The Link between Purine Metabolism and Production of Antibiotics in Streptomyces
Abstract
1. Introduction
2. Stringent Response and Morphological Differentiation
3. Secondary Metabolism in Streptomyces
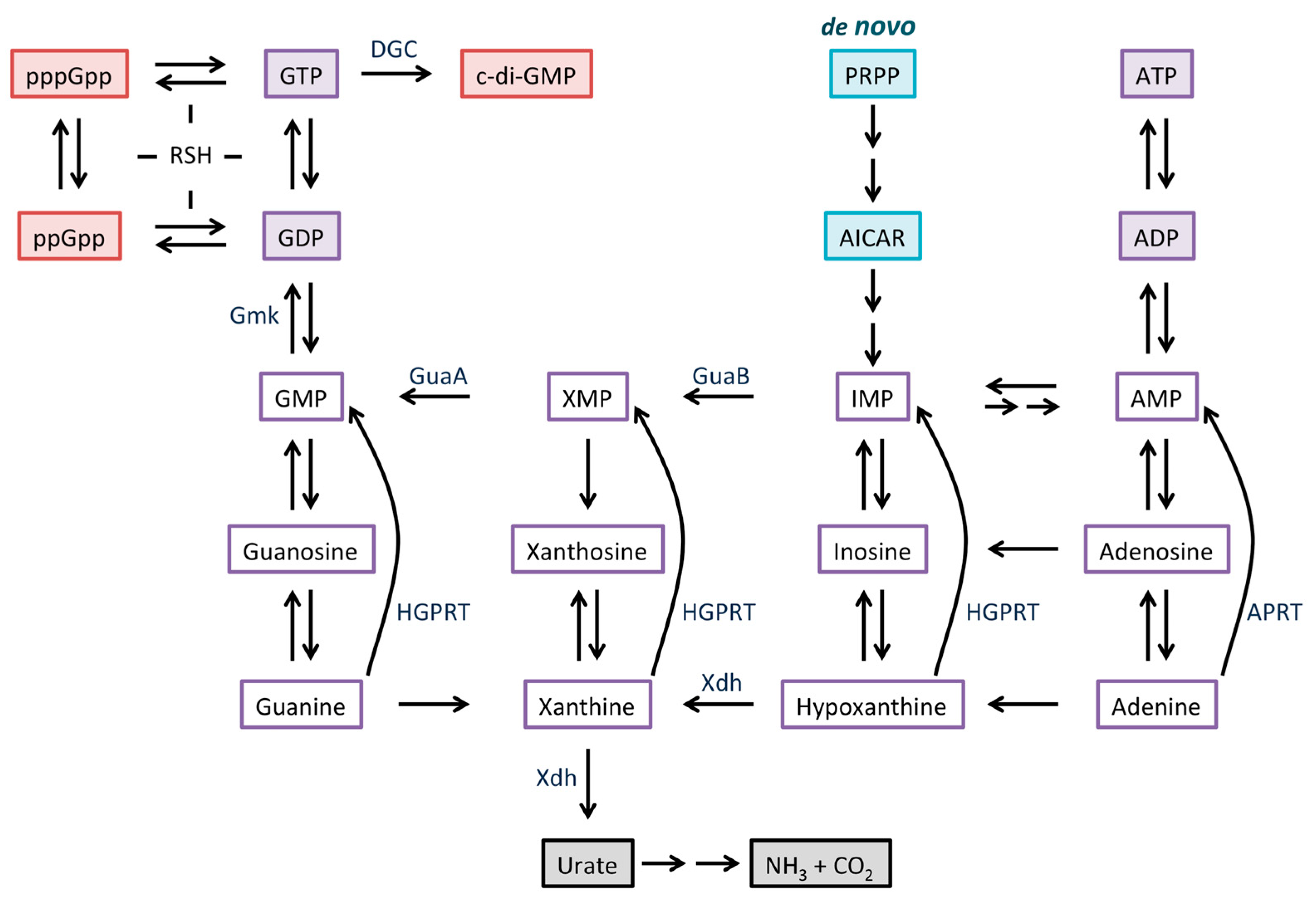
4. Link Between Purine Salvage and Production of Guanosine Nucleotide Second Messengers
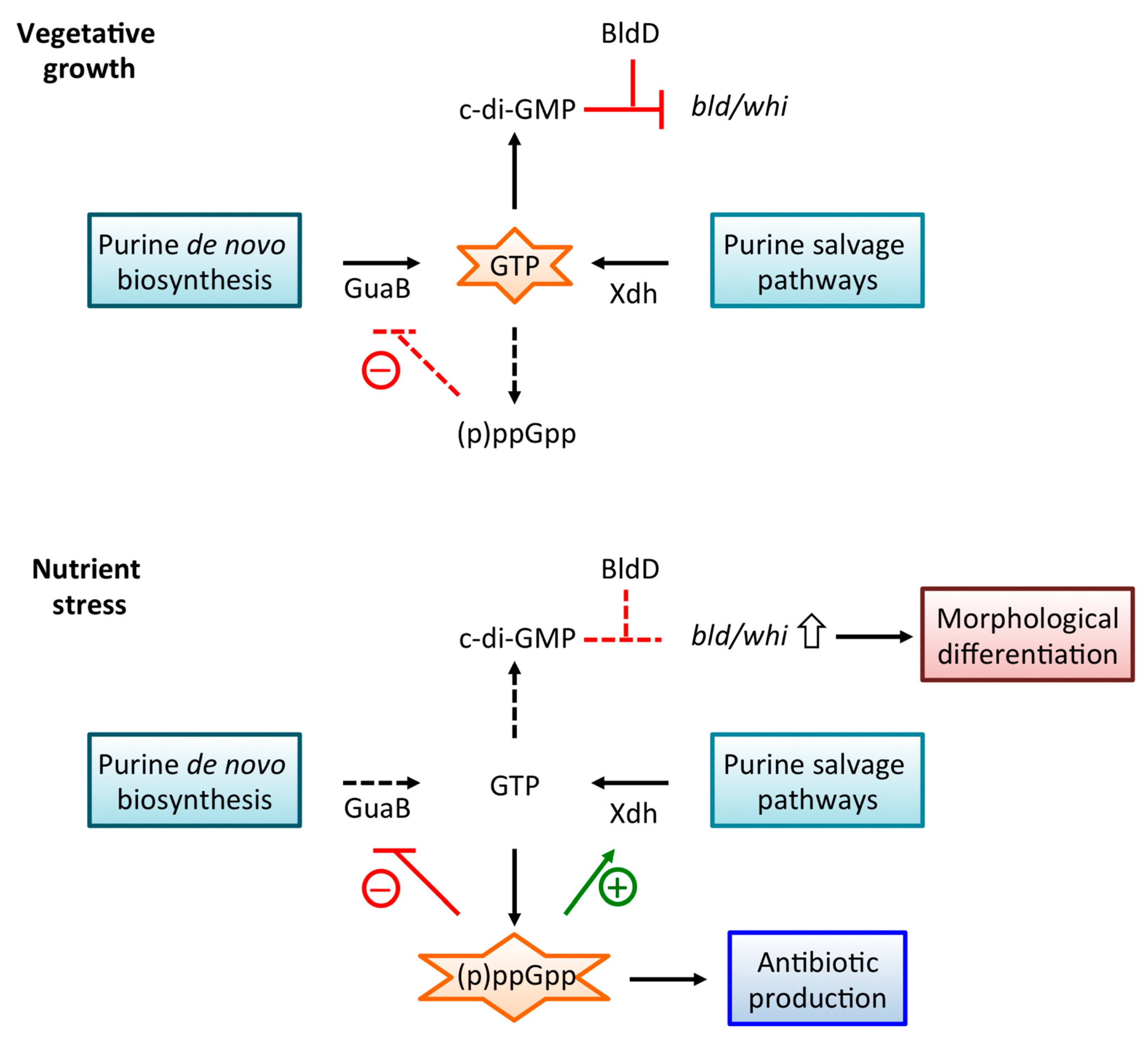
4.1. Cellular Levels of GTP and (p)ppGpp are Inter-Dependent
4.2. Production of Xdh As a Mechanism to Promote Purine salvage
5. Guanosine Metabolism Impinges on Antibiotic Production
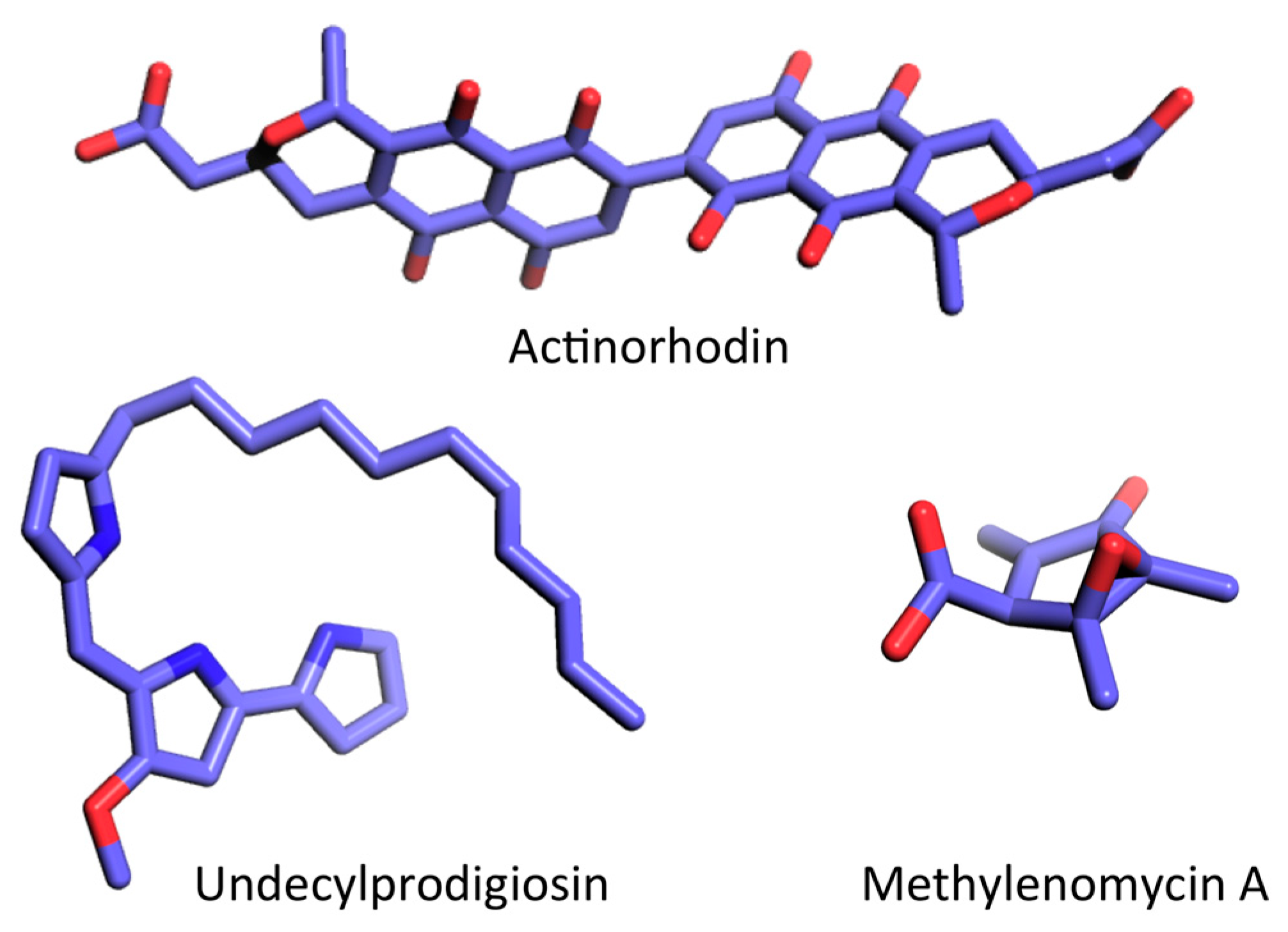
5.1. Production of Actinorhodin (ACT) and Undecylprodigiosin (RED)
5.2. Production of Other Antibiotics
5.3. Regulation of Antibiotic Production by c-di-GMP
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hauryliuk, V.; Atkinson, G.C.; Murakami, K.S.; Tenson, T.; Gerdes, K. Recent functional insights into the role of (p)ppGpp in bacterial physiology. Nat. Rev. Microbiol. 2015, 13, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Valentini, M.; Filloux, A. Biofilms and Cyclic di-GMP (c-di-GMP) Signaling: Lessons from Pseudomonas aeruginosa and Other Bacteria. J. Biol. Chem. 2016, 291, 12547–12555. [Google Scholar] [CrossRef] [PubMed]
- Den Hengst, C.D.; Tran, N.T.; Bibb, M.J.; Chandra, G.; Leskiw, B.K.; Buttner, M.J. Genes essential for morphological development and antibiotic production in Streptomyces coelicolor are targets of BldD during vegetative growth. Mol. Microbiol. 2010, 78, 361–379. [Google Scholar] [CrossRef]
- Procopio, R.E.; Silva, I.R.; Martins, M.K.; Azevedo, J.L.; Araujo, J.M. Antibiotics produced by Streptomyces. Braz. J. Infect. Dis. 2012, 16, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Cashel, M.; Gallant, J. Two compounds implicated in the function of the RC gene of Escherichia coli. Nature 1969, 221, 838–841. [Google Scholar] [CrossRef] [PubMed]
- Potrykus, K.; Cashel, M. (p)ppGpp: Still magical? Annu. Rev. Microbiol. 2008, 62, 35–51. [Google Scholar] [CrossRef]
- Gaca, A.O.; Colomer-Winter, C.; Lemos, J.A. Many means to a common end: The intricacies of (p)ppGpp metabolism and its control of bacterial homeostasis. J. Bacteriol. 2015, 197, 1146–1156. [Google Scholar] [CrossRef]
- Zhang, Y.; Zbornikova, E.; Rejman, D.; Gerdes, K. Novel (p)ppGpp Binding and Metabolizing Proteins of Escherichia coli. mBio 2018, 9. [Google Scholar] [CrossRef]
- Kriel, A.; Bittner, A.N.; Kim, S.H.; Liu, K.; Tehranchi, A.K.; Zou, W.Y.; Rendon, S.; Chen, R.; Tu, B.P.; Wang, J.D. Direct regulation of GTP homeostasis by (p)ppGpp: A critical component of viability and stress resistance. Mol. Cell 2012, 48, 231–241. [Google Scholar] [CrossRef]
- Corrigan, R.M.; Bellows, L.E.; Wood, A.; Grundling, A. ppGpp negatively impacts ribosome assembly affecting growth and antimicrobial tolerance in Gram-positive bacteria. Proc. Natl. Acad. Sci. USA 2016, 113, 1710–1719. [Google Scholar] [CrossRef]
- Wang, B.; Dai, P.; Ding, D.; Del Rosario, A.; Grant, R.A.; Pentelute, B.L.; Laub, M.T. Affinity-based capture and identification of protein effectors of the growth regulator ppGpp. Nat. Chem. Biol. 2019, 15, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Agirrezabala, X.; Fernandez, I.S.; Kelley, A.C.; Carton, D.G.; Ramakrishnan, V.; Valle, M. The ribosome triggers the stringent response by RelA via a highly distorted tRNA. EMBO Rep. 2013, 14, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.J.; Barker, M.M.; Ross, W.; Schneider, D.A.; Webb, C.; Foster, J.W.; Gourse, R.L. DksA: A critical component of the transcription initiation machinery that potentiates the regulation of rRNA promoters by ppGpp and the initiating NTP. Cell 2004, 118, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Ross, W.; Vrentas, C.E.; Sanchez-Vazquez, P.; Gaal, T.; Gourse, R.L. The magic spot: A ppGpp binding site on E. coli RNA polymerase responsible for regulation of transcription initiation. Mol. Cell 2013, 50, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Krasny, L.; Gourse, R.L. An alternative strategy for bacterial ribosome synthesis: Bacillus subtilis rRNA transcription regulation. EMBO J. 2004, 23, 4473–4483. [Google Scholar] [CrossRef] [PubMed]
- Vrentas, C.E.; Gaal, T.; Berkmen, M.B.; Rutherford, S.T.; Haugen, S.P.; Vassylyev, D.G.; Ross, W.; Gourse, R.L. Still looking for the magic spot: The crystallographically defined binding site for ppGpp on RNA polymerase is unlikely to be responsible for rRNA transcription regulation. J. Mol. Biol. 2008, 377, 551–564. [Google Scholar] [CrossRef]
- Geiger, T.; Wolz, C. Intersection of the stringent response and the CodY regulon in low GC Gram-positive bacteria. Int. J. Med. Microbiol. 2014, 304, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Kriel, A.; Brinsmade, S.R.; Tse, J.L.; Tehranchi, A.K.; Bittner, A.N.; Sonenshein, A.L.; Wang, J.D. GTP dysregulation in Bacillus subtilis cells lacking (p)ppGpp results in phenotypic amino acid auxotrophy and failure to adapt to nutrient downshift and regulate biosynthesis genes. J. Bacteriol. 2014, 196, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Krasny, L.; Tiserova, H.; Jonak, J.; Rejman, D.; Sanderova, H. The identity of the transcription +1 position is crucial for changes in gene expression in response to amino acid starvation in Bacillus Subtilis. Mol. Microbiol. 2008, 69, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Ryu, Y.G.; Kim, E.S.; Kim, D.W.; Kim, S.K.; Lee, K.J. Differential stringent responses of Streptomyces coelicolor M600 to starvation of specific nutrients. J. Microbiol. Biotechnol. 2007, 17, 305–312. [Google Scholar]
- Hesketh, A.; Sun, J.; Bibb, M. Induction of ppGpp synthesis in Streptomyces coelicolor A3(2) grown under conditions of nutritional sufficiency elicits actII-ORF4 transcription and actinorhodin biosynthesis. Mol. Microbiol. 2001, 39, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Ryu, Y.G.; Jin, W.; Kim, J.Y.; Kim, J.Y.; Lee, S.H.; Lee, K.J. Stringent factor regulates antibiotics production and morphological differentiation of Streptomyces clavuligerus. J. Microbiol. Biotechnol. 2004, 14, 1170–1175. [Google Scholar]
- Ochi, K. Metabolic initiation of differentiation and secondary metabolism by Streptomyces griseus: Significance of the stringent response (ppGpp) and GTP content in relation to A factor. J. Bacteriol. 1987, 169, 3608–3616. [Google Scholar] [CrossRef] [PubMed]
- Strauch, E.; Takano, E.; Baylis, H.A.; Bibb, M.J. The stringent response in Streptomyces coelicolor A3(2). Mol. Microbiol. 1991, 5, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Riesenberg, D.; Bergter, F.; Kari, C. Effect of serine hydroxamate and methyl alpha-D-glucopyranoside treatment on nucleoside polyphosphate pools, RNA and protein accumulation in Streptomyces hygroscopicus. J. Gen. Microbiol. 1984, 130, 2549–2558. [Google Scholar] [PubMed]
- Bascaran, V.; Sanchez, L.; Hardisson, C.; Brana, A.F. Stringent response and initiation of secondary metabolism in Streptomyces clavuligerus. J. Gen. Microbiol. 1991, 137, 1625–1634. [Google Scholar] [CrossRef]
- Gomez-Escribano, J.P.; Martin, J.F.; Hesketh, A.; Bibb, M.J.; Liras, P. Streptomyces clavuligerus relA-null mutants overproduce clavulanic acid and cephamycin C: Negative regulation of secondary metabolism by (p)ppGpp. Microbiology 2008, 154, 744–755. [Google Scholar] [CrossRef]
- Ochi, K. Changes in Nucleotide Pools during Sporulation of Streptomyces-Griseus in Submerged Culture. J. Gen. Microbiol. 1987, 133, 2787–2795. [Google Scholar] [CrossRef][Green Version]
- McCormick, J.R.; Flardh, K. Signals and regulators that govern Streptomyces development. FEMS Microbiol. Rev. 2012, 36, 206–231. [Google Scholar] [CrossRef]
- Hull, T.D.; Ryu, M.H.; Sullivan, M.J.; Johnson, R.C.; Klena, N.T.; Geiger, R.M.; Gomelsky, M.; Bennett, J.A. Cyclic Di-GMP phosphodiesterases RmdA and RmdB are involved in regulating colony morphology and development in Streptomyces Coelicolor. J. Bacteriol. 2012, 194, 4642–4651. [Google Scholar] [CrossRef]
- Tran, N.T.; Den Hengst, C.D.; Gomez-Escribano, J.P.; Buttner, M.J. Identification and characterization of CdgB, a diguanylate cyclase involved in developmental processes in Streptomyces coelicolor. J. Bacteriol. 2011, 193, 3100–3108. [Google Scholar] [CrossRef] [PubMed]
- Tschowri, N.; Schumacher, M.A.; Schlimpert, S.; Chinnam, N.B.; Findlay, K.C.; Brennan, R.G.; Buttner, M.J. Tetrameric c-di-GMP mediates effective transcription factor dimerization to control Streptomyces development. Cell 2014, 158, 1136–1147. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.K.; Higgins, M.B.; Rabinowitz, J.D. Antifolate-Induced Depletion of Intracellular Glycine and Purines Inhibits Thymineless Death in E. coli. ACS Chem. Biol. 2010, 5, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Bosso, J.A.; Mauldin, P.D.; Salgado, C.D. The association between antibiotic use and resistance: The role of secondary antibiotics. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 1125–1129. [Google Scholar] [CrossRef] [PubMed]
- Abdelmohsen, U.R.; Grkovic, T.; Balasubramanian, S.; Kamel, M.S.; Quinn, R.J.; Hentschel, U. Elicitation of secondary metabolism in actinomycetes. Biotechnol. Adv. 2015, 33, 798–811. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Ochi, K. Novel approach for improving the productivity of antibiotic-producing strains by inducing combined resistant mutations. Appl. Env. Microbiol. 2001, 67, 1885–1892. [Google Scholar] [CrossRef] [PubMed]
- Shentu, X.P.; Cao, Z.Y.; Xiao, Y.; Tang, G.; Ochi, K.; Yu, X.P. Substantial improvement of toyocamycin production in Streptomyces diastatochromogenes by cumulative drug-resistance mutations. PloS ONE 2018, 13, 0203006. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Bedre, R.; Thapa, S.S.; Sabrin, A.; Wang, G.; Dassanayake, M.; Grove, A. Global Awakening of Cryptic Biosynthetic Gene Clusters in Burkholderia thailandensis. ACS Chem. Biol. 2017, 12, 3012–3021. [Google Scholar] [CrossRef] [PubMed]
- Nett, M.; Ikeda, H.; Moore, B.S. Genomic basis for natural product biosynthetic diversity in the actinomycetes. Nat. Prod. Rep. 2009, 26, 1362–1384. [Google Scholar] [CrossRef]
- Liu, G.; Chater, K.F.; Chandra, G.; Niu, G.; Tan, H. Molecular regulation of antibiotic biosynthesis in Streptomyces. Microbiol. Mol. Biol. Rev. 2013, 77, 112–143. [Google Scholar] [CrossRef]
- Huang, J.; Shi, J.; Molle, V.; Sohlberg, B.; Weaver, D.; Bibb, M.J.; Karoonuthaisiri, N.; Lih, C.J.; Kao, C.M.; Buttner, M.J.; et al. Cross-regulation among disparate antibiotic biosynthetic pathways of Streptomyces coelicolor. Mol. Microbiol. 2005, 58, 1276–1287. [Google Scholar] [CrossRef] [PubMed]
- Dalebroux, Z.D.; Svensson, S.L.; Gaynor, E.C.; Swanson, M.S. ppGpp conjures bacterial virulence. Microbiol. Mol. Biol Rev. 2010, 74, 171–199. [Google Scholar] [CrossRef] [PubMed]
- Mantsala, P.; Zalkin, H. Cloning and sequence of Bacillus subtilis purA and guaA, involved in the conversion of IMP to AMP and GMP. J. Bacteriol. 1992, 174, 1883–1890. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hedstrom, L.; Liechti, G.; Goldberg, J.B.; Gollapalli, D.R. The antibiotic potential of prokaryotic IMP dehydrogenase inhibitors. Curr. Med. Chem. 2011, 18, 1909–1918. [Google Scholar] [CrossRef] [PubMed]
- Xi, H.; Schneider, B.L.; Reitzer, L. Purine catabolism in Escherichia coli and function of xanthine dehydrogenase in purine salvage. J. Bacteriol. 2000, 182, 5332–5341. [Google Scholar] [CrossRef] [PubMed]
- Jewett, M.W.; Lawrence, K.A.; Bestor, A.; Byram, R.; Gherardini, F.; Rosa, P.A. GuaA and GuaB are essential for Borrelia burgdorferi survival in the tick-mouse infection cycle. J. Bacteriol. 2009, 191, 6231–6241. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Sutchu, S.; Rosa, P.A.; Byram, R.; Jewett, M.W. Borrelia burgdorferi harbors a transport system essential for purine salvage and mammalian infection. Infect. Immun. 2012, 80, 3086–3093. [Google Scholar] [CrossRef] [PubMed]
- Drecktrah, D.; Lybecker, M.; Popitsch, N.; Rescheneder, P.; Hall, L.S.; Samuels, D.S. The Borrelia burgdorferi RelA/SpoT Homolog and Stringent Response Regulate Survival in the Tick Vector and Global Gene Expression during Starvation. PLoS Pathog. 2015, 11, 1005160. [Google Scholar] [CrossRef] [PubMed]
- Novak, E.A.; Sultan, S.Z.; Motaleb, M.A. The cyclic-di-GMP signaling pathway in the Lyme disease spirochete, Borrelia burgdorferi. Front. Cell Infect. Microbiol. 2014, 4, 56. [Google Scholar] [CrossRef]
- Krol, E.; Becker, A. ppGpp in Sinorhizobium meliloti: Biosynthesis in response to sudden nutritional downshifts and modulation of the transcriptome. Mol. Microbiol. 2011, 81, 1233–1254. [Google Scholar] [CrossRef]
- Sivapragasam, S.; Grove, A. Streptomyces coelicolor XdhR is a direct target of (p)ppGpp that controls expression of genes encoding xanthine dehydrogenase to promote purine salvage. Mol. Microbiol. 2016, 100, 701–718. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.M.; Beresford, M.; Epton, H.A.; Sigee, D.C.; Shama, G.; Andrew, P.W.; Roberts, I.S. Listeria monocytogenes relA and hpt mutants are impaired in surface-attached growth and virulence. J. Bacteriol 2002, 184, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Gaca, A.O.; Kajfasz, J.K.; Miller, J.H.; Liu, K.Q.; Wang, J.D.; Abranches, J.; Lemos, J.A. Basal Levels of (p)ppGpp in Enterococcus faecalis: The Magic beyond the Stringent Response. mBio 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, L.C.; Schou, S.; Nygaard, P.; Saxild, H.H. Xanthine metabolism in Bacillus subtilis: Characterization of the xpt-pbuX operon and evidence for purine- and nitrogen-controlled expression of genes involved in xanthine salvage and catabolism. J. Bacteriol. 1997, 179, 2540–2550. [Google Scholar] [CrossRef] [PubMed]
- Sivapragasam, S.; Deochand, D.K.; Meariman, J.K.; Grove, A. The Stringent Response Induced by Phosphate Limitation Promotes Purine Salvage in Agrobacterium fabrum. Biochemistry 2017, 56, 5831–5843. [Google Scholar] [CrossRef]
- Hillerich, B.; Westpheling, J. A new TetR family transcriptional regulator required for morphogenesis in Streptomyces coelicolor. J. Bacteriol. 2008, 190, 61–67. [Google Scholar] [CrossRef]
- Sprusansky, O.; Zhou, L.; Jordan, S.; White, J.; Westpheling, J. Identification of three new genes involved in morphogenesis and antibiotic production in Streptomyces coelicolor. J. Bacteriol. 2003, 185, 6147–6157. [Google Scholar] [CrossRef]
- Fu, J.; Zong, G.; Zhang, P.; Zhao, Z.; Ma, J.; Pang, X.; Cao, G. XdhR negatively regulates actinorhodin biosynthesis in Streptomyces coelicolor M145. FEMS Microbiol. Lett. 2017, 364. [Google Scholar] [CrossRef]
- Deutscher, M.P. Maturation and degradation of ribosomal RNA in bacteria. Prog. Mol. Biol. Transl. Sci. 2009, 85, 369–391. [Google Scholar]
- Hsu, D.; Shih, L.M.; Zee, Y.C. Degradation of rRNA in Salmonella strains: A novel mechanism to regulate the concentrations of rRNA and ribosomes. J. Bacteriol. 1994, 176, 4761–4765. [Google Scholar] [CrossRef][Green Version]
- Molin, S.; Von Meyenburg, K.; Maaloe, O.; Hansen, M.T.; Pato, M.L. Control of ribosome synthesis in Escherichia coli: Analysis of an energy source shift-down. J. Bacteriol. 1977, 131, 7–17. [Google Scholar]
- Gramajo, H.C.; Takano, E.; Bibb, M.J. Stationary-phase production of the antibiotic actinorhodin in Streptomyces coelicolor A3(2) is transcriptionally regulated. Mol. Microbiol. 1993, 7, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Takano, E.; Gramajo, H.C.; Strauch, E.; Andres, N.; White, J.; Bibb, M.J. Transcriptional regulation of the redD transcriptional activator gene accounts for growth-phase-dependent production of the antibiotic undecylprodigiosin in Streptomyces coelicolor A3(2). Mol. Microbiol. 1992, 6, 2797–2804. [Google Scholar] [CrossRef] [PubMed]
- Chakraburtty, R.; Bibb, M. The ppGpp synthetase gene (relA) of Streptomyces coelicolor A3(2) plays a conditional role in antibiotic production and morphological differentiation. J. Bacteriol. 1997, 179, 5854–5861. [Google Scholar] [CrossRef] [PubMed]
- Hesketh, A.; Chen, W.J.; Ryding, J.; Chang, S.; Bibb, M. The global role of ppGpp synthesis in morphological differentiation and antibiotic production in Streptomyces coelicolor A3(2). Genome Biol. 2007, 8, 161. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Tanaka, Y.; Ochi, K. The G243D mutation (afsB mutation) in the principal sigma factor sigmaHrdB alters intracellular ppGpp level and antibiotic production in Streptomyces coelicolor A3(2). Microbiology 2010, 156, 2384–2392. [Google Scholar] [CrossRef][Green Version]
- Saito, N.; Xu, J.; Hosaka, T.; Okamoto, S.; Aoki, H.; Bibb, M.J.; Ochi, K. EshA accentuates ppGpp accumulation and is conditionally required for antibiotic production in Streptomyces coelicolor A3(2). J. Bacteriol. 2006, 188, 4952–4961. [Google Scholar] [CrossRef]
- Ochi, K.; Zhang, D.; Kawamoto, S.; Hesketh, A. Molecular and functional analysis of the ribosomal L11 and S12 protein genes (rplK and rpsL) of Streptomyces coelicolor A3(2). Mol. Gen. Genet. 1997, 256, 488–498. [Google Scholar] [CrossRef]
- Hoyt, S.; Jones, G.H. relA is required for actinomycin production in Streptomyces antibioticus. J. Bacteriol. 1999, 181, 3824–3829. [Google Scholar]
- Kelly, K.S.; Ochi, K.; Jones, G.H. Pleiotropic effects of a relC mutation in Streptomyces antibioticus. J. Bacteriol. 1991, 173, 2297–2300. [Google Scholar] [CrossRef]
- Jin, W.; Ryu, Y.G.; Kang, S.G.; Kim, S.K.; Saito, N.; Ochi, K.; Lee, S.H.; Lee, K.J. Two relAlspoT homologous genes are involved in the morphological and physiological differentiation of Streptomyces Clavuligerus. Microbiol. 2004, 150, 1485–1493. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xu, Z.; You, D.; Tang, L.Y.; Zhou, Y.; Ye, B.C. Metabolic Engineering Strategies Based on Secondary Messengers (p)ppGpp and C-di-GMP To Increase Erythromycin Yield in Saccharopolyspora erythraea. ACS Synth. Biol. 2019, 8, 332–345. [Google Scholar] [CrossRef] [PubMed]
- Elliot, M.; Damji, F.; Passantino, R.; Chater, K.; Leskiw, B. The bldD gene of Streptomyces coelicolor A3(2): A regulatory gene involved in morphogenesis and antibiotic production. J. Bacteriol. 1998, 180, 1549–1555. [Google Scholar] [PubMed]
- Park, S.S.; Yang, Y.H.; Song, E.; Kim, E.J.; Kim, W.S.; Sohng, J.K.; Lee, H.C.; Liou, K.K.; Kim, B.G. Mass spectrometric screening of transcriptional regulators involved in antibiotic biosynthesis in Streptomyces coelicolor A3(2). J. Indust. Microbiol. Biotechnol. 2009, 36, 1073–1083. [Google Scholar] [CrossRef] [PubMed]
| Species | Factor | Link to Guanosine Metabolism or Antibiotic Production |
|---|---|---|
| S. coelicolor | RelA | (p)ppGpp synthesis, ACT/RED production [64] |
| S. coelicolor | RelC (RplK) | (p)ppGpp synthesis, ACT/RED production [68] |
| S. coelicolor | EshA | (p)ppGpp synthesis, ACT production [67] |
| S. coelicolor | XdhR | Xdh and (p)ppGpp synthesis, ACT production [51,56,58] |
| S. coelicolor | RmdA, RmdB | c-di-GMP degradation, ACT production [30] |
| S. coelicolor | CdgA, CdgB | c-di-GMP synthesis, ACT production [31] |
| S. antibioticus | RelA | (p)ppGpp synthesis, actinomycin production [69] |
| S. antibioticus | RelC | (p)ppGpp synthesis, actinomycin production [70] |
| S. clavuligerus | RelA | (p)ppGpp synthesis, clavulanic acid and cephamycin C production [27,71] |
| S. griseus | Rel GuaA | (p)ppGpp synthesis, streptomycin production [23] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sivapragasam, S.; Grove, A. The Link between Purine Metabolism and Production of Antibiotics in Streptomyces. Antibiotics 2019, 8, 76. https://doi.org/10.3390/antibiotics8020076
Sivapragasam S, Grove A. The Link between Purine Metabolism and Production of Antibiotics in Streptomyces. Antibiotics. 2019; 8(2):76. https://doi.org/10.3390/antibiotics8020076
Chicago/Turabian StyleSivapragasam, Smitha, and Anne Grove. 2019. "The Link between Purine Metabolism and Production of Antibiotics in Streptomyces" Antibiotics 8, no. 2: 76. https://doi.org/10.3390/antibiotics8020076
APA StyleSivapragasam, S., & Grove, A. (2019). The Link between Purine Metabolism and Production of Antibiotics in Streptomyces. Antibiotics, 8(2), 76. https://doi.org/10.3390/antibiotics8020076





