Direct Measurement of Performance: A New Era in Antimicrobial Stewardship
Abstract
1. Introduction: Importance of Antimicrobial Stewardship Metrics
2. Dynamics of Antimicrobial Stewardship and Infection Prevention and Control Programs
3. Comparison of Various Antimicrobial Stewardship Metrics
3.1. Clostridioides difficile Infection
3.1.1. CDI Diagnosis
3.1.2. Relatively Low Incidence of CDI
3.1.3. Multifactorial Etiology of CDI
3.1.4. Difficulty of Designing a Successful ASP Intervention for CDI
3.2. Incidence Rates of Infections or Colonization with MDR Bacteria
3.2.1. Extended-Spectrum Beta-Lactamase-Producing Enterobacteriaceae (ESBLE)
3.2.2. Methicillin-Resistant Staphylococcus aureus (MRSA)
3.2.3. Carbapenem-Resistant Enterobacteriaceae (CRE)
3.2.4. Antimicrobial-Resistant P. aeruginosa and A. baumannii
3.3. Quality of Care
3.3.1. Appropriate Definitive Antimicrobial Therapy
3.3.2. Appropriate Empirical Antimicrobial Therapy
3.4. Cost of Healthcare
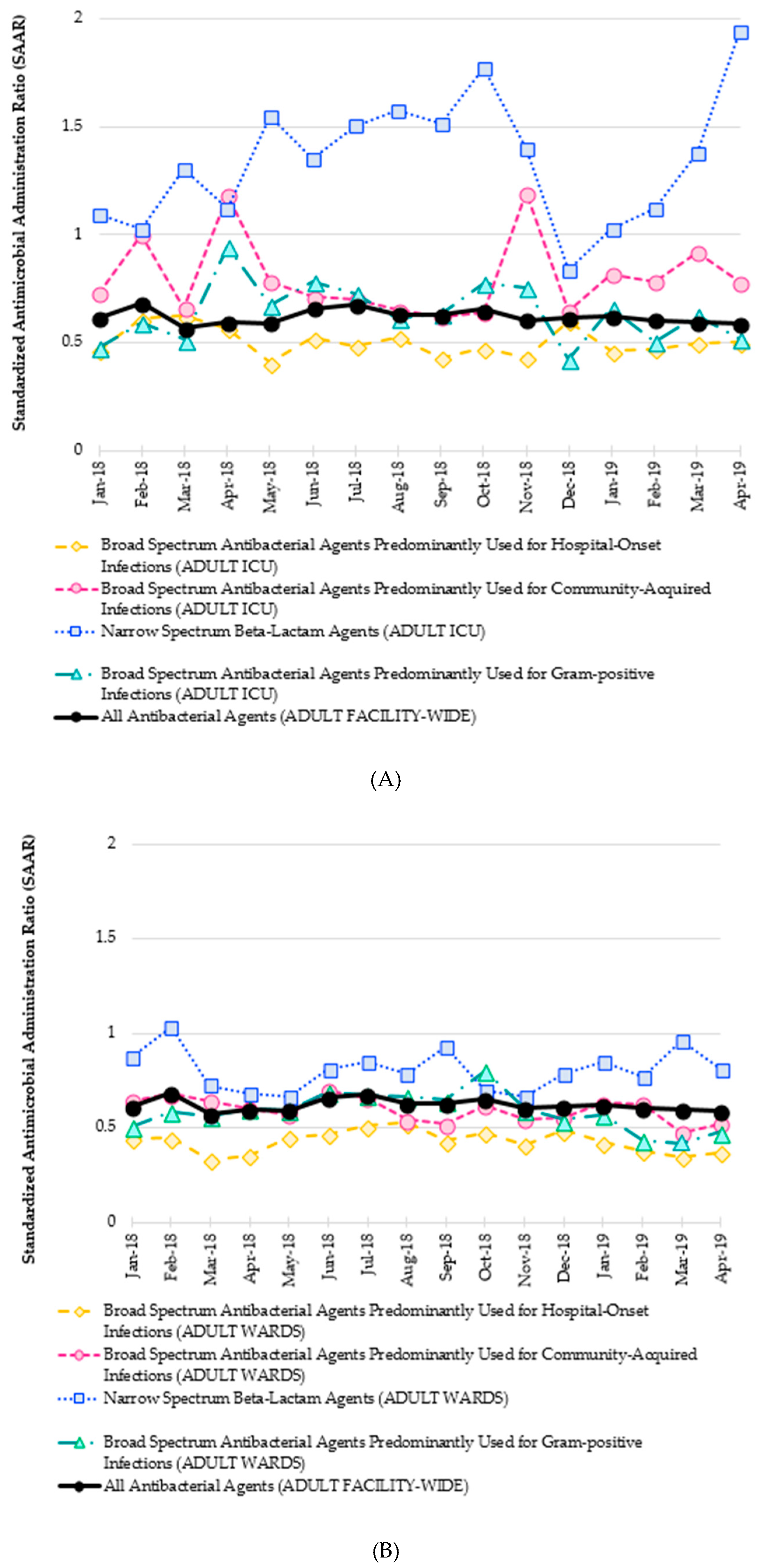
3.5. Antimicrobial Use
3.5.1. Direct and Specific ASP Metric
3.5.2. Antimicrobial Use of Broad-Spectrum Agents
3.5.3. Benefits of Reducing Antimicrobial Use
3.5.4. Measurement of Antimicrobial Use
4. Proposed Novel Antimicrobial Use (AU) Metrics
4.1. Adjustment of AU by Quality of Care
4.2. Adjustment of AU by Institutional Microbiological Burden
5. NHSN Antimicrobial Use and Resistance Module
5.1. Antimicrobial Use (AU) Option
5.2. Antimicrobial Resistance (AR) Option
6. Discussion
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Barlam, T.F.; Cosgrove, S.E.; Abbo, L.M.; MacDougall, C.; Schuetz, A.N.; Septimus, E.J.; Srinivasan, A.; Dellit, T.H.; Falck-Ytter, Y.T.; Fishman, N.O.; et al. Implementing an antibiotic stewardship program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin. Infect. Dis. 2016, 62, e51–e77. [Google Scholar] [CrossRef] [PubMed]
- Moehring, R.W.; Anderson, D.J.; Cochran, R.L.; Hicks, L.A.; Srinivasan, A.; Dodds Ashley, E.S. Structured Taskforce of Experts Working at Reliable Standards for Stewardship (STEWARDS) Panel. Expert consensus on metrics to assess the impact of patient-level antimicrobial stewardship interventions in acute-care settings. Clin. Infect. Dis. 2017, 64, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Science, M.; Timberlake, K.; Morris, A.; Read, S.; Le Saux, N. Groupe Antibiothérapie en Pédiatrie Canada Alliance for Stewardship of Antimicrobials in Pediatrics (GAP Can ASAP); Quality metrics for antimicrobial stewardship programs; Pediatrics: Elk Grove, IL, USA, 2019; p. 143. [Google Scholar]
- Nagel, J.L.; Stevenson, J.G.; Eiland, E.H.; Kaye, K.S. Demonstrating the value of antimicrobial stewardship programs to hospital administrators. Clin. Infect. Dis. 2014, 59 (Suppl. 3), S146–S153. [Google Scholar] [CrossRef] [PubMed]
- Brotherton, A.L. Metrics of antimicrobial stewardship programs. Med. Clin. N. Am. 2018, 102, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Bennett, N.; Schulz, L.; Boyd, S.; Newland, J.G. Understanding inpatient antimicrobial stewardship metrics. Am. J. Health Syst. Pharm. 2018, 75, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Tumbarello, M.; Trecarichi, E.M.; Bassetti, M.; De Rosa, F.G.; Spanu, T.; Di Meco, E.; Losito, A.R.; Parisini, A.; Pagani, N.; Cauda, R. Identifying patients harboring extended-spectrum-beta-lactamase-producing Enterobacteriaceae on hospital admission: Derivation and validation of a scoring system. Antimicrob. Agents Chemother. 2011, 55, 3485–3490. [Google Scholar] [CrossRef] [PubMed]
- Augustine, M.R.; Testerman, T.L.; Justo, J.A.; Bookstaver, P.B.; Kohn, J.; Albrecht, H.; Al-Hasan, M.N. Clinical risk score for prediction of extended-spectrum beta-lactamase-producing Enterobacteriaceae in bloodstream isolates. Infect. Control Hosp. Epidemiol. 2017, 38, 266–272. [Google Scholar] [CrossRef]
- Khanna, S.; Pardi, D.S.; Aronson, S.L.; Kammer, P.P.; Orenstein, R.; St Sauver, J.L.; Zinsmeister, A.R. The epidemiology of community-acquired Clostridium difficile infection: A population-based study. Am. J. Gastroenterol. 2012, 107, 89–95. [Google Scholar] [CrossRef]
- Lambert, P.J.; Dyck, M.; Thompson, L.H.; Hammond, G.W. Population-based surveillance of Clostridium difficile infection in Manitoba, Canada, by using interim surveillance definitions. Infect. Control Hosp. Epidemiol. 2009, 30, 945–951. [Google Scholar] [CrossRef]
- Pitout, J.D.; Hanson, N.D.; Church, D.L.; Laupland, K.B. Population-based laboratory surveillance for Escherichia coli-producing extended-spectrum beta-lactamases: Importance of community isolates with blaCTX-M genes. Clin. Infect. Dis. 2004, 38, 1736–1741. [Google Scholar] [CrossRef][Green Version]
- Thaden, J.T.; Fowler, V.G.; Sexton, D.J.; Anderson, D.J. Increasing incidence of extended-spectrum beta-lactamase-producing Escherichia coli in community hospitals throughout the Southeastern United States. Infect. Control Hosp. Epidemiol. 2016, 37, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. World Health Organization, 2017. Available online: https://www.who.int/medicines/publications/global-priority-list-antibiotic-resistant-bacteria/en/ (accessed on 30 June 2019).
- Karino, S.; Kaye, K.S.; Navalkele, B.; Nishan, B.; Salim, M.; Solanki, S.; Pervaiz, A.; Tashtoush, N.; Shaikh, H.; Koppula, S.; et al. Epidemiology of acute kidney injury among patients receiving concomitant vancomycin and piperacillin-tazobactam: Opportunities for antimicrobial stewardship. Antimicrob. Agents Chemother. 2016, 60, 3743–3750. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hammond, D.A.; Smith, M.N.; Li, C.; Hayes, S.M.; Lusardi, K.; Bookstaver, P.B. Systematic review and meta-analysis of acute kidney injury associated with concomitant vancomycin and piperacillin/tazobactam. Clin. Infect. Dis. 2017, 64, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Piacenti, F.J.; Leuthner, K.D. Antimicrobial stewardship and Clostridium difficile-associated diarrhea. J. Pharm. Pract. 2013, 26, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Longtin, Y.; Trottier, S.; Brochu, G.; Paquet-Bolduc, B.; Garenc, C.; Loungnarath, V.; Beaulieu, C.; Goulet, D.; Longtin, J. Impact of the type of diagnostic assay on Clostridium difficile infection and complication rates in a mandatory reporting program. Clin. Infect. Dis. 2013, 56, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Grein, J.D.; Ochner, M.; Hoang, H.; Jin, A.; Morgan, M.A.; Murthy, A.R. Comparison of testing approaches for Clostridium difficile infection at a large community hospital. Clin. Microbiol. Infect. 2014, 20, 65–69. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kelly, S.G.; Yarrington, M.; Zembower, T.R.; Sutton, S.H.; Silkaitis, C.; Postelnick, M.; Mikolajczak, A.; Bolon, M.K. Inappropriate Clostridium difficile testing and consequent overtreatment and inaccurate publicly reported metrics. Infect. Control Hosp. Epidemiol. 2016, 37, 1395–1400. [Google Scholar] [CrossRef]
- Graber, C.J. Clostridium difficile infection: Stewardship’s lowest hanging fruit? Lancet Infect. Dis. 2017, 17, 123–124. [Google Scholar] [CrossRef]
- Al-Hasan, M.N.; Wilson, J.W.; Lahr, B.D.; Thomsen, K.M.; Eckel-Passow, J.E.; Vetter, E.A.; Tleyjeh, I.M.; Baddour, L.M. Beta-lactam and fluoroquinolone combination antibiotic therapy for bacteremia caused by gram-negative bacilli. Antimicrob. Agents Chemother. 2009, 53, 1386–1394. [Google Scholar] [CrossRef]
- Seddon, M.M.; Bookstaver, P.B.; Justo, J.A.; Kohn, J.; Rac, H.; Haggard, E.; Mediwala, K.N.; Dash, S.; Al-Hasan, M.N. Role of early de-escalation of antimicrobial therapy on risk of Clostridioides difficile infection following Enterobacteriaceae bloodstream infections. Clin. Infect. Dis. 2019, 69, 414–420. [Google Scholar] [CrossRef]
- Tucker, K.; Lashkova, L.; Flemming, T.; Justo, J.; Kohn, J.; Al-Hasan, M.N.; Sanasi, K.; Bookstaver, P.B. Impact of antimicrobial stewardship initiatives on carbapenem utilization and antimicrobial resistance. In Proceedings of the 55th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, CA, USA, 17–21 September 2015. Abstract #2243. [Google Scholar]
- Palacios-Baena, Z.R.; Delgado-Valverde, M.; Valiente Méndez, A.; Almirante, B.; Gómez-Zorrilla, S.; Borrell, N.; Corzo, J.E.; Gurguí, M.; de la Calle, C.; García-Álvarez, L.; et al. Impact of de-escalation on prognosis of patients with bacteraemia due to Enterobacteriaceae: A post-hoc analysis from a multicenter prospective cohort. Clin. Infect. Dis. 2018. [Google Scholar] [CrossRef] [PubMed]
- Tariq, R.; Singh, S.; Gupta, A.; Pardi, D.S.; Khanna, S. Association of gastric acid suppression with recurrent Clostridium difficile infection: A systematic review and meta-analysis. JAMA Intern. Med. 2017, 177, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Stevenson, M.; Riley, T.V. Antibiotics and hospital-acquired Clostridium difficile-associated diarrhoea: A systematic review. J. Antimicrob. Chemother. 2003, 51, 1339–1350. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.A.; Khanafer, N.; Daneman, N.; Fisman, D.N. Meta-analysis of antibiotics and the risk of community-associated Clostridium difficile infection. Antimicrob. Agents Chemother. 2013, 57, 2326–2332. [Google Scholar] [CrossRef]
- Pagels, C.M.; McCreary, E.K.; Rose, W.E.; Dodds Ashley, E.S.; Bookstaver, P.B.; Dilworth, T.J. Designing antimicrobial stewardship initiatives to enhance scientific dissemination. J. Am. Coll. Clin. Pharm. 2019, 1–7. [Google Scholar] [CrossRef]
- McFarland, L.V.; Surawicz, C.M.; Stamm, W.E. Risk factors for Clostridium difficile carriage and, C. difficile-associated diarrhea in a cohort of hospitalized patients. J. Infect. Dis. 1990, 162, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Lawes, T.; Lopez-Lozano, J.M.; Nebot, C.A.; Macartney, G.; Subbarao-Sharma, R.; Wares, K.D.; Sinclair, C.; Gould, I.M. Effect of a national 4C antibiotic stewardship intervention on the clinical and molecular epidemiology of Clostridium difficile infections in a region of Scotland: A non-linear time-series analysis. Lancet Infect. Dis. 2017, 17, 194–206. [Google Scholar] [CrossRef]
- Harris, A.D.; Sbarra, A.N.; Leekha, S.; Jackson, S.S.; Johnson, J.K.; Pineles, L.; Thom, K.A. Electronically available comorbid conditions for risk prediction of healthcare-associated Clostridium difficile infection. Infect. Control Hosp. Epidemiol. 2018, 39, 297–301. [Google Scholar] [CrossRef]
- Al-Hasan, M.N.; Lahr, B.D.; Eckel-Passow, J.E.; Baddour, L.M. Antimicrobial resistance trends of Escherichia coli bloodstream isolates: A population-based study, 1998–2007. J. Antimicrob. Chemother. 2009, 64, 169–174. [Google Scholar] [CrossRef]
- Waltner-Toews, R.I.; Paterson, D.L.; Qureshi, Z.A.; Sidjabat, H.E.; Adams-Haduch, J.M.; Shutt, K.A.; Jones, M.; Tian, G.B.; Pasculle, A.W.; Doi, Y. Clinical characteristics of bloodstream infections due to ampicillin-sulbactam-resistant, non-extended-spectrum-beta-lactamase-producing Escherichia coli and the role of TEM-1 hyperproduction. Antimicrob. Agents Chemother. 2011, 55, 495–501. [Google Scholar] [CrossRef]
- Baggs, J.; Fridkin, S.K.; Pollack, L.A.; Srinivasan, A.; Jernigan, J.A. Estimating National trends in inpatient antibiotic use among US hospitals from 2006 to 2012. JAMA Intern. Med. 2016, 176, 1639–1648. [Google Scholar] [CrossRef]
- Logan, L.K.; Gandra, S.; Mandal, S.; Klein, E.Y.; Levinson, J.; Weinstein, R.A.; Laxminarayan, R. Prevention Epicenters Program, US Centers for Disease Control and Prevention. Multidrug- and carbapenem-resistant Pseudomonas aeruginosa in children, United States, 1999–2012. J. Pediatr. Infect. Dis. Soc. 2017, 6, 352–359. [Google Scholar]
- Knothe, H.; Shah, P.; Krcmery, V.; Antal, M.; Mitsuhashi, S. Transferable resistance to cefotaxime, cefoxitin, cefamandole and cefuroxime in clinical isolates of Klebsiella pneumoniae and Serratia marcescens. Infection 1983, 11, 315–317. [Google Scholar] [CrossRef]
- Rodriguez-Bano, J.; Navarro, M.D.; Romero, L.; Martínez-Martínez, L.; Muniain, M.A.; Perea, E.J.; Pérez-Cano, R.; Pascual, A. Epidemiology and clinical features of infections caused by extended-spectrum beta-lactamase-producing Escherichia coli in nonhospitalized patients. J. Clin. Microbiol. 2004, 42, 1089–1094. [Google Scholar] [CrossRef]
- Rottier, W.C.; Bamberg, Y.R.; Dorigo-Zetsma, J.W.; van der Linden, P.D.; Ammerlaan, H.S.; Bonten, M.J. Predictive value of prior colonization and antibiotic use for third-generation cephalosporin-resistant enterobacteriaceae bacteremia in patients with sepsis. Clin. Infect. Dis. 2015, 60, 1622–1630. [Google Scholar] [CrossRef]
- Morgan, D.J.; Meddings, J.; Saint, S.; Lautenbach, E.; Shardell, M.; Anderson, D.; Milstone, A.M.; Drees, M.; Pineles, L.; Safdar, N.; et al. SHEA Research Network. Does nonpayment for hospital-acquired catheter-associated urinary tract infections lead to overtesting and increased antimicrobial prescribing? Clin. Infect. Dis. 2012, 55, 923–929. [Google Scholar] [CrossRef]
- Lawes, T.; Lopez-Lozano, J.M.; Nebot, C.A.; Macartney, G.; Subbarao-Sharma, R.; Dare, C.R.; Wares, K.D.; Gould, I.M. Effects of national antibiotic stewardship and infection control strategies on hospital-associated and community-associated meticillin-resistant Staphylococcus aureus infections across a region of Scotland: A non-linear time-series study. Lancet Infect. Dis. 2015, 15, 1438–1449. [Google Scholar] [CrossRef]
- Jain, R.; Kralovic, S.M.; Evans, M.E.; Ambrose, M.; Simbartl, L.A.; Obrosky, D.S.; Render, M.L.; Freyberg, R.W.; Jernigan, J.A.; Muder, R.R.; et al. Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections. N. Engl. J. Med. 2011, 364, 1419–1430. [Google Scholar] [CrossRef]
- Klein, E.Y.; Mojica, N.; Jiang, W.; Cosgrove, S.E.; Septimus, E.; Morgan, D.J.; Laxminarayan, R. Trends in methicillin-resistant Staphylococcus aureus hospitalizations in the United States, 2010–2014. Clin. Infect. Dis. 2017, 65, 1921–1923. [Google Scholar] [CrossRef]
- Kourtis, A.P.; Hatfield, K.; Baggs, J.; Mu, Y.; See, I.; Epson, E.; Nadle, J.; Kainer, M.A.; Dumyati, G.; Petit, S.; et al. Vital signs: Epidemiology and recent trends in methicillin-resistant and in methicillin-susceptible Staphylococcus aureus bloodstream infections—United States. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 214–219. [Google Scholar] [CrossRef]
- FDA Updates Warnings for Fluoroquinolone Antibiotics. Available online: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm513183.htm (accessed on 4 June 2019).
- Orsi, G.B.; Bencardino, A.; Vena, A.; Carattoli, A.; Venditti, C.; Falcone, M.; Giordano, A.; Venditti, M. Patient risk factors for outer membrane permeability and KPC-producing carbapenem-resistant Klebsiella pneumoniae isolation: Results of a double case-control study. Infection 2013, 41, 61–67. [Google Scholar] [CrossRef]
- Al-Hasan, M.N.; Wilson, J.W.; Lahr, B.D.; Eckel-Passow, J.E.; Baddour, L.M. Incidence of Pseudomonas aeruginosa bacteremia: A population-based study. Am. J. Med. 2008, 121, 702–708. [Google Scholar] [CrossRef]
- Gransden, W.R.; Leibovici, L.; Eykyn, S.J.; Pitlik, S.D.; Samra, Z.; Konisberger, H.; Drucker, M.; Phillips, I. Risk factors and a clinical index for diagnosis of Pseudomonas aeruginosa bacteremia. Clin. Microbiol. Infect. 1995, 1, 119–123. [Google Scholar] [CrossRef][Green Version]
- Hammer, K.L.; Justo, J.A.; Bookstaver, P.B.; Kohn, J.; Albrecht, H.; Al-Hasan, M.N. Differential effect of prior beta-lactams and fluoroquinolones on risk of bloodstream infections secondary to Pseudomonas aeruginosa. Diagn. Microbiol. Infect. Dis. 2017, 87, 87–91. [Google Scholar] [CrossRef]
- Cheong, H.S.; Kang, C.I.; Wi, Y.M.; Kim, E.S.; Lee, J.S.; Ko, K.S.; Chung, D.R.; Lee, N.Y.; Song, J.H.; Peck, K.R. Clinical significance and predictors of community-onset Pseudomonas aeruginosa bacteremia. Am. J. Med. 2008, 121, 709–714. [Google Scholar] [CrossRef]
- Schechner, V.; Nobre, V.; Kaye, K.S.; Leshno, M.; Giladi, M.; Rohner, P.; Harbarth, S.; Anderson, D.J.; Karchmer, A.W.; Schwaber, M.J.; et al. Gram-negative bacteremia upon hospital admission: When should Pseudomonas aeruginosa be suspected? Clin. Infect. Dis. 2009, 48, 580–586. [Google Scholar] [CrossRef]
- Decraene, V.; Ghebrehewet, S.; Dardamissis, E.; Huyton, R.; Mortimer, K.; Wilkinson, D.; Shokrollahi, K.; Singleton, S.; Patel, B.; Turton, J.; et al. An outbreak of multidrug-resistant Pseudomonas aeruginosa in a burns service in the North of England: Challenges of infection prevention and control in a complex setting. J. Hosp. Infect. 2018, 100, e239–e245. [Google Scholar] [CrossRef]
- Milan, A.; Furlanis, L.; Cian, F.; Bressan, R.; Luzzati, R.; Lagatolla, C.; Deiana, M.L.; Knezevich, A.; Tonin, E.; Dolzani, L. Epidemic dissemination of a carbapenem-resistant Acinetobacter baumannii clone carrying armA two years after its first isolation in an Italian hospital. Microb. Drug Resist. 2016, 22, 668–674. [Google Scholar] [CrossRef]
- Paramythiotou, E.; Lucet, J.C.; Timsit, J.F.; Vanjak, D.; Paugam-Burtz, C.; Trouillet, J.L.; Belloc, S.; Kassis, N.; Karabinis, A.; Andremont, A. Acquisition of multidrug-resistant Pseudomonas aeruginosa in patients in intensive care units: Role of antibiotics with antipseudomonal activity. Clin. Infect. Dis. 2004, 38, 670–677. [Google Scholar] [CrossRef]
- Montero, M.; Sala, M.; Riu, M.; Belvis, F.; Salvado, M.; Grau, S.; Horcajada, J.P.; Alvarez-Lerma, F.; Terradas, R.; Orozco-Levi, M.; et al. Risk factors for multidrug-resistant Pseudomonas aeruginosa acquisition. Impact of antibiotic use in a double case-control study. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 335–339. [Google Scholar] [CrossRef]
- Nakamura, A.; Miyake, K.; Misawa, S.; Kuno, Y.; Horii, T.; Kondo, S.; Tabe, Y.; Ohsaka, A. Meropenem as predictive risk factor for isolation of multidrug-resistant Pseudomonas aeruginosa. J. Hosp. Infect. 2013, 83, 153–155. [Google Scholar] [CrossRef]
- Cobos-Trigueros, N.; Sole, M.; Castro, P.; Torres, J.L.; Hernández, C.; Rinaudo, M.; Fernández, S.; Soriano, Á.; Nicolás, J.M.; Mensa, J.; et al. Acquisition of Pseudomonas aeruginosa and its resistance phenotypes in critically ill medical patients: Role of colonization pressure and antibiotic exposure. Crit. Care 2015, 19, 218. [Google Scholar] [CrossRef]
- Al-Jaghbeer, M.J.; Justo, J.A.; Owens, W.; Kohn, J.; Bookstaver, P.B.; Hucks, J.; Al-Hasan, M.N. Risk factors for pneumonia due to beta-lactam-susceptible and beta-lactam-resistant Pseudomonas aeruginosa: A case-case-control study. Infection 2018, 46, 487–494. [Google Scholar] [CrossRef]
- Cain, S.E.; Kohn, J.; Bookstaver, P.B.; Albrecht, H.; Al-Hasan, M.N. Stratification of the impact of inappropriate empirical antimicrobial therapy for Gram-negative bloodstream infections by predicted prognosis. Antimicrob. Agents Chemother. 2015, 59, 245–250. [Google Scholar] [CrossRef]
- Retamar, P.; Portillo, M.M.; Lopez-Prieto, M.D.; Rodríguez-López, F.; de Cueto, M.; García, M.V.; Gómez, M.J.; Del Arco, A.; Muñoz, A.; Sánchez-Porto, A.; et al. SAEI/SAMPAC Bacteremia Group. Impact of inadequate empirical therapy on the mortality of patients with bloodstream infections: A propensity score-based analysis. Antimicrob. Agents Chemother. 2012, 56, 472–478. [Google Scholar] [CrossRef]
- Al-Hasan, M.N.; Rac, H. Transition from intravenous to oral antimicrobial therapy in patients with uncomplicated and complicated bloodstream infections. Clin. Microbiol. Infect. 2019. [Google Scholar] [CrossRef]
- Paul, M.; Shani, V.; Muchtar, E.; Kariv, G.; Robenshtok, E.; Leibovici, L. Systematic review and meta-analysis of the efficacy of appropriate empiric antibiotic therapy for sepsis. Antimicrob. Agents Chemother. 2010, 54, 4851–4863. [Google Scholar] [CrossRef]
- Shorr, A.F.; Micek, S.T.; Welch, E.C.; Doherty, J.A.; Reichley, R.M.; Kollef, M.H. Inappropriate antibiotic therapy in Gram-negative sepsis increases hospital length of stay. Crit. Care Med. 2011, 39, 46–51. [Google Scholar] [CrossRef]
- Battle, S.E.; Bookstaver, P.B.; Justo, J.A.; Kohn, J.; Albrecht, H.; Al-Hasan, M.N. Association between inappropriate empirical antimicrobial therapy and hospital length of stay in Gram-negative bloodstream infections: Stratification by prognosis. J. Antimicrob. Chemother. 2017, 72, 299–304. [Google Scholar] [CrossRef]
- Sogaard, M.; Norgaard, M.; Dethlefsen, C.; Schonheyder, H.C. Temporal changes in the incidence and 30-day mortality associated with bacteremia in hospitalized patients from 1992 through 2006: A population-based cohort study. Clin. Infect. Dis. 2011, 52, 61–69. [Google Scholar] [CrossRef]
- Nimmich, E.B.; Bookstaver, P.B.; Kohn, J.; Justo, J.A.; Hammer, K.L.; Albrecht, H.; Al-Hasan, M.N. Development of Institutional Guidelines for Management of Gram-Negative Bloodstream Infections: Incorporating Local Evidence. Hosp. Pharm. 2017, 52, 691–697. [Google Scholar] [CrossRef]
- Bookstaver, P.B.; Nimmich, E.B.; Smith, T.J.; Justo, J.A.; Kohn, J.; Hammer, K.L.; Troficanto, C.; Albrecht, H.A.; Al-Hasan, M.N. Cumulative effect of an antimicrobial stewardship and rapid diagnostic testing bundle on early streamlining of antimicrobial therapy in Gram-negative bloodstream infections. Antimicrob. Agents Chemother. 2017, 61, e00189-17. [Google Scholar] [CrossRef]
- Al-Hasan, M.N.; Lahr, B.D.; Eckel-Passow, J.E.; Baddour, L.M. Predictive scoring model of mortality in Gram-negative bloodstream infection. Clin. Microbiol. Infect. 2013, 19, 948–954. [Google Scholar] [CrossRef]
- Al-Hasan, M.N.; Juhn, Y.J.; Bang, D.W.; Yang, H.J.; Baddour, L.M. External validation of bloodstream infection mortality risk score in a population-based cohort. Clin. Microbiol. Infect. 2014, 20, 886–891. [Google Scholar] [CrossRef]
- Huang, A.M.; Newton, D.; Kunapuli, A.; Gandhi, T.N.; Washer, L.L.; Isip, J.; Collins, C.D.; Nagel, J.L. Impact of rapid organism identification via matrix-assisted laser desorption/ionization time-of-flight combined with antimicrobial stewardship team intervention in adult patients with bacteremia and candidemia. Clin. Infect. Dis. 2013, 57, 1237–1245. [Google Scholar] [CrossRef]
- MacVane, S.H.; Nolte, F.S. Benefits of adding a rapid PCR-based blood culture identification panel to an established antimicrobial stewardship program. J. Clin. Microbiol. 2016, 54, 2455–2463. [Google Scholar] [CrossRef]
- Banerjee, R.; Teng, C.B.; Cunningham, S.A.; Ihde, S.M.; Steckelberg, J.M.; Moriarty, J.P.; Shah, N.D.; Mandrekar, J.N.; Patel, R. Randomized trial of rapid multiplex polymerase chain reaction-based blood culture identification and susceptibility testing. Clin. Infect. Dis. 2015, 61, 1071–1080. [Google Scholar] [CrossRef]
- Mediwala, K.N.; Kohn, J.E.; Bookstaver, P.B.; Justo, J.A.; Rac, H.; Tucker, K.; Lashkova, L.; Dash, S.; Al-Hasan, M.N. Syndrome-specific versus prospective audit and feedback interventions for reducing use of broad-spectrum antimicrobial agents. Am. J. Infect. Control 2019. [Google Scholar] [CrossRef]
- Sick, A.C.; Lehmann, C.U.; Tamma, P.D.; Lee, C.K.; Agwu, A.L. Sustained savings from a longitudinal cost analysis of an internet-based preapproval antimicrobial stewardship program. Infect. Control Hosp. Epidemiol. 2013, 34, 573–580. [Google Scholar] [CrossRef]
- Beardsley, J.R.; Williamson, J.C.; Johnson, J.W.; Luther, V.P.; Wrenn, R.H.; Ohl, C.C. Show me the money: Long-term financial impact of an antimicrobial stewardship program. Infect. Control Hosp. Epidemiol. 2012, 33, 398–400. [Google Scholar] [CrossRef]
- Ozkurt, Z.; Erol, S.; Kadanali, A.; Ertek, M.; Ozden, K.; Tasyaran, M.A. Changes in antibiotic use, cost and consumption after an antibiotic restriction policy applied by infectious disease specialists. Jpn. J. Infect. Dis. 2005, 58, 338–343. [Google Scholar]
- Akpan, M.R.; Ahmad, R.; Shebl, N.A.; Ashiru-Oredope, D. A review of quality measures for assessing the impact of antimicrobial stewardship programs in hospitals. Antibiotics 2016, 5, 5. [Google Scholar] [CrossRef]
- Bartlett, J.M.; Siola, P.L. Implementation and first-year results of an antimicrobial stewardship program at a community hospital. Am. J. Health Syst. Pharm. 2014, 71, 943–949. [Google Scholar] [CrossRef]
- Tamma, P.D.; Avdic, E.; Keenan, J.F.; Zhao, Y.; Anand, G.; Cooper, J.; Dezube, R.; Hsu, S.; Cosgrove, S.E. What is the more effective antibiotic stewardship intervention: Preprescription authorization or postprescription review with feedback? Clin. Infect. Dis. 2017, 64, 537–543. [Google Scholar]
- Al-Hasan, M.N.; Eckel-Passow, J.E.; Baddour, L.M. Influence of referral bias on the clinical characteristics of patients with Gram-negative bloodstream Infection. Epidemiol. Infect. 2011, 139, 1750–1756. [Google Scholar] [CrossRef]
- Antimicrobial Use and Resistance Module. 2019. Available online: https://www.cdc.gov/nhsn/PDFs/pscManual/11pscAURcurrent.pdf (accessed on 1 June 2019).
- van Santen, K.L.; Edwards, J.R.; Webb, A.K.; Pollack, L.A.; O’Leary, E.; Neuhauser, M.M.; Srinivasan, A.; Pollock, D.A. The standardized antimicrobial administration ratio: A new metric for measuring and comparing antibiotic use. Clin. Infect. Dis. 2018, 67, 179–185. [Google Scholar] [CrossRef]
| Adjusted AU | Formula |
|---|---|
| APBL | |
| Carbapenems | |
| Anti-MRSA agents | |
| Anti-VRE agents |
| Adjusted AU | Formula |
|---|---|
| APBL | |
| Carbapenems | |
| Anti-MRSA agents | |
| Anti-VRE agents |
| Category | Commonly Used Antimicrobials |
|---|---|
| Broad-spectrum agents predominantly used for hospital-onset infections | Piperacillin/tazobactam, ceftazidime, cefepime, meropenem, imipenem/cilastatin, aztreonam, gentamicin, tobramycin |
| Broad-spectrum agents predominantly used for community-acquired infections | Ceftriaxone, cefotaxime, cefuroxime, cefdinir, ertapenem, ciprofloxacin, levofloxacin, moxifloxacin |
| Agents predominantly used for resistant gram-positive infections | Vancomycin, daptomycin, linezolid, ceftaroline |
| Narrow-spectrum beta-lactam agents | Penicillin G, ampicillin, amoxicillin, ampicillin/sulbactam, amoxicillin/clavulanate, nafcillin, dicloxacillin, cefazolin, cephalexin, cefoxitin |
| Agents posing the highest risk for C. difficile infection | Clindamycin, cefepime, ceftriaxone, cefdinir, ciprofloxacin, levofloxacin, moxifloxacin |
| Antifungal agents predominantly used for invasive candidiasis | Fluconazole, voriconazole, posaconazole, caspofungin, micafungin, anidulafungin |
| ASP Metrics | Description |
|---|---|
Antimicrobial use of broad-spectrum agents:
|
|
Antimicrobial resistance of predominantly hospital-onset bacteria:
|
|
| Incidence rate of CRE |
|
| Global Metrics | Description |
|---|---|
| Incidence rate of hospital-onset Clostridioides difficile infection |
|
| Incidence rate of ESBLE infections or colonization |
|
| Incidence rate of MRSA infections or colonization |
|
| Sepsis or bloodstream infection case-fatality rate |
|
| Cost of healthcare |
|
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Hasan, M.N.; Winders, H.R.; Bookstaver, P.B.; Justo, J.A. Direct Measurement of Performance: A New Era in Antimicrobial Stewardship. Antibiotics 2019, 8, 127. https://doi.org/10.3390/antibiotics8030127
Al-Hasan MN, Winders HR, Bookstaver PB, Justo JA. Direct Measurement of Performance: A New Era in Antimicrobial Stewardship. Antibiotics. 2019; 8(3):127. https://doi.org/10.3390/antibiotics8030127
Chicago/Turabian StyleAl-Hasan, Majdi N., Hana Rac Winders, P. Brandon Bookstaver, and Julie Ann Justo. 2019. "Direct Measurement of Performance: A New Era in Antimicrobial Stewardship" Antibiotics 8, no. 3: 127. https://doi.org/10.3390/antibiotics8030127
APA StyleAl-Hasan, M. N., Winders, H. R., Bookstaver, P. B., & Justo, J. A. (2019). Direct Measurement of Performance: A New Era in Antimicrobial Stewardship. Antibiotics, 8(3), 127. https://doi.org/10.3390/antibiotics8030127







