfu/gm | Amp (µg/mL) High Dose | Median Log10 cfu/gm | Amp (µg/mL) High Dose | Median Log10 cfu/mL | ||||
| Range | Range | Range | ||||||||
| (No. Sterile/Total) | (No. Sterile/Total) | (No. Sterile/Total) | ||||||||
| High Dose | Low Dose | High Dose | Low Dose | High Dose | Low Dose | |||||
| 2 | 6.4 ± 3.8 | 217.4 ± 127.3 | 4.3 | - | 69 ± 46.7 | 3.6 | - | 2428 ± 928.1 | 5.2 | - |
| (2.8–5.2) | (2.8–4.6) | (2.3–5.6) | ||||||||
| (0/6) | (0/6) | (0/5) | ||||||||
| 4 | 1 ± 0.2 | 0.1 ± 0 | 4.6 | - | 14 ± 7.2 | 3.4 | - | 395 ± 172.5 | 5.1 | - |
| (2.9–5.2) | (3.0–4.1) | (4.9–5.9) | ||||||||
| (0/6) | (0/6) | (0/5) | ||||||||
| 24 | 0.3 ± 0.2 | 0.1 ± 0 | 3.8 | 5.5 | 0.1 ± 0 | 3.6 | 4.8 | 173.3 ± 145.6 | 4.3 | 4.7 |
| (3.3–4.2) | (3.4–5.9) | (1.6–4.4) | (4.4–5.9) | (2.3–4.8) | (3.0–6.2) | |||||
| (0/6) | (0/6) | (0/6) | (0/6) | (0/6) | (0/5) | |||||
| 26 | 24.5 ± 9.6 | 972.1 ± 818.5 | 3.2 | - | 203 ± 97.1 | 2.8 | - | 9044 ± 2933 | 2.2 | - |
| (2.4–3.7) | (1.3–3.7) | (1.3–2.5) | ||||||||
| (0/6) | (0/6) | (2/4) | ||||||||
| 28 | 8.3 ± 3.9 | 495 ± 409.7 | 2.9 | - | 73.9 ± 22.4 | 2.3 | - | 6356 ± 2425.4 | 3.0 | - |
| (1.4–3.0) | (1.4–3.2) | (2.3–4.1) | ||||||||
| (1/6) | (0/6) | (0/3) | ||||||||
| 48 | 0.1 ± 0.1 | 0.1 ± 0 | 2.8 | 4.3 * | 0.1 ± 0 | 3.5 | 3.7 | 84.9 ± 33.7 | 2.0 | 4.1 |
| (2.3–3.3) | (3.6–4.7) | (2.8–4.0) | (2.0–4.8) | (-) | (3.0–4.3) | |||||
| (0/6) | (0/6) | (0/6) | (0/6) | (4/5) | (0/5) | |||||
| 50 | 31.8 ± 7.7 | 2180.1 ± 1977 | 2.3 | - | 298 ± 74.7 | 1.1 | - | 7010 ± 1885.7 | 2.5 | - |
| (2.2–2.6) | (0.8–1.4) | (2.0–2.7) | ||||||||
| (1/6) | (1/6) | (4/6) | ||||||||
| 52 | 18.6 ± 4.2 | 983.5 ± 356.3 | 2.3 | - | 203.9 ± 61.7 | 2.6 | - | 10338 ± 3974.2 | 2.3 | - |
| (0.8–3.4) | (1.7–2.8) | (2.3–2.3) | ||||||||
| (0/6) | (4/6) | (2/5) | ||||||||
| 72 | 0.2 ± 0.1 | 0.1 ± 0 | 2.1 | 3.6 | 0.1 ± 0 | 2.5 | 2.7 | 103 ± 55.1 | 2.2 | 2.3 |
| (0.8–3.4) | (2.1–3.8) | (1.1–3.6) | (1.9–4.0) | (2.0–2.3) | (2.0–3.3) | |||||
| (4/6) | (0/6) | (3/6) | (0/6) | (4/6) | (2/6) | |||||
| Control | <LLOQ ϕ | <LLOQ | 2.6 | 4.1 | <LLOQ | 3.2 | 3.8 ** | <LLOQ | 2.7 | 3.7 |
| (1.4–3.5) | (4.0–4.2) | (2.1–5.2) | (2.6–5.7) | (2.6–2.8) | (2.6–5.0) | |||||
| (1/6) | (0/6) | (0/6) | (0/6) | (3/5) | (0/6) | |||||
| Ciprofloxacin Concentration (µg/mL) and Bacterial Counts (cfu/gm or mL) @ | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time (h) | Plasma | Bladder | Kidneys | Urine # | ||||||
| Cipro (µg/mL) High Dose | Cipro (µg/mL) High Dose | Median Log10 cfu/gm | Cipro (µg/mL) High Dose | Median Log10 cfu/gm | Cipro (µg/mL) High Dose | Median Log10 cfu/mL | ||||
| Range | Range | Range | ||||||||
| (No. Sterile/Total) | (No. Sterile/Total) | (No. Sterile/Total) | ||||||||
| High Dose | Low Dose | High Dose | Low Dose | High Dose | Low Dose | |||||
| 24 | 0.1 ± 0 | 223.7 ± 106.4 | 3.3 | 4.6 | 4.2 ± 3.7 | 1.9 | 3.4 | 198.5 ± 100.8 | 2.0 | 4.4 |
| (1.4–3.7) | (3.3–5.6) | (1.3–2.2) | (2.5–4.7) | (-) | (2–5.6) | |||||
| (0/8) | (0/6) | (3/8) | (0/6) | (4/5) | (0/6) | |||||
| 26 | 1.0 ± 0.2 | 705.7 ± 604.5 | 0.9 | - | 72.8 ± 66.5 | 2.1 | - | 965.4 ± 48.6 | 2.0 | - |
| (0.8–2.4) | (1.1–2.4) | (-) | ||||||||
| (4/9) | (7/9) | (6/7) | ||||||||
| 28 | 0.9 ± 0.1 | 796.1 ± 271.2 | 2.1 | - | 59.9 ± 85 | 2.0 | - | NA | 0 | - |
| (1.6–3.0) | (1.3–2.7) | (-) | ||||||||
| (2/8) | (3/8) | (3/3) | ||||||||
| 32 | 0.2 ± 0.1 | 495.7 ± 281.6 | 2.2 | - | 8.4 ± 5.9 | 2.4 | - | 451.4 ± 112 | 1.0 | - |
| (1.4–2.7) | (0.8–3.3) | (-) | ||||||||
| (4/8) | (3/8) | (6/7) | ||||||||
| 48 | 0.1 ± 0 | 173.9 ± 14.3 | 2.3 | 4.4 * | 5.1 ± 3.7 | 2.9 | 2.3 | 378.9 ± 199.5 | 0 | 4.7 |
| (1.5–3.0) | (3.5–4.7) | (1.7–3.3) | (0.8–4.1) | (-) | (2.5–5.0) | |||||
| (0/8) | (0/6) | (3/8) | (0/6) | (6/6) | (1/5) | |||||
| 72 | 0.1 ± 0 | 160 ± 18.5 | 2.8 | 3.3 | 1.8 ± 0.6 | 2.1 | 2.3 | 237.3 ± 77.1 | 0 | 3.8 |
| (0.8–3.5) | (2.7–3.8) | (1.1–2.8) | (1.6–3.7) | (-) | (-) | |||||
| (0/9) | (0/6) | (3/9) | (0/6) | (7/7) | (5/6) | |||||
| Control | <LLOQ ϕ | <LLOQ | 1.7 | 5.0 | <LLOQ | 4.3 | 2.1 | <LLOQ | 2.6 | 4.1 |
| (1.7–2.7) | (1.3–4.5) | (1.4–4.6) | (1.1–4.3) | (2.4–2.7) | (2.5–5.5) | |||||
| (0/3) | (0/6) | (1/3) | (0/6) | (0/2) | (1/6) | |||||
| Fosfomycin Concentration (µg/mL) and Bacterial Counts (cfu/gm or mL) @ | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time (h) | Plasma | Bladder | Kidneys | Urine# | ||||||
| Fosfo (µg/mL) High Dose | Fosfo (µg/mL) High Dose | Median Log10 cfu/gm | Fosfo (µg/mL) High Dose | Median Log10 cfu/gm | Fosfo (µg/mL) High Dose | Median Log10 cfu/mL | ||||
| Range | Range | Range | ||||||||
| (No. Sterile/Total) | (No. Sterile/Total) | (No. Sterile/Total) | ||||||||
| High Dose | Low Dose | High Dose | Low Dose | High Dose | Low Dose | |||||
| 24 | 2.0 ± 0.6 | <LLOQ | 2.8 | 4.5 * | 39 ± 8.16 | 1.4 | 2.9 | 1134.4 ± 465.2 | 0 | 4.8 |
| (1.8–3.3) | (3.8–4.8) | (1.4–2.4) | (2.2–3.7) | (-) | (4.1–5.4) | |||||
| (2/9) | (0/6) | (6/9) | (0/6) | (7/7) | (1/5) | |||||
| 26 | 181.7 ± 38.5 | 44087 ± 37613.2 | 2.8 | - | 2232.2 ± 590.6 | 2.0 | - | 73509.4 ± 25767.2 | 0 | - |
| (1.8–3.6) | (1.4–2.7) | (-) | ||||||||
| (1/9) | (5/9) | (8/8) | ||||||||
| 28 | 81.9 ± 22.4 | 36688.8 ± 57680.9 | 2.7 | - | 2517 ± 1187.3 | 1.9 | - | 66556.3 ± 24606.8 | 0 | - |
| (1.5–4.3) | (1.1–4.3) | (-) | ||||||||
| (2/9) | (0/9) | (7/7) | ||||||||
| 32 | 37.1 ± 11.9 | 22844.9 ± 38340.4 | 2.1 | - | 809 ± 516.9 | 1.9 | - | 27835 ± 10871 | 0 | - |
| (1.8–3.4) | (1.1–3.9) | (-) | ||||||||
| (1/8) | (5/8) | (4/4) | ||||||||
| 48 | 2.6 ± 1 | 480.7 ± 319.5 | 2.3 | 3.7 * | 44.9 ± 18.2 | 2.0 | 2.5 | 1380.6 ± 1096.9 | 2.0 | 2.6 |
| (1.9–3.4) | (2.9–4.1) | (0.8–3.3) | (2.1–4.1) | (-) | (1.0–2.9) | |||||
| (1/9) | (0/6) | (1/9) | (0/6) | (8/9) | (2/6) | |||||
| 72 | 1.1 ± 0 | <LLOQ | 3.1 | 4.0 * | 31.2 ±0 | 2.2 | 2.4 | 1235.9 ± 894.3 | 3.1 | 2.4 |
| (2.0–3.4) | (3.3–4.1) | (1.3–2.5) | (1.4–3.1) | (2.0–3.5) | (2.0–2.8) | |||||
| (0/9) | (0/6) | (5/9) | (0/6) | (3/9) | (3/6) | |||||
| Control | <LLOQ ϕ | <LLOQ | 2.9 | 4.3 | <LLOQ | 3.1 | 4.7 | <LLOQ | 2.9 | 3.2 |
| (1.7–4.1) | (4.0–4.4) | (2.3–4.3) | (3.3–5.5) | (2.3–3.1) | (3.0–5.8) | |||||
| (0/6) | (0/6) | (0/6) | (0/6) | (3/5) | (0/6) | |||||
| Time (Weeks) | Days Post-Infection | Bacterial Counts (cfu/gm or mL) @ | |||||
|---|---|---|---|---|---|---|---|
| Bladder | Kidneys | Urine # | |||||
| Median Log10 cfu/gm | Median Log10 cfu/gm | Median Log10 cfu/mL | |||||
| Range | Range | Range | |||||
| (No. Sterile/Total) | (No. Sterile/Total) | (No. Sterile/Total) | |||||
| Treatment | Control | Treatment | Control | Treatment | Control | ||
| First week | Day 4 | 2.8 | 4.3 ** | 2.4 | 4.2 | 2 | 3.4 |
| (1.6–3.8) | (3.9–4.4) | (1.8–3.9) | (3.5–5.2) | (2.0–3.3) | (2.4–5.1) | ||
| (0/6) | (0/6) | (0/6) | (0/6) | (0/5) | (0/6) | ||
| Day 7 | 2.4 | 3.9 | 3.3 | 4.6 | 2.7 | 3.4 | |
| (0.8–3.5) | (3.7–7.2) | (1.7–3.8) | (2.1–7.2) | (2.3–3.0) | (2.8–3.7) | ||
| (0/6) | (0/6) | (0/6) | (0/6) | (0/2) | (0/5) | ||
| Second week | Day 4 | 2.9 | 3.0 | 1.5 | 2.2 | 0 | 3.1 |
| (2.5–3.1) | (2.5–3.0) | (1.4–1.5) | (1.4–3.3) | (-) | (3.1–3.2) | ||
| (4/6) | (3/6) | (4/6) | (1/6) | (6/6) | (4/6) | ||
| Day 7 | 2.3 | 2.5 | 1.4 | 1.6 | 0 | 2.4 | |
| (0.5–2.6) | (2.1–2.9) | (1.3–2.0) | (1.1–1.6) | (-) | (-) | ||
| (2/6) | (2/6) | (3/6) | (3/6) | (6/6) | (5/6) | ||
| Third week | Day 4 | 2.4 | 2.3 | 2 | 2.2 | 0 | 0 |
| (2.2–3.0) | (0.5–2.7) | (1.8–2.8) | (1.1–2.5) | ||||
| (3/6) | (0/6) | (3/6) | (2/6) | ||||
| Day 7 | 1.7 | 1.8 | 1.1 | 1.2 | 0 | 0 | |
| (1.6–1.9) | (1.6–1.8) | (0.8–1.2) | (-) | ||||
| (2/6) | (3/6) | (4/6) | (5/6) | ||||
| Treatment Group (Dose/kg) | 1 × MIC Resistant Colonies |
|---|---|
| Ampicillin | MIC–4 µg/mL |
| High dose (200 mg/kg) | One urine sample in control group (1.4 × 103 cfu/mL) |
| Low dose (20 mg/kg) | None |
| Ciprofloxacin | MIC–0.03 µg/mL |
| High dose (50 mg/kg) | One bladder (1.8 × 102 cfu/mL) and one kidney (8.7 × 101 cfu/mL) samples in 28 h. group; |
| Two bladders (2 × 101 and 7 × 100 cfu/mL) and one kidney (4.26 × 102 cfu/mL) samples in 32 h. group | |
| Low dose (5 mg/kg) | One kidney sample each in 24 h. (1.3 × 101 cfu/mL) and 72 h. (7 × 100 cfu/mL) group |
| Fosfomycin | MIC–64 µg/mL |
| High dose (1000 mg/kg) | One urine sample in 24 h. (6.7 × 101 cfu/mL) group |
| Low dose (100 mg/kg) | None |
| Long-term study (500 mg/kg) | One bladder (1 × 101 cfu/ml) sample in day 4 of week 3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chockalingam, A.; Stewart, S.; Xu, L.; Gandhi, A.; Matta, M.K.; Patel, V.; Sacks, L.; Rouse, R. Evaluation of Immunocompetent Urinary Tract Infected Balb/C Mouse Model For the Study of Antibiotic Resistance Development Using Escherichia Coli CFT073 Infection. Antibiotics 2019, 8, 170. https://doi.org/10.3390/antibiotics8040170
Chockalingam A, Stewart S, Xu L, Gandhi A, Matta MK, Patel V, Sacks L, Rouse R. Evaluation of Immunocompetent Urinary Tract Infected Balb/C Mouse Model For the Study of Antibiotic Resistance Development Using Escherichia Coli CFT073 Infection. Antibiotics. 2019; 8(4):170. https://doi.org/10.3390/antibiotics8040170
Chicago/Turabian StyleChockalingam, Ashok, Sharron Stewart, Lin Xu, Adarsh Gandhi, Murali K. Matta, Vikram Patel, Leonard Sacks, and Rodney Rouse. 2019. "Evaluation of Immunocompetent Urinary Tract Infected Balb/C Mouse Model For the Study of Antibiotic Resistance Development Using Escherichia Coli CFT073 Infection" Antibiotics 8, no. 4: 170. https://doi.org/10.3390/antibiotics8040170
APA StyleChockalingam, A., Stewart, S., Xu, L., Gandhi, A., Matta, M. K., Patel, V., Sacks, L., & Rouse, R. (2019). Evaluation of Immunocompetent Urinary Tract Infected Balb/C Mouse Model For the Study of Antibiotic Resistance Development Using Escherichia Coli CFT073 Infection. Antibiotics, 8(4), 170. https://doi.org/10.3390/antibiotics8040170





