Carbapenem-Resistant Enterobacteriaceae Posing a Dilemma in Effective Healthcare Delivery
Abstract
1. Introduction
- production of carbapenemase enzymes (Carbapenem-hydrolysing enzymes) namely: the class A carbapenemases (the Klebsiella pneumoniae carbapenemase types), the class B or Metallo-beta-lactamases (MBLs), and class D Oxacillinases (e.g., OXA-48-like enzymes)
- production of extended spectrum beta-lactamases (ESBLs)
- production of AmpC enzymes (mostly, plasmid-mediated)
- porins loss leading to drug decreased permeability
2. Results
Characteristics of Study Population
3. Discussion
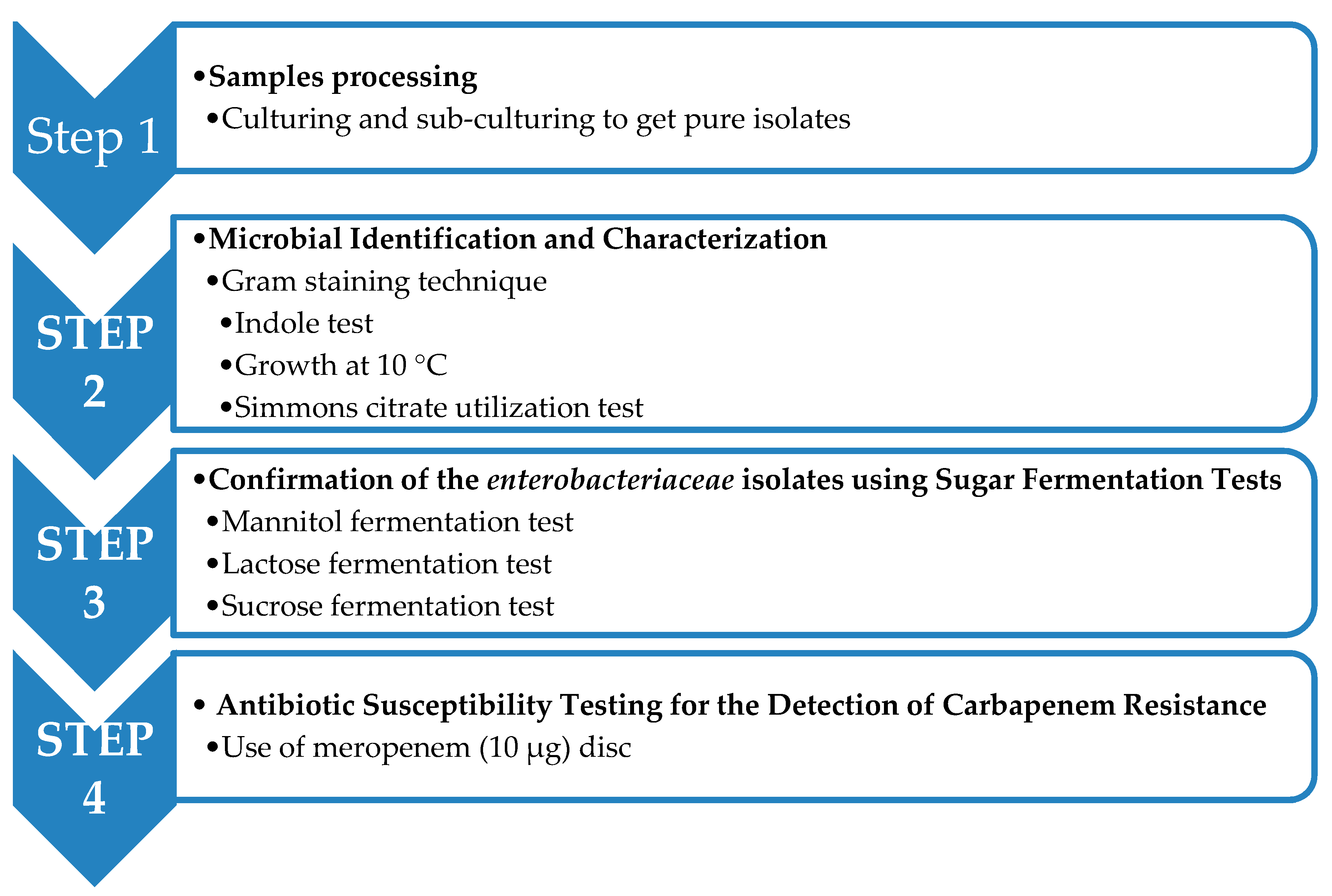
4. Materials and Methods
4.1. Study Area and Site, Study Population, and Ethical Clearance
4.2. Sample Size Calculation
4.3. Microbiological Investigations
Sample Processing
4.4. Microbial Identification and Characterization
4.4.1. Gram Staining Technique
4.4.2. Biochemical Tests
4.4.3. Indole Test
4.4.4. Confirmation of the Enterobacteriaceae Isolates Using Sugar Fermentation Tests
4.4.5. Growth at 10 °C
4.4.6. Simmons Citrate Utilization Test
4.4.7. Storage of the Isolates
4.4.8. Antibiotic Susceptibility Testing for the Detection of Carbapenem Resistance
4.5. Statistical Data Analysis and Interpretation
5. Conclusions
6. Patients
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pitout, J.D. Multiresistant Enterobacteriaceae: New threat of an old problem. Expert Rev. Anti Infect. Ther. 2008, 6, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Toussaint, K.A.; Gallagher, J.C. β-Lactam/β-Lactamase Inhibitor Combinations: From Then to Now. Ann. Pharmacother. 2015, 49, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Singh, P.; Rajurkar, M. Multidrug Resistant and Extensively Drug Resistant Bacteria: A Study. J. Pathog. 2016, 2016, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.N.; Khan, A.U. Breaking the Spell: Combating Multidrug Resistant ‘Superbugs’. Front. Microbiol. 2016, 7, 4636. [Google Scholar] [CrossRef] [PubMed]
- WHO. Antibiotic Resistance—World Health Organization. 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 28 May 2018).
- Lutgring, J.D.; Limbago, B.M. The problem of carbapenemase-producing- carbapenem-resistant-Enterobacteriaceae detection. J. Clin. Microbiol. 2016, 54, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Codjoe, F.S.; Donkor, E.S. Carbapenem Resistance: A Review. Med. Sci. 2017, 6, 1. [Google Scholar] [CrossRef]
- Bonomo, R.A.; Burd, E.M.; Conly, J.; Limbago, B.M.; Poirel, L.; Segre, J.A.; Westblade, L.F. Carbapenemase-Producing Organisms: A Global Scourge. Clin. Infect. Dis. 2018, 66, 1290–1297. [Google Scholar] [CrossRef]
- Doumith, M.; Ellington, M.J.; Livermore, D.M.; Woodford, N. Molecular mechanisms disrupting porin expression in ertapenem resistant Klebsiella and Enterobacter spp. clinical isolates from the UK. J. Antimicrob. Chemother. 2009, 63, 659–667. [Google Scholar] [CrossRef]
- Trivedi, M.K.; Patil, S.; Shettigar, H.; Bairwa, K.; Jana, S. Phenotypic and Biotypic Characterization of Klebsiella oxytoca: An Impact of Biofield Treatment. J. Microb. Biochem. Technol. 2015, 7, 202–205. [Google Scholar] [CrossRef]
- Joainig, M.M.; Gorkiewicz, G.; Leitner, E.; Weberhofer, P.; Zollner-Schwetz, I.; Lippe, I.; Feierl, G.; Krause, R.; Hinterleitner, T.; Zechner, E.L.; et al. Cytotoxic Effects of Klebsiella oxytoca Strains Isolated from Patients with Antibiotic-Associated Hemorrhagic Colitis or Other Diseases Caused by Infections and from Healthy Subjects. J. Clin. Microbiol. 2010, 48, 817–824. [Google Scholar] [CrossRef]
- Herzog, K.A.T.; Schneditz, G.; Leitner, E.; Feierl, G.; Hoffmann, K.M.; Zollner-Schwetz, I.; Krause, R.; Gorkiewicz, G.; Zechner, E.L.; Högenauer, C. Genotypes of Klebsiella oxytoca Isolates from Patients with Nosocomial Pneumonia Are Distinct from Those of Isolates from Patients with Antibiotic-Associated Hemorrhagic Colitis. J. Clin. Microbiol. 2014, 52, 1607–1616. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, I.; Arzai, A.; Haruna, M.; Sharif, A.; Getso, M. Detection of multi drug resistant bacteria in major hospitals in Kano, North-West, Nigeria. Braz. J. Microbiol. 2014, 45, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, R.; Robinson, M.L.; Balasubramanian, U.; Kulkarni, V.; Kagal, A.; Raichur, P.; Khadse, S.; Kadam, D.; Valvi, C.; Kinikar, A.; et al. Drug-resistant Enterobacteriaceae colonization is associated with healthcare utilization and antimicrobial use among inpatients in Pune, India. BMC Infect. Dis. 2018, 18, 504. [Google Scholar] [CrossRef] [PubMed]
- Wartiti, M.A.; Bahmani, F.Z.; Elouenass, M.; Benouda, A. Prevalence of carbapenemase producing Enterobacteriaceae in a university hospital in Morocco: A 19 months prospective study. Int. Arabic. J. Antimicrob. Agents 2012, 2, 372–377. [Google Scholar]
- Okoche, D.; Asiimwe, B.B.; Katabazi, F.A.; Kato, L.; Najjuka, C.F. Prevalence and Characterization of Carbapenem-Resistant Enterobacteriaceae Isolated from Mulago National Referral Hospital, Uganda. PLoS ONE 2015, 10, e0135745. [Google Scholar] [CrossRef] [PubMed]
- Baran, I.; Aksu, N. Phenotypic and genotypic characteristics of carbapenem-resistant Enterobacteriaceae in a tertiary-level reference hospital in Turkey. Ann. Clin. Microbiol. Antimicrob. 2016, 15, 20. [Google Scholar] [CrossRef] [PubMed]
- Sonda, T.; Kumburu, H.; Van Zwetselaar, M.; Alifrangis, M.; Mmbaga, B.T.; Lund, O.; Aarestrup, F.M.; Kibiki, G. Prevalence and risk factors for CTX-M gram-negative bacteria in hospitalized patients at a tertiary care hospital in Kilimanjaro, Tanzania. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 897–906. [Google Scholar] [CrossRef]
- Seni, J.; Mwakyoma, A.A.; Mashuda, F.; Marando, R.; Ahmed, M.; DeVinney, R.; Pitout, J.D.D.; Mshana, S.E. Deciphering risk factors for blood stream infections, bacteria species and antimicrobial resistance profiles among children under five years of age in North-Western Tanzania: A multicentre study in a cascade of referral health care system. BMC Pediatr. 2019, 19, 32. [Google Scholar] [CrossRef]
- Nicolas-Chanoine, M.H.; Vigan, M.; Laouénan, C.; Robert, J.; E-carb Study Group. Risk factors for Carbapenem-resistant Enterobacteriaceae infections: A French case-control-control study. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 383–393. [Google Scholar] [CrossRef]
- Kang, J.S.; Yi, J.; Ko, M.K.; Lee, S.O.; Lee, J.E.; Kim, K.H. Prevalence and Risk Factors of Carbapenem-resistant Enterobacteriaceae Acquisition in an Emergency Intensive Care Unit in a Tertiary Hospital in Korea: A Case-Control Study. J. Korean Med. Sci. 2019, 34, 140. [Google Scholar] [CrossRef]
- Chotiprasitsakul, D.; Srichatrapimuk, S.; Kirdlarp, S.; Pyden, A.D.; Santanirand, P. Epidemiology of carbapenem-resistant Enterobacteriaceae: A 5-year experience at a tertiary care hospital. Infect. Drug Resist. 2019, 12, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Karuniawati, A.; Saharman, Y.R.; Lestari, D. Detection of Carbapenemase Encoding genes in Enterobacteriacae, Pseudomonas aeruginosa and Acinetobacter baumanii Isolated from patients at Intensive care unit Cipto Mangunkusumo Hospital in 2011. Acta Med. Indones. 2013, 45, 101–144. [Google Scholar] [PubMed]
- Michelle, K.; Paczosa, J.M. Klebsiella pneumoniae: Going on the Offense with a Strong Defense. Microbiol. Mol. Biol. Rev. 2016, 80, 629–661. [Google Scholar]
- Ridolfo, A.L.; Rimoldi, S.G.; Pagani, C.; Marino, A.F.; Piol, A.; Rimoldi, M.; Olivieri, P.; Galli, M.; Dolcetti, L.; Gismondo, M.R. Diffusion and transmission of carbapenem-resistant Klebsiella pneumoniae in the medical and surgical wards of a university hospital in Milan, Italy. J. Infect. Public Health 2016, 9, 24–33. [Google Scholar] [CrossRef][Green Version]
- Mohamudha, P.R.; Harish, B.N.; Parija, S.C. Molecular description of plasmid-mediated AmpC β-lactamases among nosocomial isolates of Escherichia coli and Klebsiella pneumoniae from six different hospitals in India. Indian. J. Med. Res. 2012, 135, 114–119. [Google Scholar]
- Mate, H.; Devi, S.; Devi, M.; Damrolien, S.; Devi, N.L.; Devi, P.P. Prevalence of Carbapenem resistance in a tertiary hospital in north east India. J. Dent. Med. Sci. 2014, 13, 56–60. [Google Scholar]
- Adje, D.U.; Oli, A.N. Community Pharmacy in Warri, Nigeria—A Survey of Practice Details. Sch. Acad. J. Pharm. 2013, 2, 391–397. [Google Scholar]
- Pfeifer, Y.; Cullik, A.; Witte, W. Nosocomial infections. Int. J. Med. Microbiol. 2010, 300, 371–379. [Google Scholar] [CrossRef]
- Nordmann, P. Gram-negative bacteria with resistance to carbapenems. Med. Sci. (Paris) 2010, 26, 950–959. [Google Scholar] [CrossRef]
- Ejiofor, O.S.; Ajunwa, O.M.; Ezeudu, C.E.; Emechebe, G.O.; Okeke, K.N.; Ifezulike, C.C.; Ekejindu, I.M.; Okoyeh, J.N.; Osuala, E.O.; Oli, A.N. The Bacteriology and Its Virulence Factors in Neonatal Infections: Threats to Child Survival Strategies. J. Pathog. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Papp-Wallace, K.M.; Endimiani, A.; Taracila, M.A.; Bonomo, R.A. Carbapenems: Past, Present, and Future. Antimicrob. Agents Chemother. 2011, 55, 4943–4960. [Google Scholar] [CrossRef] [PubMed]
- Nordmann, P.; Cuzon, G.; Naas, T. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect. Dis. 2009, 9, 228–236. [Google Scholar] [CrossRef]
- Oli, A.N.; Eze, D.E.; Gugu, T.H.; Ezeobi, I.; Maduagwu, U.N.; Ihekwereme, C.P. Multi-antibiotic resistant extended-spectrum beta-lactamase producing bacteria pose a challenge to the effective treatment of wound and skin infections. Pan Afr. Med. J. 2017, 27, 66. [Google Scholar] [CrossRef] [PubMed]
- Nordmann, P.; Gniadkowski, M.; Giske, C.; Poirel, L.; Woodford, N.; Miriagou, V. Identification and screening of carbapenemase-producing Enterobacteriaceae. Clin. Microbiol. Infect. 2012, 18, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Vading, M.; Samuelsen, Ø.; Haldorsen, B.; Sundsfjord, A.S.; Giske, C.G. Comparison of disk diffusion, Etest and VITEK2 for detection of carbapenemase-producing Klebsiella pneumoniae with the EUCAST and CLSI breakpoint systems. Clin. Microbiol. Infect. 2011, 17, 668–674. [Google Scholar]
- The European Committee on Antimicrobial Susceptibility Testing—EUCAST. Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 6.0, 2016; EUCAST: Sweden, 2016; Available online: http://www.eucast.org/clinical_breakpoints/ (accessed on 5 September 2017).

| Variable | Patients Tested | Number with Infection (% Infected) | Number of Resistant Organisms * (% Resistant) | ||||
|---|---|---|---|---|---|---|---|
| Location (residence) | K. p. 37 (24) | K. o. 12 (9) | E. coli 29 (19) | K. p. 7 (19) | K. o. 9 (75) | E. coli 6 (21) | |
| Rural | 67 | 17 (25) | 3 (4) | 11 (16) | 2 (12) | 3 (100) | 3 (27) |
| Sub-Urban | 33 | 9 (27) | 4 (12) | 8 (24) | 3 (33) | 3 (75) | 1 (25) |
| Urban | 53 | 11 (21) | 5 (9) | 10 (19) | 2 (18) | 3 (60) | 2 (20) |
| Sex | |||||||
| Male | 75 | 21 (28) | 6 (8) | 13 (17) | 3 (11) | 6 (100) | 2 (15) |
| Female | 78 | 16 (21) | 6 (8) | 16 (21) | 4 (25) | 3 (50) | 4 (25) |
| Age Group (yrs) | |||||||
| 1–20 | 21 | 6 (29) | 1 (5) | 3 (14) | 2 (33) | 1 (100) | 1 (33) |
| 21–40 | 81 | 20 (25) | 6 (7) | 16 (20) | 3 (15) | 4 (67) | 1 (6) |
| 41–60 | 32 | 7 (22) | 3 (9) | 7 (22) | 1 (14) | 3 (100) | 2 (29) |
| 61–80 | 18 | 4 (22) | 2 (11) | 3 (17) | 1 (25) | 1 (50) | 2 (67) |
| Marital status | |||||||
| Single | 63 | 16 (25) | 4 (6) | 15 (24) | 2 (13) | 4 (100) | 3 (20) |
| Widowed | 6 | 1 (17) | 0 (0) | 1 (17) | 1 (100) | 0 (0) | 1 (100) |
| Divorced | 0 | 0 | 0 | 0 | 0 (0) | 0 (0) | 0 (0) |
| Still Married | 84 | 20 (24) | 8 (10) | 13 (15) | 4 (20) | 5 (63) | 2 (15) |
| Highest Education Completed | |||||||
| No formal Education | 9 | 3 (33) | 0 (0) | 1 (11) | 2 (67) | 0 (0) | 1 (100) |
| Primary school | 24 | 4 (17) | 2 (8) | 5 (21) | 1 (25) | 2 (100) | 1 (20) |
| Senior Secondary | 66 | 20 (33) | 3 (5) | 15 (23) | 1 (5) | 2 (67) | 2 (13) |
| Tertiary Education | 47 | 10 (21) | 6 (13) | 7 (15) | 3 (30) | 4 (67) | 1 (14) |
| Higher Degree | 7 | 0 (0) | 1 (14) | 1 (14) | 0 (0) | 1 (100) | 1 (100) |
| Clinic Setting | |||||||
| In patients | 57 | 6 (11) | 10 (18) | 16 (28) | 5 (83) | 7 (70) | 5 (31) |
| Out patients | 96 | 31 (32) | 2 (2) | 13 (14) | 2 (6) | 2 (100) | 1 (8) |
| Enterobacteriaceae Isolated | Number of Samples | Total = 153 | |||
|---|---|---|---|---|---|
| Urine N = 55 | Sputum N = 51 | Anal Swab N = 13 | Wound/Pus Swab N = 34 | ||
| E. coli | 12 (21.82) | 2 (3.92) | 10 (76.92) | 5 (14.71) | 29 (18.95) |
| Klebsiella pneumoniae | 15 (27.27) | 18 (35.29) | 1 (7.69) | 3 (8.82) | 37 (24.18) |
| Klebsiella oxytoca | 4 (7.27) | 0 (0.00) | 1 (7.69) | 7 (20.59) | 12 (7.84) |
| Total | 31 (39.74) | 20 (25.64) | 12 (15.38) | 15 (19.23) | 78 (50.98) |
| A. CLINIC SOURCES OF THE SAMPLES | ||||
| Clinics | Resistant (%) | Intermediate (%) | Susceptible (%) | Total (%) |
| GOPD | 0 (0.00) | 6 (33.33) | 12 (66.67) | 18 (23.08) |
| Gyn. & Antenatal clinic | 0 (0.00) | 1 (12.50) | 7 (87.50) | 8 (10.26) |
| Medical Ward | 0 (0.00) | 2 (33.33) | 4 (66.67) | 6 (7.69) |
| Surgical Ward | 3 (11.54) | 5 (19.23) | 18 (69.23) | 26 (33.33 |
| Chest Clinic | 3 (15.00) | 2 (10.00) | 15 (75.00) | 20 (25.64) |
| Total | 6 (7.69) | 16 (20.51) | 56 (71.79) | 78 (100) |
| B. ANATOMICAL SITE SOURCES OF THE SAMPLES | ||||
| Urine | 0 (0.00) | 9 (29.03) | 22 (70.97) | 31 (39.74) |
| Wound/Pus swab | 2 (13.33) | 4 (26.67) | 9 (60.00) | 15 (19.23) |
| Sputum | 3 (15.00) | 2 (10.00) | 15 (75.00) | 20 (25.64) |
| Anal swab | 1 (8.33) | 1 (8.33) | 10 (83.33) | 12 (15.38) |
| Total | 6 (7.69) | 16 (20.51) | 56 (71.79) | 78 (100) |
| Organism | Resistant | Susceptible | Total |
|---|---|---|---|
| E. coli | 6 (20.69) | 23 (79.31) | 29 (37.18) |
| K. pneumoniae | 7 (18.92) | 30 (81.08) | 37 (47.44) |
| K. oxytoca | 9 (75.00) | 3 (25.00) | 12 (15.38) |
| Total | 22 (28.21) | 56 (71.79) | 78 (100) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oli, A.N.; Itumo, C.J.; Okam, P.C.; Ezebialu, I.U.; Okeke, K.N.; Ifezulike, C.C.; Ezeobi, I.; Emechebe, G.O.; Okezie, U.M.; Adejumo, S.A.; et al. Carbapenem-Resistant Enterobacteriaceae Posing a Dilemma in Effective Healthcare Delivery. Antibiotics 2019, 8, 156. https://doi.org/10.3390/antibiotics8040156
Oli AN, Itumo CJ, Okam PC, Ezebialu IU, Okeke KN, Ifezulike CC, Ezeobi I, Emechebe GO, Okezie UM, Adejumo SA, et al. Carbapenem-Resistant Enterobacteriaceae Posing a Dilemma in Effective Healthcare Delivery. Antibiotics. 2019; 8(4):156. https://doi.org/10.3390/antibiotics8040156
Chicago/Turabian StyleOli, Angus Nnamdi, Chimaobi Johnpaul Itumo, Princeston Chukwuemeka Okam, Ifeanyichukwu U. Ezebialu, Kenneth Nchekwube Okeke, Christian Chukwuemeka Ifezulike, Ifeanyi Ezeobi, George Ogonna Emechebe, Ugochukwu Moses Okezie, Samson A. Adejumo, and et al. 2019. "Carbapenem-Resistant Enterobacteriaceae Posing a Dilemma in Effective Healthcare Delivery" Antibiotics 8, no. 4: 156. https://doi.org/10.3390/antibiotics8040156
APA StyleOli, A. N., Itumo, C. J., Okam, P. C., Ezebialu, I. U., Okeke, K. N., Ifezulike, C. C., Ezeobi, I., Emechebe, G. O., Okezie, U. M., Adejumo, S. A., & Okoyeh, J. N. (2019). Carbapenem-Resistant Enterobacteriaceae Posing a Dilemma in Effective Healthcare Delivery. Antibiotics, 8(4), 156. https://doi.org/10.3390/antibiotics8040156





