The Effect of 2-Thiocyanatopyridine Derivative 11026103 on Burkholderia Cenocepacia: Resistance Mechanisms and Systemic Impact
Abstract
1. Introduction
2. Results and Discussion
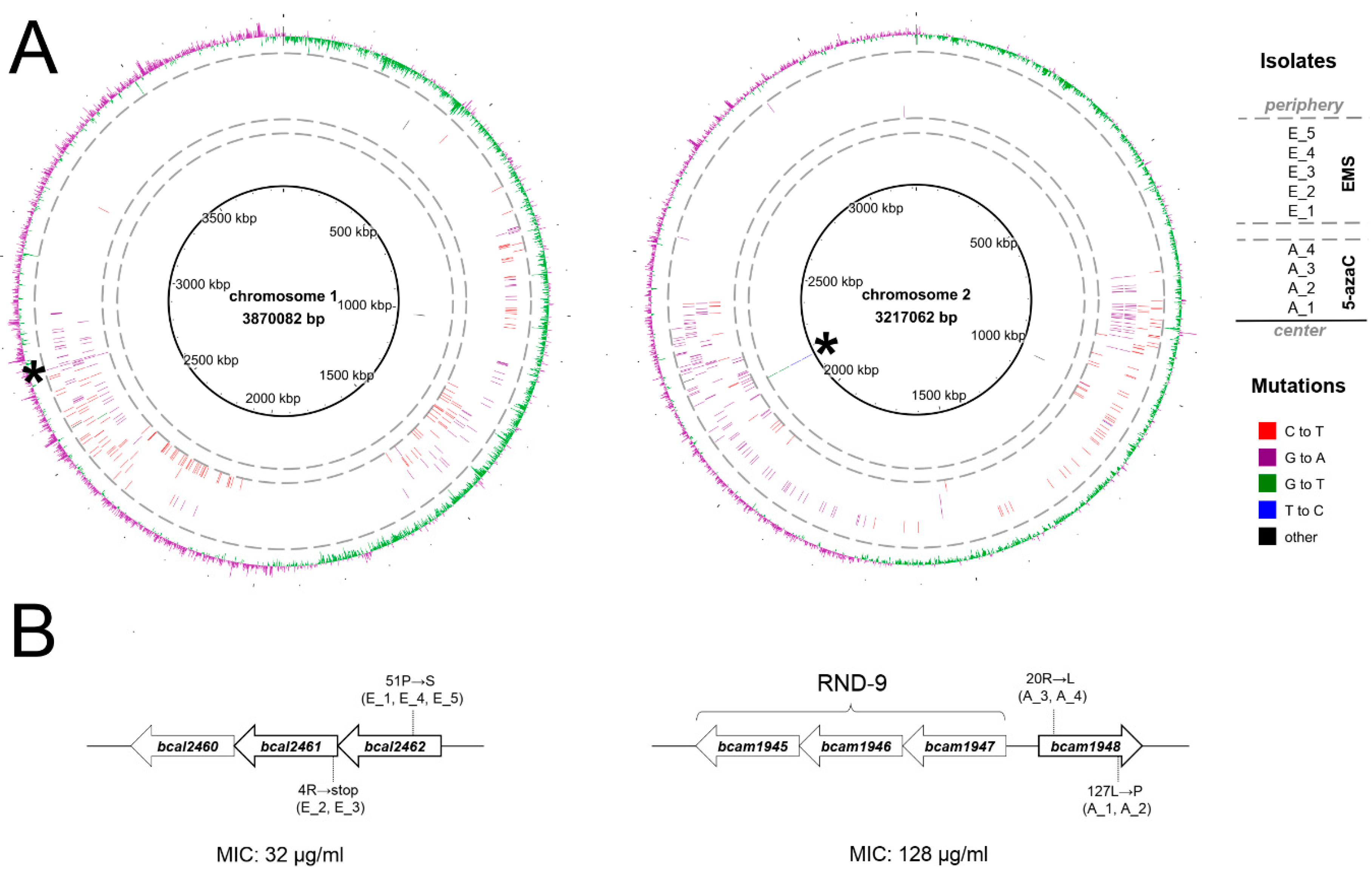
2.1. Selection and Genetic Characterization of B. cenocepacia Mutants Resistant to 11026103
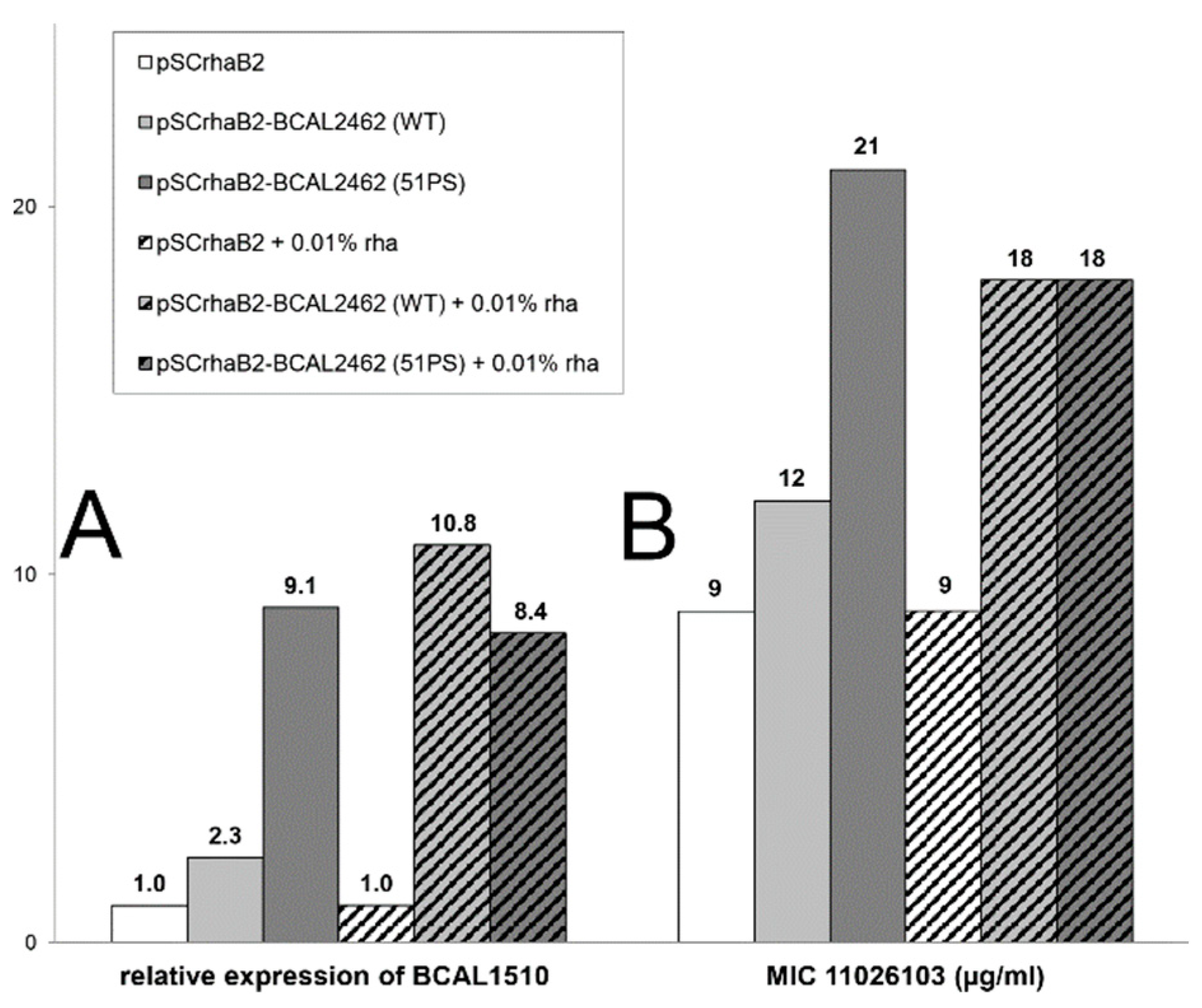
2.2. Resistance of B. cenocepacia to 11026103 Is Solely Efflux-Mediated
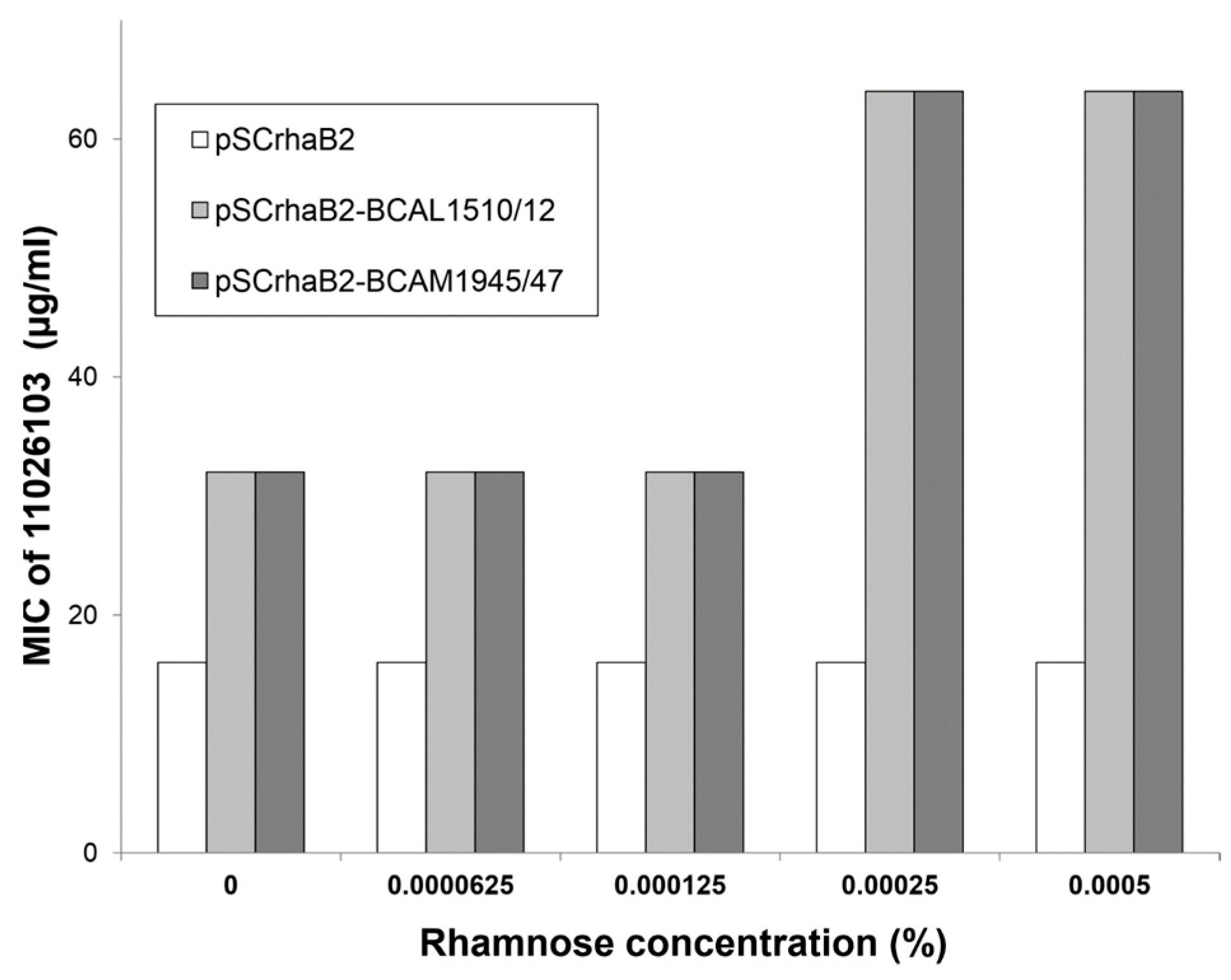
2.3. Knockdowns in Transport and Metabolic Genes Increase Resistance to 11026103
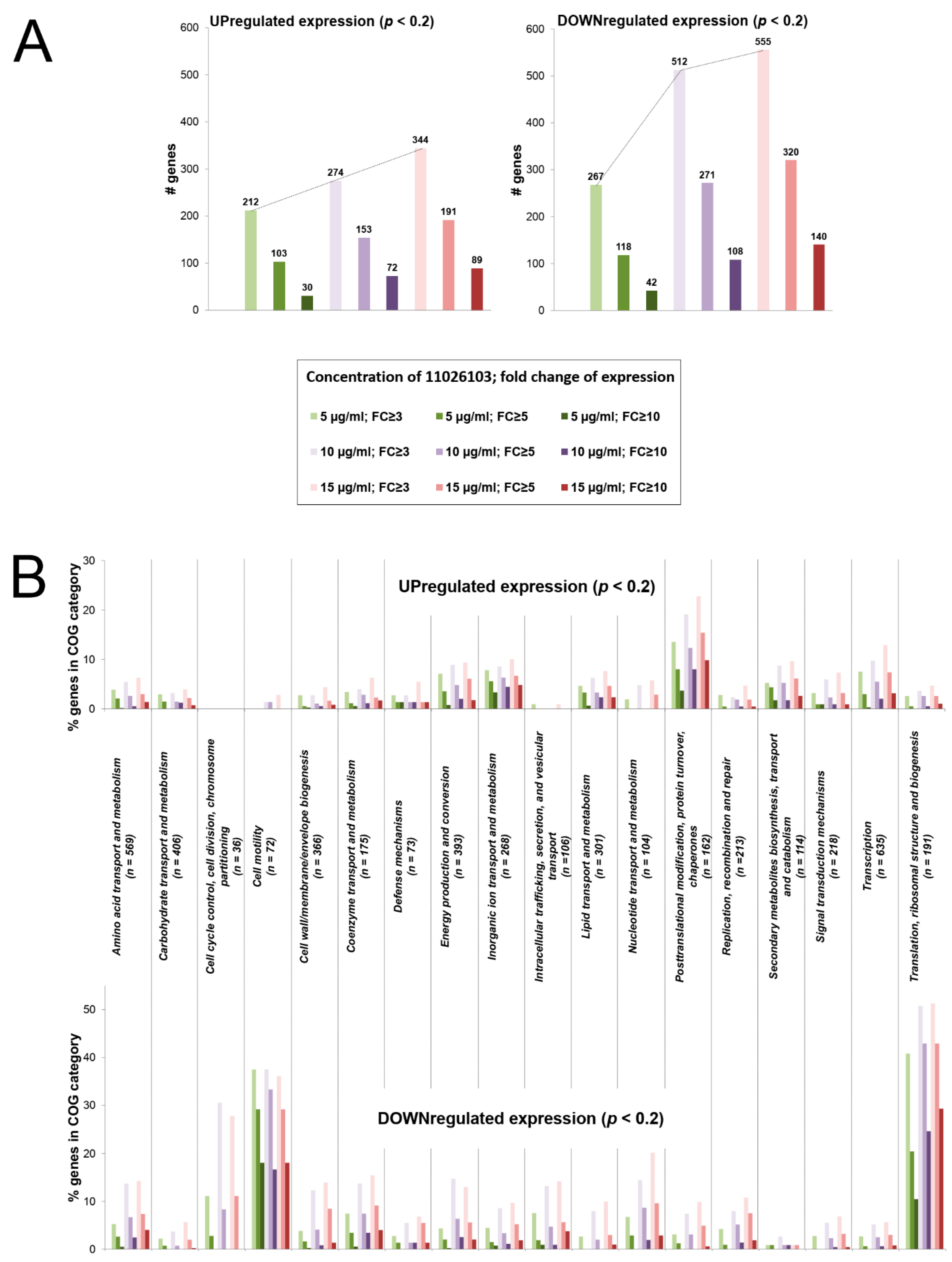
2.4. Transcriptomic Response of B. cenocepacia Elicited by 11026103
3. Materials and Methods
3.1. Bacterial Strains, Plasmids, and Growth Conditions
3.2. Chemical Mutagenesis
3.3. MIC Determination
3.4. Construction of pSCrhaB2 Derivatives and Genetic Modification of B. cenocepacia
3.5. Whole-Genome Sequencing and Data Analysis
3.6. RNA-Seq and Data Analysis
3.7. Competitive Fitness Assay and Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mahenthiralingam, E.; Urban, T.A.; Goldberg, J.B. The multifarious, multireplicon Burkholderia cepacia complex. Nat. Rev. Microbiol. 2005, 3, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.K.; Govan, J.R. Burkholderia cepacia: Another twist and a further threat. Thorax 1998, 53, 333–334. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nunvar, J.; Capek, V.; Fiser, K.; Fila, L.; Drevinek, P. What matters in chronic Burkholderia cenocepacia infection in cystic fibrosis: Insights from comparative genomics. PLoS Pathog. 2017, 13, e1006762. [Google Scholar] [CrossRef] [PubMed]
- Nzula, S.; Vandamme, P.; Govan, J.R. Influence of taxonomic status on the in vitro antimicrobial susceptibility of the Burkholderia cepacia complex. J. Antimicrob. Chemother. 2002, 50, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Aaron, S.D.; Vandemheen, K.L.; Ferris, W.; Fergusson, D.; Tullis, E.; Haase, D.; Berthiaume, Y.; Brown, N.; Wilcox, P.; Yozghatlian, V.; et al. Combination antibiotic susceptibility testing to treat exacerbations of cystic fibrosis associated with multiresistant bacteria: A randomised, double-blind, controlled clinical trial. Lancet 2005, 366, 463–471. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, Y.; Tabibi, S.; Alba, L.; Garber, E.; Saiman, L. Antimicrobial susceptibility and synergy studies of Burkholderia cepacia complex isolated from patients with cystic fibrosis. Antimicrob. Agents Chemother. 2007, 51, 1085–1088. [Google Scholar] [CrossRef] [PubMed]
- Salina, E.; Ryabova, O.; Kaprelyants, A.; Makarov, V. New 2-thiopyridines as potential candidates for killing both actively growing and dormant Mycobacterium tuberculosis cells. Antimicrob. Agents Chemother. 2014, 58, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Scoffone, V.C.; Spadaro, F.; Udine, C.; Makarov, V.; Fondi, M.; Fani, R.; De Rossi, E.; Riccardi, G.; Buroni, S. Mechanism of resistance to an antitubercular 2-thiopyridine derivative that is also active against Burkholderia cenocepacia. Antimicrob. Agents Chemother. 2014, 58, 2415–2417. [Google Scholar] [CrossRef]
- Buroni, S.; Pasca, M.R.; Flannagan, R.S.; Bazzini, S.; Milano, A.; Bertani, I.; Venturi, V.; Valvano, M.A.; Riccardi, G. Assessment of three Resistance-Nodulation-Cell Division drug efflux transporters of Burkholderia cenocepacia in intrinsic antibiotic resistance. BMC Microbiol. 2009, 9, 200. [Google Scholar] [CrossRef]
- Cupples, C.G.; Miller, J.H. A set of lacZ mutations in Escherichia coli that allow rapid detection of each of the six base substitutions. Proc. Natl. Acad. Sci. USA 1989, 86, 5345–5349. [Google Scholar] [CrossRef]
- Scoffone, V.C.; Ryabova, O.; Makarov, V.; Iadarola, P.; Fumagalli, M.; Fondi, M.; Fani, R.; De Rossi, E.; Riccardi, G.; Buroni, S. Efflux-mediated resistance to a benzothiadiazol derivative effective against Burkholderia cenocepacia. Front. Microbiol. 2015, 6, 815. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alikhan, N.F.; Petty, N.K.; Ben Zakour, N.L.; Beatson, S.A. BLAST Ring Image Generator (BRIG): Simple prokaryote genome comparisons. BMC Genom. 2011, 12, 402. [Google Scholar] [CrossRef] [PubMed]
- Burns, P.A.; Allen, F.L.; Glickman, B.W. DNA sequence analysis of mutagenicity and site specificity of ethyl methanesulfonate in Uvr+ and UvrB− strains of Escherichia coli. Genetics 1986, 113, 811–819. [Google Scholar] [PubMed]
- Du, W.L.; Dubarry, N.; Passot, F.M.; Kamgoué, A.; Murray, H.; Lane, D.; Pasta, F. Orderly replication and segregation of the four replicons of Burkholderia cenocepacia J2315. PLoS Genet. 2016, 12, e1006172. [Google Scholar] [CrossRef] [PubMed]
- Menard, A.; de Los Santos, P.E.; Graindorge, A.; Cournoyer, B. Architecture of Burkholderia cepacia complex sigma70 gene family: Evidence of alternative primary and clade-specific factors, and genomic instability. BMC Genom. 2007, 8, 308. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, R.; Ravi, J.; Datta, P.; Chen, T.; Schnappinger, D.; Bassler, K.E.; Balázsi, G.; Gennaro, M.L. Reconstruction and topological characterization of the sigma factor regulatory network of Mycobacterium tuberculosis. Nat. Commun. 2016, 7, 11062. [Google Scholar] [CrossRef] [PubMed]
- Badran, A.H.; Liu, D.R. Development of potent in vivo mutagenesis plasmids with broad mutational spectra. Nat. Commun. 2015, 6, 8425. [Google Scholar] [CrossRef] [PubMed]
- Hogan, A.M.; Scoffone, V.C.; Makarov, V.; Gislason, A.S.; Tesfu, H.; Stietz, M.S.; Brassinga, A.K.C.; Domaratzki, M.; Li, X.; Azzalin, A.; et al. Competitive fitness of essential gene knockdowns reveals a broad-spectrum antibacterial inhibitor of the cell division protein FtsZ. Antimicrob. Agents Chemother. 2018. [Google Scholar] [CrossRef]
- Bloodworth, R.A.; Gislason, A.S.; Cardona, S.T. Burkholderia cenocepacia conditional growth mutant library created by random promoter replacement of essential genes. Microbiologyopen 2013, 2, 243–258. [Google Scholar] [CrossRef]
- Nichols, R.J.; Sen, S.; Choo, Y.J.; Beltrao, P.; Zietek, M.; Chaba, R.; Lee, S.; Kazmierczak, K.M.; Lee, K.J.; Wong, A.; et al. Phenotypic landscape of a bacterial cell. Cell 2011, 144, 143–156. [Google Scholar] [CrossRef]
- Peters, J.M.; Colavin, A.; Shi, H.; Czarny, T.L.; Larson, M.H.; Wong, S.; Hawkins, J.S.; Lu, C.H.S.; Koo, B.M.; Marta, E.; et al. A comprehensive, CRISPR-based functional analysis of essential genes in bacteria. Cell 2016, 165, 1493–1506. [Google Scholar] [CrossRef] [PubMed]
- Salina, E.G.; Ryabova, O.; Vocat, A.; Nikonenko, B.; Cole, S.T.; Makarov, V. New 1-hydroxy-2-thiopyridine derivatives active against both replicating and dormant Mycobacterium tuberculosis. J. Infect. Chemother. 2017, 23, 794–797. [Google Scholar] [CrossRef] [PubMed]
- Salina, E.G.; Huszár, S.; Zemanová, J.; Keruchenko, J.; Riabova, O.; Kazakova, E.; Grigorov, A.; Azhikina, T.; Kaprelyants, A.; Mikušová, K.; et al. Copper-related toxicity in replicating and dormant Mycobacterium tuberculosis caused by 1-hydroxy-5-R-pyridine-2(1H)-thiones. Metallomics 2018, 10, 992–1002. [Google Scholar] [CrossRef] [PubMed]
- Kempes, C.P.; van Bodegom, P.M.; Wolpert, D.; Libby, E.; Amend, J.; Hoehler, T. Drivers of bacterial maintenance and minimal energy requirements. Front. Microbiol. 2017, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Shaw, K.J.; Miller, N.; Liu, X.; Lerner, D.; Wan, J.; Bittner, A.; Morrow, B.J. Comparison of the changes in global gene expression of Escherichia coli induced by four bactericidal agents. J. Mol. Microbiol. Biotechnol. 2003, 5, 105–122. [Google Scholar] [CrossRef] [PubMed]
- Lemke, J.J.; Sanchez-Vazquez, P.; Burgos, H.L.; Hedberg, G.; Ross, W.; Gourse, R.L. Direct regulation of Escherichia coli ribosomal protein promoters by the transcription factors ppGpp and DksA. Proc. Natl. Acad. Sci. USA 2011, 108, 5712–5717. [Google Scholar] [CrossRef] [PubMed]
- Cardona, S.T.; Valvano, M.A. An expression vector containing a rhamnose-inducible promoter provides tightly regulated gene expression in Burkholderia cenocepacia. Plasmid 2005, 54, 219–228. [Google Scholar] [CrossRef]
- Holden, M.T.; Seth-Smith, H.M.; Crossman, L.C.; Sebaihia, M.; Bentley, S.D.; Cerdeño-Tárraga, A.M.; Thomson, N.R.; Bason, N.; Quail, M.A.; Sharp, S.; et al. The genome of Burkholderia cenocepacia J2315, an epidemic pathogen of cystic fibrosis patients. J. Bacteriol. 2009, 191, 261–277. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Winsor, G.L.; Khaira, B.; Van Rossum, T.; Lo, R.; Whiteside, M.D.; Brinkman, F.S. The Burkholderia Genome Database: Facilitating flexible queries and comparative analyses. Bioinformatics 2008, 24, 2803–2804. [Google Scholar] [CrossRef]
- Bazzini, S.; Udine, C.; Sass, A.; Pasca, M.R.; Longo, F.; Emiliani, G.; Fondi, M.; Perrin, E.; Decorosi, F.; Viti, C.; et al. Deciphering the role of RND efflux transporters in Burkholderia cenocepacia. PLoS ONE 2011, 6, e18902. [Google Scholar] [CrossRef] [PubMed]
- Buroni, S.; Matthijs, N.; Spadaro, F.; Van Acker, H.; Scoffone, V.C.; Pasca, M.R.; Riccardi, G.; Coenye, T. Differential Roles of RND Efflux Pumps in Antimicrobial Drug Resistance of Sessile and Planktonic Burkholderia cenocepacia Cells. Antimicrob. Agents Chemother. 2014, 58, 7424–7429. [Google Scholar] [CrossRef] [PubMed]
- Coenye, T.; Van Acker, H.; Peeters, E.; Sass, A.; Buroni, S.; Riccardi, G.; Mahenthiralingam, E. Molecular mechanisms of chlorhexidine tolerance in Burkholderia cenocepacia biofilms. Antimicrob. Agents Chemother. 2011, 55, 1912–1919. [Google Scholar] [CrossRef] [PubMed]
- Rushton, L.; Sass, A.; Baldwin, A.; Dowson, C.G.; Donoghue, D.; Mahenthiralingam, E. Key role for efflux in the preservative susceptibility and adaptive resistance of Burkholderia cepacia complex bacteria. Antimicrob. Agents Chemother. 2013, 57, 2972–2980. [Google Scholar] [CrossRef] [PubMed]
- Perrin, E.; Maggini, V.; Maida, I.; Gallo, E.; Lombardo, K.; Madarena, M.P.; Buroni, S.; Scoffone, V.C.; Firenzuoli, F.; Mengoni, A.; et al. Antimicrobial activity of six essential oils against Burkholderia cepacia complex: Insights into mechanism(s) of action. Future Microbiol. 2018, 13, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Venter, H.; Mowla, R.; Ohene-Agyei, T.; Ma, S. RND-type drug efflux pumps from Gram-negative bacteria: Molecular mechanism and inhibition. Front. Microbiol. 2015, 6, 377. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunvar, J.; Hogan, A.M.; Buroni, S.; Savina, S.; Makarov, V.; Cardona, S.T.; Drevinek, P. The Effect of 2-Thiocyanatopyridine Derivative 11026103 on Burkholderia Cenocepacia: Resistance Mechanisms and Systemic Impact. Antibiotics 2019, 8, 159. https://doi.org/10.3390/antibiotics8040159
Nunvar J, Hogan AM, Buroni S, Savina S, Makarov V, Cardona ST, Drevinek P. The Effect of 2-Thiocyanatopyridine Derivative 11026103 on Burkholderia Cenocepacia: Resistance Mechanisms and Systemic Impact. Antibiotics. 2019; 8(4):159. https://doi.org/10.3390/antibiotics8040159
Chicago/Turabian StyleNunvar, Jaroslav, Andrew M. Hogan, Silvia Buroni, Svetlana Savina, Vadim Makarov, Silvia T. Cardona, and Pavel Drevinek. 2019. "The Effect of 2-Thiocyanatopyridine Derivative 11026103 on Burkholderia Cenocepacia: Resistance Mechanisms and Systemic Impact" Antibiotics 8, no. 4: 159. https://doi.org/10.3390/antibiotics8040159
APA StyleNunvar, J., Hogan, A. M., Buroni, S., Savina, S., Makarov, V., Cardona, S. T., & Drevinek, P. (2019). The Effect of 2-Thiocyanatopyridine Derivative 11026103 on Burkholderia Cenocepacia: Resistance Mechanisms and Systemic Impact. Antibiotics, 8(4), 159. https://doi.org/10.3390/antibiotics8040159




