Trends in Neisseria gonorrhoeae Antimicrobial Resistance over a Ten-Year Surveillance Period, Johannesburg, South Africa, 2008–2017
Abstract
1. Introduction
2. Results
2.1. Characteristics of Participants
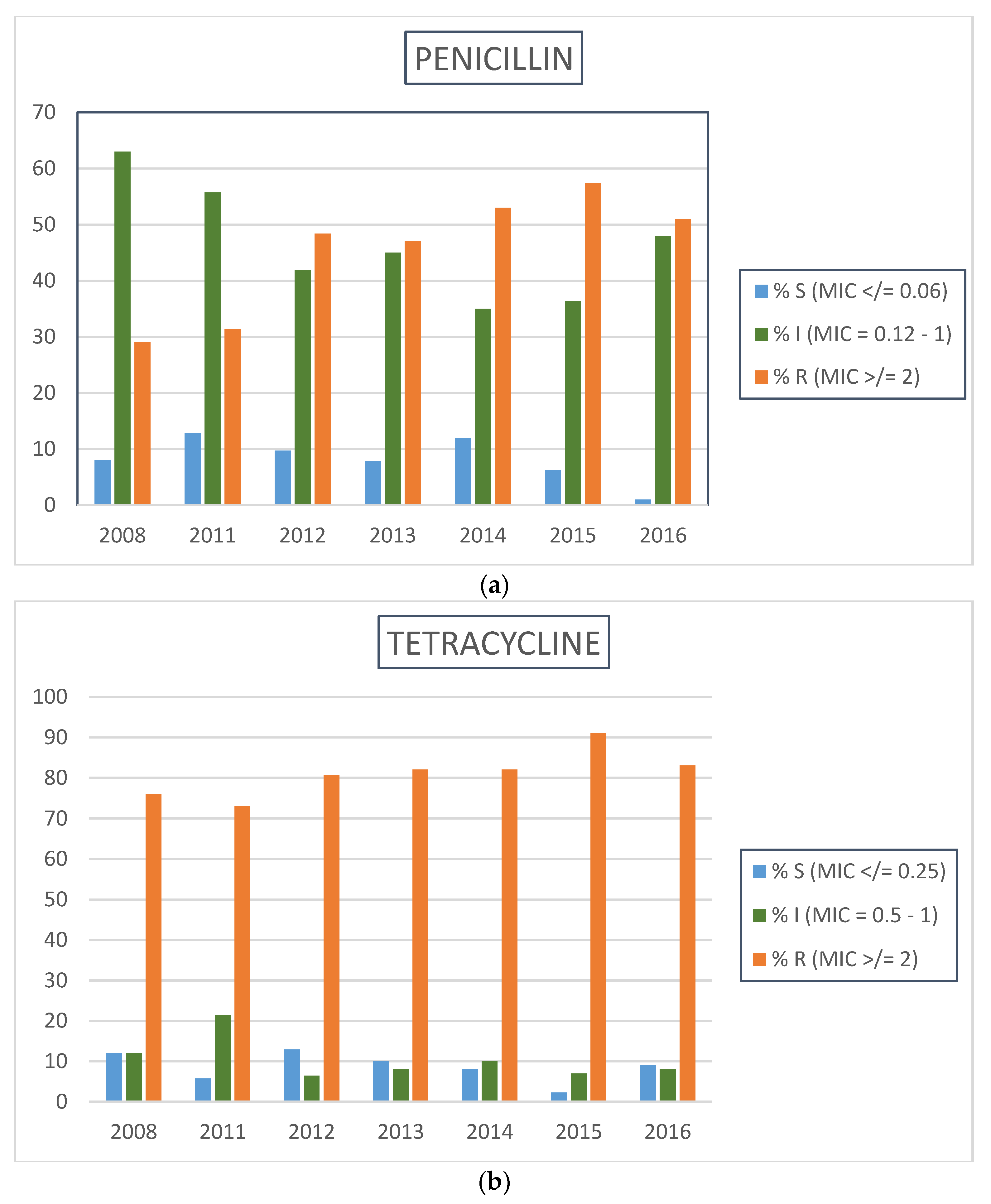
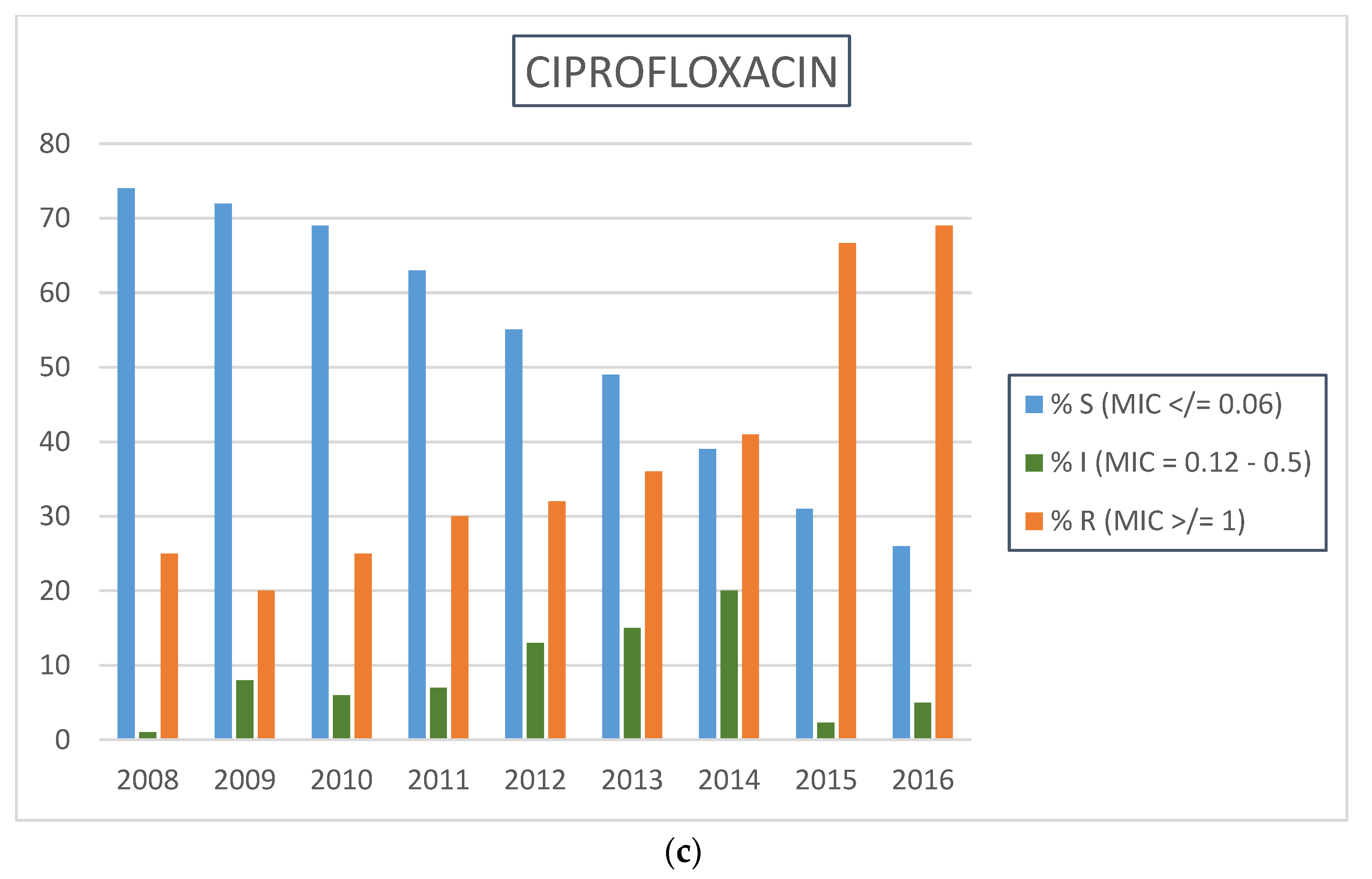
2.2. Neisseria gonorrhoeae Antimicrobial Resistance Profiles by Calendar Year
3. Discussion
4. Methods
4.1. Patient Recruitment
4.2. Sample Collection
4.3. Laboratory Procedures
4.4. Data Management and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Emergence of Multi-Drug Resistant Neisseria gonorrhoeae; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Newman, L.; Rowley, J.; Vander Hoorn, S.; Wijesooriya, N.S.; Unemo, M.; Low, N.; Stevens, G.; Gottlieb, S.; Kiarie, J.; Temmerman, M. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS ONE 2015, 10, e0143304. [Google Scholar] [CrossRef] [PubMed]
- Goire, N.; Lahra, M.M.; Chen, M.; Donovan, B.; Fairley, C.K.; Guy, R.; Kaldor, J.; Regan, D.; Ward, J.; Nissen, M.D.; et al. Molecular approaches to enhance surveillance of gonococcal antimicrobial resistance. Nat. Rev. Microbiol. 2014, 12, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Unemo, M.; Shafer, W.M. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: Past, evolution, and future. Clin. Microbiol. Rev. 2014, 27, 587–613. [Google Scholar] [CrossRef] [PubMed]
- McMillan, A.; Young, H. The treatment of pharyngeal gonorrhoea with a single oral dose of cefixime. Int. J. STD AIDS 2007, 18, 253–254. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Yasuda, M.; Yokoi, S.; Ito, S.-I.; Takahashi, Y.; Ishihara, S.; Maeda, S.-I.; Deguchi, T. Remarkable increase in central Japan in 2001–2002 of Neisseria gonorrhoeae isolates with decreased susceptibility to penicillin, tetracycline, oral cephalosporins, and fluoroquinolones. Antimicrob. Agents Chemother. 2004, 48, 3185–3187. [Google Scholar] [CrossRef] [PubMed]
- Kidd, S.; Workowski, K.A. Management of gonorrhea in adolescents and adults in the United States. Clin. Infect. Dis. 2015, 61, S785–S801. [Google Scholar] [CrossRef] [PubMed]
- Chisholm, S.A.; Dave, J.; Ison, C.A. High-level azithromycin resistance occurs in Neisseria gonorrhoeae as a result of a single point mutation in the 23s rRNA genes. Antimicrob. Agents Chemother. 2010, 54, 3812–3816. [Google Scholar] [CrossRef] [PubMed]
- National Department of Health. Epidemiological Comments; National Department of Health: Pretoria, South Africa, 2008. [Google Scholar]
- Mhlongo, S.; Magooa, P.; Muller, E.E.; Nel, N.; Radebe, F.; Wasserman, E.; Lewis, D.A. Etiology and STI/HIV coinfections among patients with urethral and vaginal discharge syndromes in South Africa. Sex. Transm. Dis. 2010, 37, 566–570. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Baseline Report on Global Sexually Transmitted Infection Surveillance; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Patel, J.B. Performance Standards for Antimicrobial Susceptibility Testing; Clinical Laboratory Standards Institute: Wayne, PA, USA, 2018; Volume M100, pp. 72–75. [Google Scholar]
- Jensen, J.S.; Cusini, M.; Gomberg, M.; Moi, H. 2016 European guideline on Mycoplasma genitalium infections. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1650–1656. [Google Scholar] [CrossRef] [PubMed]
- Muller, E.E.; Fayemiwo, S.A.; Lewis, D.A. Characterization of a novel beta-lactamase-producing plasmid in Neisseria gonorrhoeae: Sequence analysis and molecular typing of host gonococci. J. Antimicrob. Chemother. 2011, 66, 1514–1517. [Google Scholar] [CrossRef] [PubMed]
- Fayemiwo, S.A.; Muller, E.E.; Gumede, L.; Lewis, D.A. Plasmid-mediated penicillin and tetracycline resistance among Neisseria gonorrhoeae isolates in South Africa: Prevalence, detection and typing using a novel molecular assay. Sex. Transm. Dis. 2011, 38, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.A.; Scott, L.; Slabbert, M.; Mhlongo, S.; van Zijl, A.; Sello, M.; du Plessis, N.; Radebe, F.; Wasserman, E. Escalation in the relative prevalence of ciprofloxacin-resistant gonorrhoea among men with urethral discharge in two South African cities: Association with HIV seropositivity. Sex. Transm. Infect. 2008, 84, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Tapsall, J. Antimcrobial Resistance in Neisseria gonorrhoeae; World Health Organization: Geneva, Switzerland, 2001. [Google Scholar]
- National Department of Health. First-Line Comprehensive Management and Control of Sexually Transmitted Infections; National Department of Health: Pretoria, South Africa, 2008. [Google Scholar]
- Igawa, G.; Yamagishi, Y.; Lee, K.I.; Dorin, M.; Shimuta, K.; Suematsu, H.; Nakayama, S.I.; Mikamo, H.; Unemo, M.; Ohnishi, M. Neisseria cinerea with high ceftriaxone MIC is a source of ceftriaxone and cefixime resistance-mediating penA sequences in Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Duncan, M.; Tomberg, J.; Davies, C.; Unemo, M.; Nicholas, R.A. Genetics of chromosomally mediated intermediate resistance to ceftriaxone and cefixime in Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 2009, 53, 3744–3751. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.A.; Sriruttan, C.; Muller, E.E.; Golparian, D.; Gumede, L.; Fick, D.; de Wet, J.; Maseko, V.; Coetzee, J.; Unemo, M. Phenotypic and genetic characterization of the first two cases of extended-spectrum-cephalosporin-resistant Neisseria gonorrhoeae infection in South Africa and association with cefixime treatment failure. J. Antimicrob. Chemother. 2013, 68, 1267–1270. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, M.; Golparian, D.; Shimuta, K.; Saika, T.; Hoshina, S.; Iwasaku, K.; Nakayama, S.-I.; Kitawaki, J.; Unemo, M. Is Neisseria gonorrhoeae initiating a future era of untreatable gonorrhea?: Detailed characterization of the first strain with high-level resistance to ceftriaxone. Antimicrob. Agents Chemother. 2011, 55, 3538–3545. [Google Scholar] [CrossRef] [PubMed]
- National Department of Health. Sexually Transmitted Infections Management Guidelines; National Department of Health: Pretoria, South Africa, 2015. [Google Scholar]
- World Health Organization. Global Action Plan to Control the Spread and Impact of Antimicrobial Resistance in Neisseria Gonorrhoeae; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Unemo, M.; Golparian, D.; Skogen, V.; Olsen, A.O.; Moi, H.; Syversen, G.; Hjelmevoll, S.O. Neisseria gonorrhoeae strain with high-level resistance to spectinomycin due to a novel resistance mechanism (mutated ribosomal protein s5) verified in Norway. Antimicrob. Agents Chemother. 2013, 57, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Bignell, C.; Garley, J. Azithromycin in the treatment of infection with Neisseria gonorrhoeae. Sex. Transm. Infect. 2010, 86, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Soge, O.O.; Harger, D.; Schafer, S.; Toevs, K.; Raisler, K.A.; Venator, K.; Holmes, K.K.; Kirkcaldy, R.D. Emergence of increased azithromycin resistance during unsuccessful treatment of Neisseria gonorrhoeae infection with azithromycin (Portland, or, 2011). Sex. Transm. Dis. 2012, 39, 877–879. [Google Scholar] [CrossRef] [PubMed]
- Fifer, H.; Cole, M.; Hughes, G.; Padfield, S.; Smolarchuk, C.; Woodford, N.; Wensley, A.; Mustafa, N.; Schaefer, U.; Myers, R.; et al. Sustained transmission of high-level azithromycin-resistant Neisseria gonorrhoeae in England: An observational study. Lancet Infect. Dis. 2018. [Google Scholar] [CrossRef]
- Fifer, H.; Natarajan, U.; Jones, L.; Alexander, S.; Hughes, G.; Golparian, D.; Unemo, M. Failure of dual antimicrobial therapy in treatment of gonorrhea. N. Engl. J. Med. 2016, 374, 2504–2506. [Google Scholar] [CrossRef] [PubMed]
- Public Health England. UK Case of Neisseria gonorrhoeae with High-Level Resistance to Azithromycin and Resistance to Ceftriaxone Acquired Abroad; Public Health England: London, UK, 2018. [Google Scholar]
| Characteristic | Males (2254) | Females (191) | All (2445) |
|---|---|---|---|
| Age in years (median, IQR) * | 28 (24–32) | 24 (23–30) | 27 (24–32) |
| Ethnic group (black African) β (%) | 1921 (99.7) | 164 (100) | 2085 (99.7) |
| History of STI syndrome in past 12 months (%) | 580 (25.8) | 63 (31.3) | 643 (26.3) |
| Heterosexual orientation α (%) | 1907 (99.8) | 161 (99.4) | 2068 (99.8) |
| HIV positivity µ (%) | 655 (32.5) | 104 (52.0) | 759 (34.3) |
| Year of enrolment (%) | |||
| 2008 | 309 (13.7) | 29 (15.2) | 338 (13.8) |
| 2009 | 305 (13.5) | 19 (10.0) | 324 (13.2) |
| 2010 | 287 (12.7) | 29 (15.2) | 316 (12.9) |
| 2011 | 260 (11.5) | 38 (19.9) | 298 (12.2) |
| 2012 | 273 (12.1) | 21 (11.0) | 294 (12.0) |
| 2013 | 221 (9.8) | 28 (14.7) | 249 (10.2) |
| 2014 | 208 (9.2) | 27 (14.1) | 235 (9.6) |
| 2015 | 137 (6.1) | 0 (0.0) | 137 (5.6) |
| 2016 | 128 (5.6) | 0 (0.0) | 128 (5.7) |
| 2017 | 128 (5.7) | 0 (0.0) | 128 (5.7) |
| Antimicrobials and AST Method | ||
|---|---|---|
| Year | Cefixime (CXM), ceftriaxone (CTR), ciprofloxacin (CIP) E-test MIC | Azithromycin (AZM), penicillin (PEN), tetracycline (TET), spectinomycin (SPC) Agar dilution MIC |
| 2008 | 338 (CTR and CIP only) | 233 |
| 2009 | 324 | 0 |
| 2010 | 316 | 0 |
| 2011 | 298 | 70 |
| 2012 | 294 | 31 |
| 2013 | 249 | 77 |
| 2014 | 235 | 93 |
| 2015 | 136 | 125 |
| 2016 | 128 | 113 (CIP included) |
| 2017 | 128 (CXM and CTR only) | 122 (AZM and SPC only) |
| Year | No. of Isolates | MIC50 | MIC90 | Maximum MIC | % with MIC = 0.125 | *% with MIC = 0.25 | % with MIC >/= 0.5 |
|---|---|---|---|---|---|---|---|
| 2009 | 324 | <0.016 | 0.016 | 0.064 | 0 | 0 | 0 |
| 2010 | 316 | <0.016 | <0.016 | 0.016 | 0 | 0 | 0 |
| 2011 | 297 | <0.016 | <0.016 | 0.016 | 0 | 0 | 0 |
| 2012 | 294 | <0.016 | <0.016 | 0.016 | 0 | 0 | 0 |
| 2013 | 249 | 0.016 | 0.016 | 0.25 | 0 | 0.4 (n = 1) | 0 |
| 2014 | 235 | <0.016 | 0.016 | 0.047 | 0 | 0 | 0 |
| 2015 | 137 | 0.016 | 0.032 | 0.064 | 0 | 0 | 0 |
| 2016 | 128 | <0.016 | 0.016 | 0.016 | 0 | 0 | |
| 2017 | 128 | <0.016 | 0.016 | 0.125 | 0.8 (n = 1) | 0 | 0 |
| Year | No. of Isolates | MIC50 | MIC90 | Maximum MIC | % with MIC = 0.125 | % with MIC = 0.25 | % with MIC >/= 0.5 |
|---|---|---|---|---|---|---|---|
| 2008 | 338 | 0.002 | 0.004 | 0.008 | 0 | 0 | 0 |
| 2009 | 324 | 0.003 | 0.006 | 0.38 | 0 | 0.3 (n = 1) | 0.3 (n = 1) |
| 2010 | 316 | 0.002 | 0.006 | 0.032 | 0 | 0 | 0 |
| 2011 | 298 | 0.003 | 0.004 | 0.012 | 0 | 0 | 0 |
| 2012 | 294 | 0.003 | 0.004 | 0.012 | 0 | 0 | 0 |
| 2013 | 228 | 0.003 | 0.006 | 0.064 | 0 | 0 | 0 |
| 2014 | 235 | 0.004 | 0.008 | 0.047 | 0 | 0 | 0 |
| 2015 | 136 | 0.003 | 0.006 | 0.012 | 0 | 0 | 0 |
| 2016 | 135 | 0.004 | 0.008 | 0.032 | 0 | 0 | 0 |
| 2017 | 128 | 0.003 | 0.008 | 0.032 | 0 | 0 | 0 |
| Year | No. of Isolates | MIC50 | MIC90 | Maximum MIC | % Susceptible MIC </= 0.25 | % Intermediately-Resistant MIC = 0.5 | % Resistant MIC > 0.5 |
|---|---|---|---|---|---|---|---|
| 2008 | 233 | 0.128 | 0.5 | 1 | 86.3 | 9.4 | 4.3 |
| 2011 | 70 | 0.128 | 0.25 | 0.5 | 94.3 | 5.7 | 0 |
| 2012 | 31 | 0.128 | 0.25 | 0.5 | 93.6 | 6.4 | 0 |
| 2013 | 77 | 0.25 | 0.25 | 0.5 | 90.9 | 9.1 | 0 |
| 2014 | 93 | 0.064 | 0.25 | 0.5 | 97.9 | 2.1 | 0 |
| 2015 | 125 | 0.128 | 0.25 | 0.5 | 97.6 | 2.4 | 0 |
| 2016 | 113 | 0.128 | 0.25 | 0.25 | 100 | 0 | 0 |
| 2017 | 122 | 0.064 | 0.25 | 0.5 | 97.5 | 2.5 | 0 |
| Year | Number Tested | MIC50 | MIC90 | Maximum MIC | % Susceptible (MIC < 32) | % Intermediately-Resistant (MIC = 64) | % Resistant (MIC ≥ 128) |
|---|---|---|---|---|---|---|---|
| 2008 | 233 | 16 | 32 | 64 | 99.6 | 0.4 | 0 |
| 2011 | 70 | 16 | 32 | 64 | 98.6 | 1.4 | 0 |
| 2012 | 31 | 16 | 32 | 32 | 100 | 0 | 0 |
| 2013 | 77 | 16 | 32 | 32 | 100 | 0 | 0 |
| 2014 | 93 | 16 | 16 | 32 | 100 | 0 | 0 |
| 2015 | 125 | 32 | 32 | 32 | 100 | 0 | 0 |
| 2016 | 113 | 16 | 16 | 32 | 100 | 0 | 0 |
| 2017 | 122 | 16 | 16 | 32 | 100 | 0 | 0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kularatne, R.; Maseko, V.; Gumede, L.; Kufa, T. Trends in Neisseria gonorrhoeae Antimicrobial Resistance over a Ten-Year Surveillance Period, Johannesburg, South Africa, 2008–2017. Antibiotics 2018, 7, 58. https://doi.org/10.3390/antibiotics7030058
Kularatne R, Maseko V, Gumede L, Kufa T. Trends in Neisseria gonorrhoeae Antimicrobial Resistance over a Ten-Year Surveillance Period, Johannesburg, South Africa, 2008–2017. Antibiotics. 2018; 7(3):58. https://doi.org/10.3390/antibiotics7030058
Chicago/Turabian StyleKularatne, Ranmini, Venessa Maseko, Lindy Gumede, and Tendesayi Kufa. 2018. "Trends in Neisseria gonorrhoeae Antimicrobial Resistance over a Ten-Year Surveillance Period, Johannesburg, South Africa, 2008–2017" Antibiotics 7, no. 3: 58. https://doi.org/10.3390/antibiotics7030058
APA StyleKularatne, R., Maseko, V., Gumede, L., & Kufa, T. (2018). Trends in Neisseria gonorrhoeae Antimicrobial Resistance over a Ten-Year Surveillance Period, Johannesburg, South Africa, 2008–2017. Antibiotics, 7(3), 58. https://doi.org/10.3390/antibiotics7030058





