Tunable Silver-Functionalized Porous Frameworks for Antibacterial Applications
Abstract
1. Introduction
2. Results and Discussion
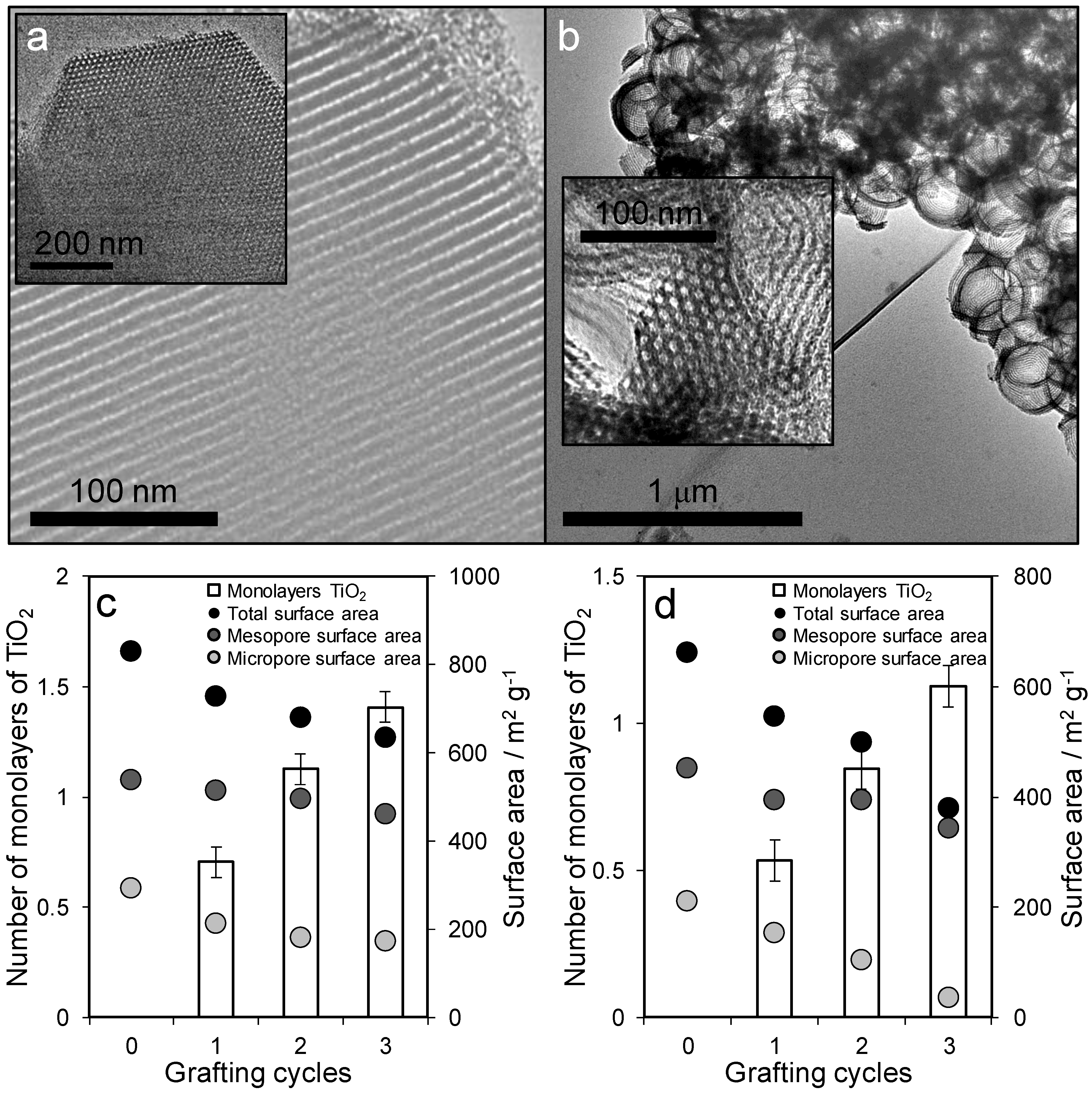
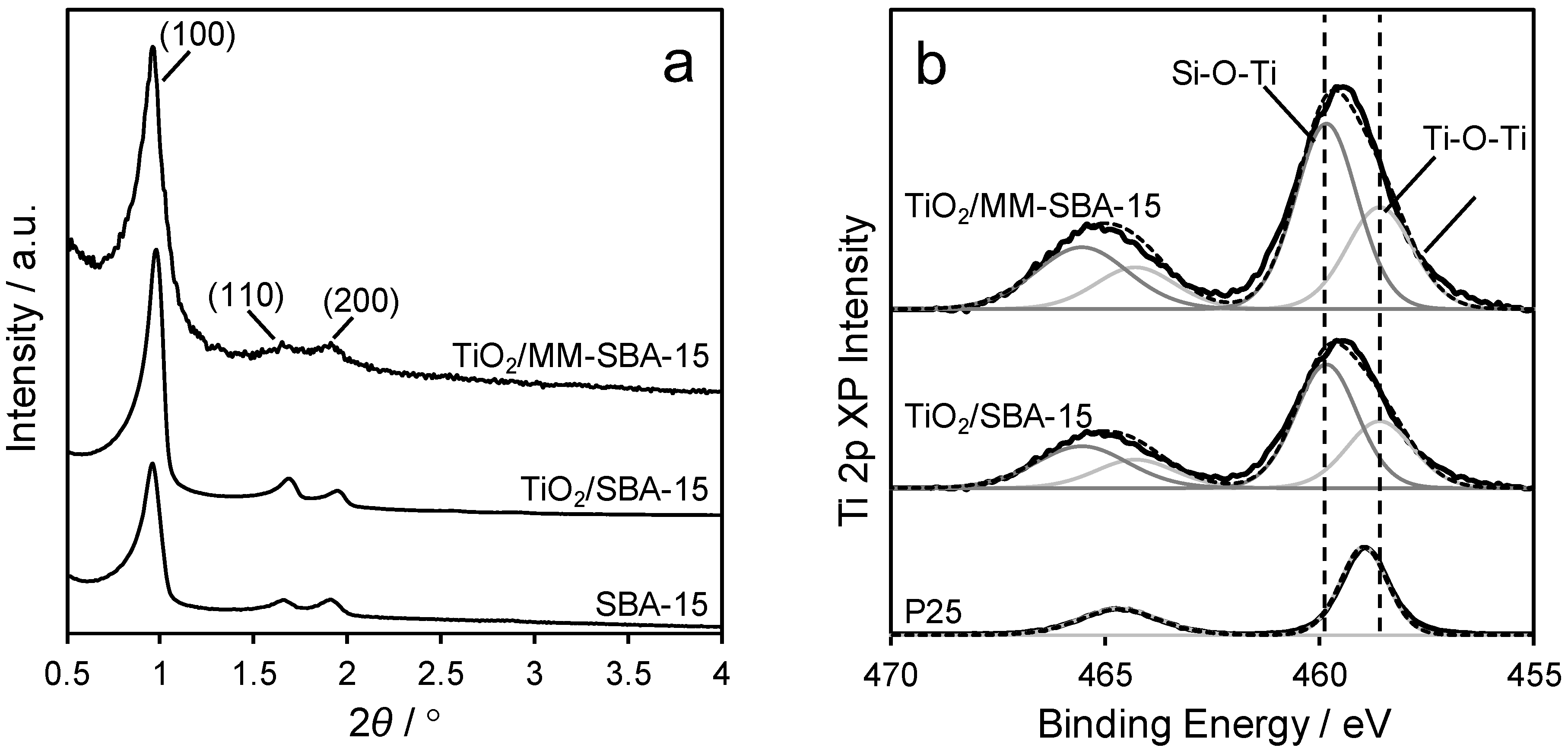
2.1. Materials Synthesis and Characterisation
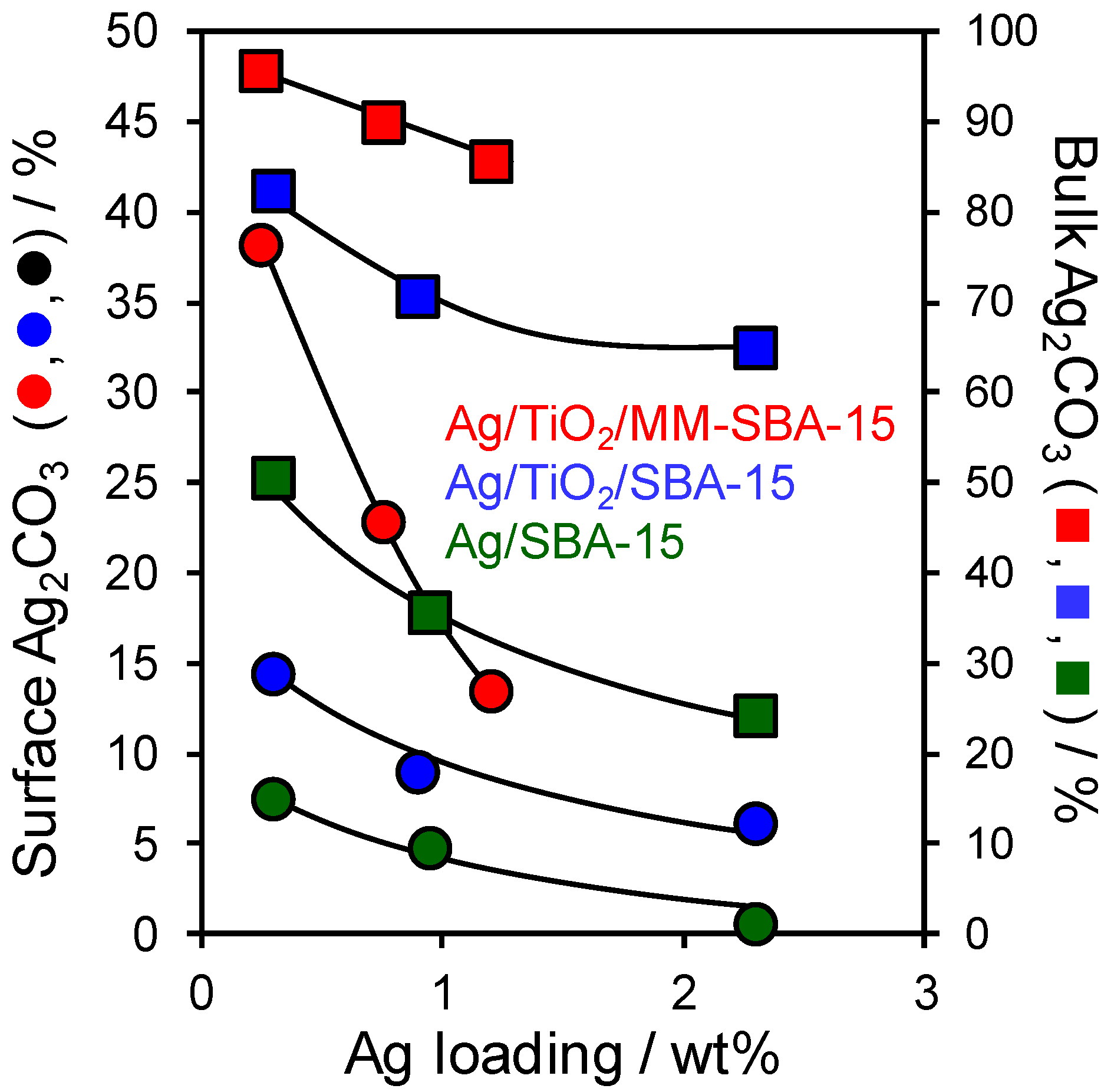
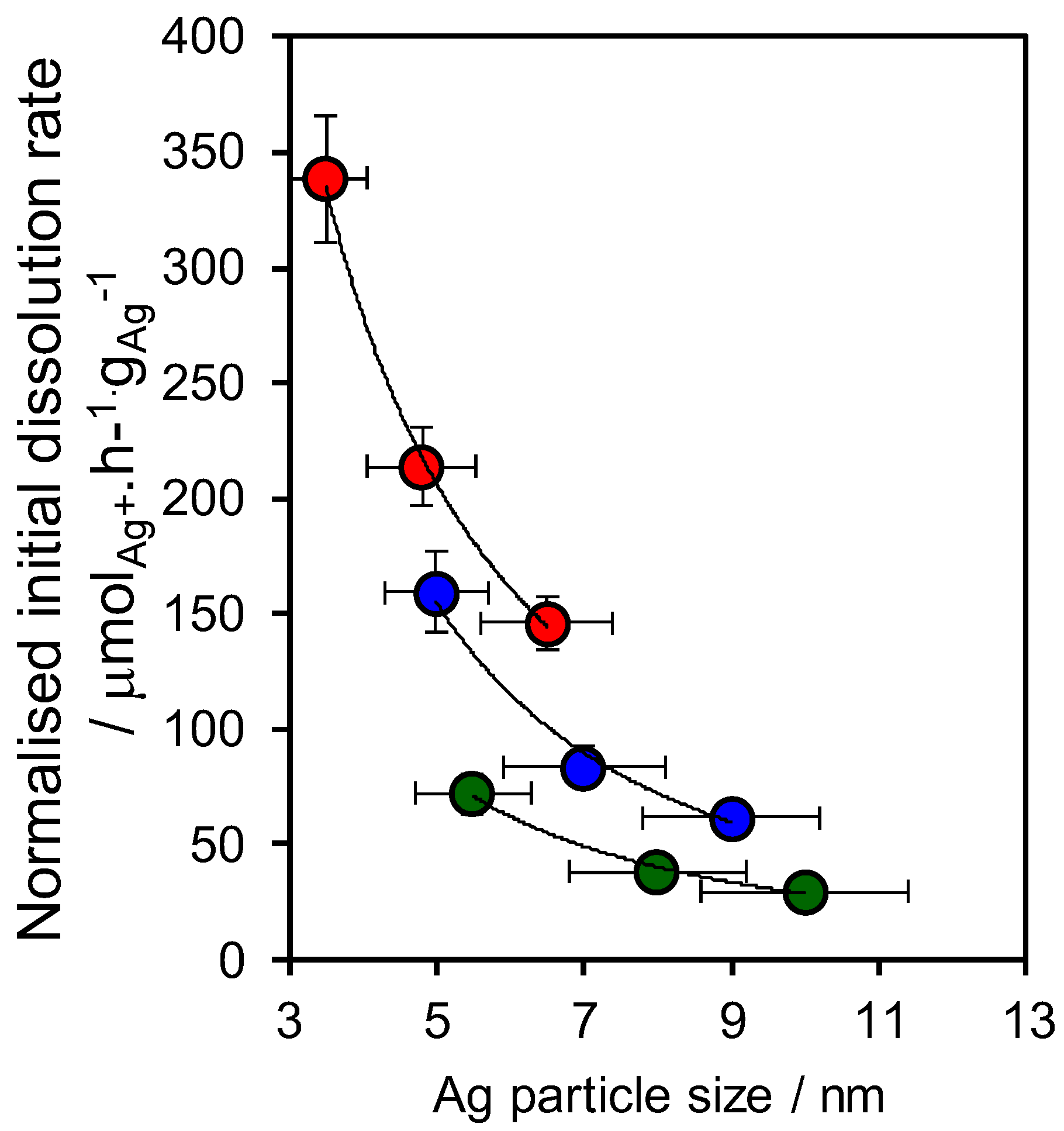
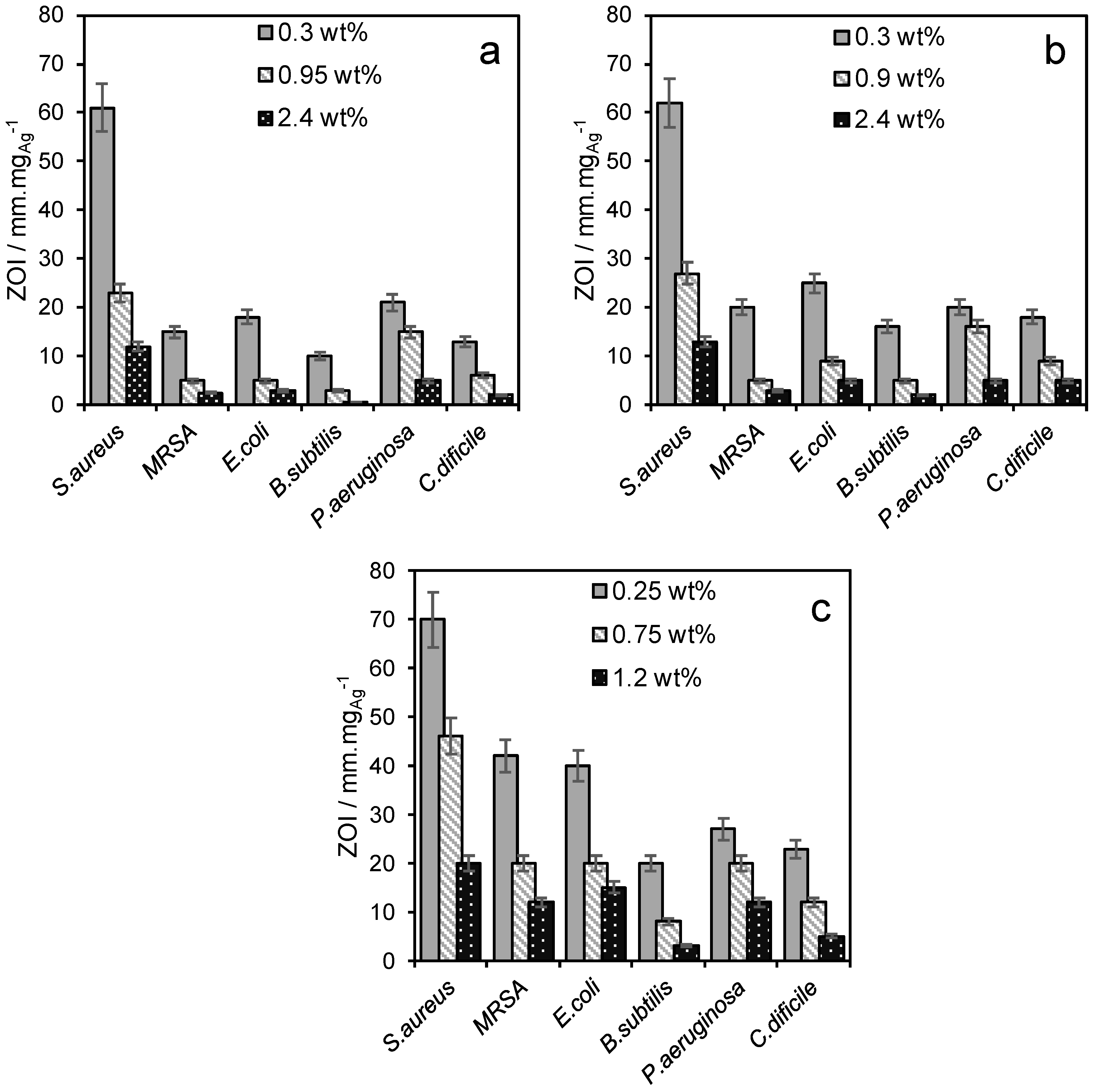
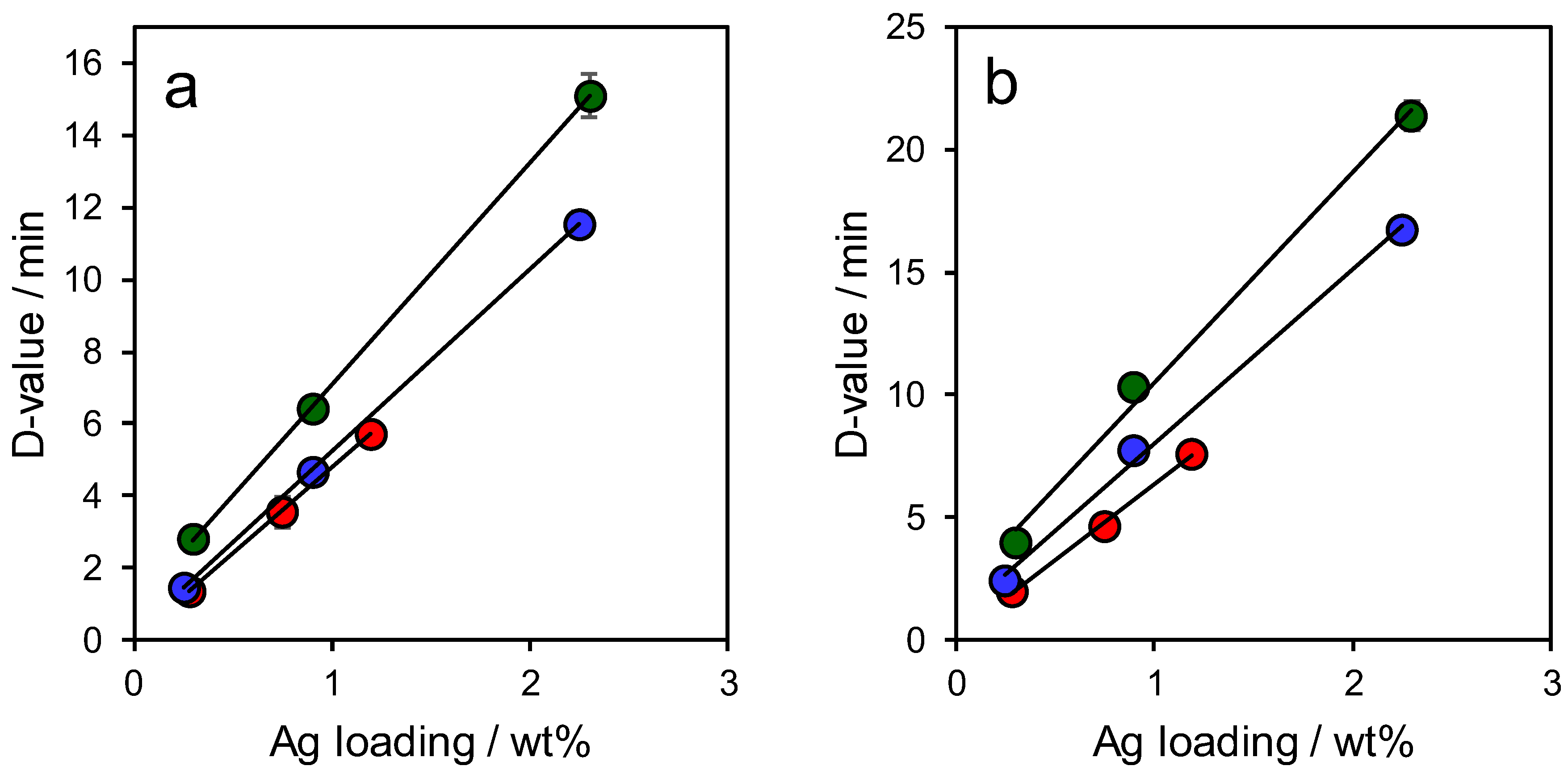
2.2. Materials Performance Assaying
3. Conclusions
4. Materials and Methods
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Zarb, P.; Coignard, B.; Griskeviciene, J.; Muller, A.; Vankerckhoven, V.; Weist, K.; Goossens, M.; Vaerenberg, S.; Hopkins, S.; Catry, B. The European Centre for Disease Prevention and Control (ECDC) pilot point prevalence survey of healthcare-associated infections and antimicrobial use. Eurosurveillance 2012, 17, 20316. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.; Schwab, F.; Zingg, W.; Gastmeier, P. Process and outcome indicators for infection control and prevention in European Acute Care Hospitals in 2011 to 2012-results of the prohibit study. Eurosurveillance 2018, 23, 1700513. [Google Scholar] [CrossRef] [PubMed]
- Cassini, A.; Plachouras, D.; Eckmanns, T.; Sin, M.A.; Blank, H.-P.; Ducomble, T.; Haller, S.; Harder, T.; Klingeberg, A.; Sixtensson, M. Burden of six healthcare-associated infections on european population health: Estimating incidence-based disability-adjusted life years through a population prevalence-based modelling study. PLoS Med. 2016, 13, e1002150. [Google Scholar] [CrossRef] [PubMed]
- Modic, M.; Nikiforov, A.; Leys, C.; Kuchakova, I.; De Vrieze, M.; Petrovska, M.; Zille, A.; Dinescu, G.; Mitu, B.; Cvelbar, U. Atmospheric Pressure Plasma and Depositions of Antibacterial Coatings; Meeting Abstracts; The Electrochemical Society: Pennington, NJ, USA, 2018; p. 1176. [Google Scholar]
- O’Neill, J. Antimicrobial resistance: Tackling a crisis for the health and wealth of nations. Rev. Antimicrob. Resist. 2014, 1–20. Available online: https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf (accessed on 28 June 2018).
- Peleg, A.Y.; Hooper, D.C. Hospital-acquired infections due to gram-negative bacteria. N. Engl. J. Med. 2010, 362, 1804–1813. [Google Scholar] [CrossRef] [PubMed]
- Service, N.H. Clostridium Difficile, Staphylococcus Aureus Bacteraemia and Escherichia coli Bacteraemia, Surveillance Update. Available online: https://www.wales.nhs.uk/sites3/page.cfm?orgid=379&pid=67899 (accessed on 30 May 2018).
- England, P.H. Annual Epidemiological Commentary Mandatory MRSA, MSSA and E. coli Bacteraemia and C. difficile Infection Data 2016/17. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/634675/Annual_epidemiological_commentary_2017.pdf (accessed on 2 July 2018).
- Bhattacharya, A.; Nsonwu, O.; Johnson, A.; Hope, R. Estimating the incidence and 30-day all-cause mortality rate of Escherichia coli bacteraemia in England by 2020/21. J. Hosp. Infect. 2018, 98, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Percival, S.L.; Bowler, P.G.; Dolman, J. Antimicrobial activity of silver-containing dressings on wound microorganisms using an in vitro biofilm model. Int. Wound J. 2007, 4, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Furr, J.R.; Russell, A.D.; Turner, T.D.; Andrews, A. Antibacterial activity of actisorb plus, actisorb and silver nitrate. J. Hosp. Infect. 1994, 27, 201–208. [Google Scholar] [CrossRef]
- Textor, T.; Fouda, M.M.; Mahltig, B. Deposition of durable thin silver layers onto polyamides employing a heterogeneous tollens’ reaction. Appl. Surf. Sci. 2010, 256, 2337–2342. [Google Scholar] [CrossRef]
- Buckley, J.J.; Gai, P.L.; Lee, A.F.; Olivi, L.; Wilson, K. Silver carbonate nanoparticles stabilised over alumina nanoneedles exhibiting potent antibacterial properties. Chem. Commun. 2008, 4013–4015. [Google Scholar] [CrossRef] [PubMed]
- Buckley, J.J.; Lee, A.F.; Olivi, L.; Wilson, K. Hydroxyapatite supported antibacterial Ag3Po4 nanoparticles. J. Mater. Chem. 2010, 20, 8056–8063. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Kim, J.; Oh, J.; Bae, S.; Lee, S.; Hong, I.S.; Kim, S.-H. Ion-release kinetics and ecotoxicity effects of silver nanoparticles. Environ. Toxicol. Chem. 2012, 31, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Chen, J.; Chen, M.; Xu, W.; Zhang, X. Antibacterial activity of silver nanoparticles colloidal sol and its application in package film. Adv. Mater. Res. 2012, 380, 254–259. [Google Scholar] [CrossRef]
- Ray, S.; Mohan, R.; Singh, J.K.; Samantaray, M.K.; Shaikh, M.M.; Panda, D.; Ghosh, P. Anticancer and antimicrobial metallopharmaceutical agents based on palladium, gold, and silver N-Heterocyclic carbene complexes. J. Am. Chem. Soc. 2007, 129, 15042–15053. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Deally, A.; Gleeson, B.; Müller-Bunz, H.; Paradisi, F.; Tacke, M. Novel benzyl-substituted N-Heterocyclic carbene–silver acetate complexes: Synthesis, cytotoxicity and antibacterial studies. Metallomics 2011, 3, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Almalioti, F.; MacDougall, J.; Hughes, S.; Hasson, M.M.; Jenkins, R.L.; Ward, B.D.; Tizzard, G.J.; Coles, S.J.; Williams, D.W.; Bamford, S. Convenient syntheses of cyanuric chloride-derived NHC ligands, their Ag (I) and Au (I) complexes and antimicrobial activity. Dalton Trans. 2013, 42, 12370–12380. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Münstedt, H. Silver ion release from antimicrobial polyamide/silver composites. Biomaterials 2005, 26, 2081–2088. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hurt, R.H. Ion release kinetics and particle persistence in aqueous nano-silver colloids. Environ. Sci. Technol. 2010, 44, 2169–2175. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Xia, Y.; Li, Q.; Ma, X.; Quan, F.; Geng, C.; Han, Z. Microwave-assisted synthesis of silver nanoparticles using sodium alginate and their antibacterial activity. Colloids Surf. A Physicochem. Eng. Asp. 2014, 444, 180–188. [Google Scholar] [CrossRef]
- Zhang, W.; Yao, Y.; Sullivan, N.; Chen, Y. Modeling the primary size effects of citrate-coated silver nanoparticles on their ion release kinetics. Environ. Sci. Technol. 2011, 45, 4422–4428. [Google Scholar] [CrossRef] [PubMed]
- Peretyazhko, T.S.; Zhang, Q.; Colvin, V.L. Size-controlled dissolution of silver nanoparticles at neutral and acidic pH conditions: Kinetics and size changes. Environ. Sci. Technol. 2014, 48, 11954–11961. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Levard, C.; Marinakos, S.M.; Cheng, Y.; Liu, J.; Michel, F.M.; Brown, G.E., Jr.; Lowry, G.V. Size-controlled dissolution of organic-coated silver nanoparticles. Environ. Sci. Technol. 2011, 46, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, M.A.; Durndell, L.J.; Hilton, A.C.; Olivi, L.; Parlett, C.M.; Wilson, K.; Lee, A.F. Tunable Ag@ Sio2 core–shell nanocomposites for broad spectrum antibacterial applications. RSC Adv. 2017, 7, 23342–23347. [Google Scholar] [CrossRef]
- Morones-Ramirez, J.R.; Winkler, J.A.; Spina, C.S.; Collins, J.J. Silver enhances antibiotic activity against gram-negative bacteria. Sci. Transl. Med. 2013, 5, 190ra81. [Google Scholar] [CrossRef] [PubMed]
- Dhainaut, J.; Dacquin, J.-P.; Lee, A.F.; Wilson, K. Hierarchical macroporous-mesoporous SBA-15 sulfonic acid catalysts for biodiesel synthesis. Green Chem. 2010, 12, 296–303. [Google Scholar] [CrossRef]
- Zhao, D.; Huo, Q.; Feng, J.; Chmelka, B.F.; Stucky, G.D. Nonionic triblock and star diblock copolymer and oligomeric surfactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]
- Landau, M.V.; Dafa, E.; Kaliya, M.L.; Sen, T.; Herskowitz, M. Mesoporous alumina catalytic material prepared by grafting wide-pore MCM-41 with an alumina multilayer. Microporous Mesoporous Mater. 2001, 49, 65–81. [Google Scholar] [CrossRef]
- Parlett, C.M.A.; Durndell, L.J.; Machado, A.; Cibin, G.; Bruce, D.W.; Hondow, N.S.; Wilson, K.; Lee, A.F. Alumina-grafted SBA-15 as a high performance support for Pd-catalysed cinnamyl alcohol selective oxidation. Catal. Today 2014, 229, 46–55. [Google Scholar] [CrossRef]
- Guo, X.-C.; Dong, P. Multistep coating of thick titania layers on monodisperse silica nanospheres. Langmuir 1999, 15, 5535–5540. [Google Scholar] [CrossRef]
- NIST. Binding Energies of Ag 3d 5/2. Available online: https://srdata.nist.gov/xps/EngElmSrchQuery.aspx?EType=PE&CSOpt=Retri_ex_dat&Elm=Ag (accessed on 6 July 2017).
- Gaarenstroom, S.W.; Winograd, N. Initial and final state effects in the esca spectra of cadmium and silver oxides. J. Chem. Phys. 1977, 67, 3500–3506. [Google Scholar] [CrossRef]
- Weaver, J.F.; Hoflund, G.B. Surface characterization study of the thermal decomposition of ago. J. Phys. Chem. 1994, 98, 8519–8524. [Google Scholar] [CrossRef]
- Waterhouse, G.I.N.; Bowmaker, G.A.; Metson, J.B. Oxidation of a polycrystalline silver foil by reaction with ozone. Appl. Surf. Sci. 2001, 183, 191–204. [Google Scholar] [CrossRef]
- Nanda, K.; Maisels, A.; Kruis, F.; Fissan, H.; Stappert, S. Higher surface energy of free nanoparticles. Phys. Rev. Lett. 2003, 91, 106102. [Google Scholar] [CrossRef] [PubMed]
- Jiawei, W.; Wenxing, C.; Chuanyi, J.; Lirong, Z.; Juncai, D.; Xusheng, Z.; Yu, W.; Wensheng, Y.; Chen, C.; Qing, P.; et al. Defect effects on TiO2 nanosheets: Stabilizing single atomic site au and promoting catalytic properties. Adv. Mater. 2018, 30, 1705369. [Google Scholar]
- Nolan, M.; Iwaszuk, A.; Lucid, A.K.; Carey, J.J.; Fronzi, M. Design of novel visible light active photocatalyst materials: Surface modified TiO2. Adv. Mater. 2016, 28, 5425–5446. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhou, W.; Xiong, T.; Wang, A.; Chen, S.; Chu, B. Enhanced electrocatalytic activity of Co@N-doped carbon nanotubes by ultrasmall defect-rich TiO2 nanoparticles for hydrogen evolution reaction. Nano Res. 2017, 10, 2599–2609. [Google Scholar] [CrossRef]
- Bhattacharyya, K.; Danon, A.; Vijayan, B.K.; Gray, K.A.; Stair, P.C.; Weitz, E. Role of the surface lewis acid and base sites in the adsorption of CO2 on titania nanotubes and platinized titania nanotubes: An in situ FT-IR study. J. Phys. Chem. C 2013, 117, 12661–12678. [Google Scholar] [CrossRef]
- Xu, C.; Liu, Y.; Huang, B.; Li, H.; Qin, X.; Zhang, X.; Dai, Y. Preparation, characterization, and photocatalytic properties of silver carbonate. Appl. Surf. Sci. 2011, 257, 8732–8736. [Google Scholar] [CrossRef]
- Slager, T.; Lindgren, B.; Mallmann, A.J.; Greenler, R.G. Infrared spectra of the oxides and carbonates of silver. J. Phys. Chem. 1972, 76, 940–943. [Google Scholar] [CrossRef]
- Vaudreuil, S.; Bousmina, M.; Kaliaguine, S.; Bonneviot, L. Synthesis of macrostructured silica by sedimentation–aggregation. Adv. Mater. 2001, 13, 1310–1312. [Google Scholar] [CrossRef]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W3. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isaacs, M.A.; Barbero, B.; Durndell, L.J.; Hilton, A.C.; Olivi, L.; Parlett, C.M.A.; Wilson, K.; Lee, A.F. Tunable Silver-Functionalized Porous Frameworks for Antibacterial Applications. Antibiotics 2018, 7, 55. https://doi.org/10.3390/antibiotics7030055
Isaacs MA, Barbero B, Durndell LJ, Hilton AC, Olivi L, Parlett CMA, Wilson K, Lee AF. Tunable Silver-Functionalized Porous Frameworks for Antibacterial Applications. Antibiotics. 2018; 7(3):55. https://doi.org/10.3390/antibiotics7030055
Chicago/Turabian StyleIsaacs, Mark A., Brunella Barbero, Lee J. Durndell, Anthony C. Hilton, Luca Olivi, Christopher M. A. Parlett, Karen Wilson, and Adam F. Lee. 2018. "Tunable Silver-Functionalized Porous Frameworks for Antibacterial Applications" Antibiotics 7, no. 3: 55. https://doi.org/10.3390/antibiotics7030055
APA StyleIsaacs, M. A., Barbero, B., Durndell, L. J., Hilton, A. C., Olivi, L., Parlett, C. M. A., Wilson, K., & Lee, A. F. (2018). Tunable Silver-Functionalized Porous Frameworks for Antibacterial Applications. Antibiotics, 7(3), 55. https://doi.org/10.3390/antibiotics7030055






