Antibacterial Activity and Toxicity of Analogs of Scorpion Venom IsCT Peptides
Abstract
:1. Introduction
2. Results
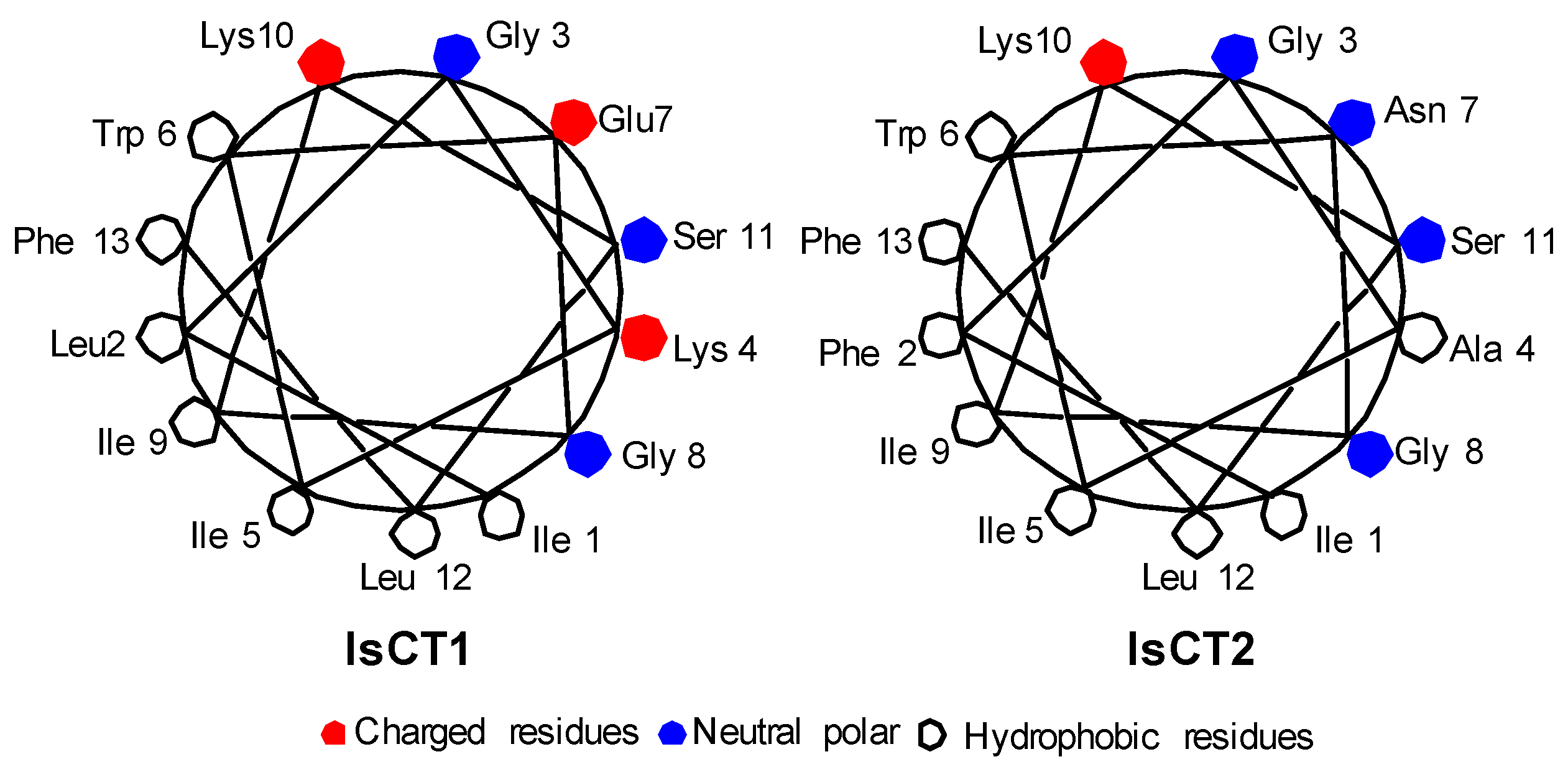
2.1. Peptide Design IsCT
2.2. Secondary Structure
2.3. Antimicrobial Activities
2.4. Hemolytic Activity
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Peptide Synthesis and Purification
4.3. Characterization of Helical Structure
4.4. Bacteria Strains
4.5. Measurement of Antibacterial Activity
4.6. Measurement of Hemolytic Activity
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nhung, N.T.; Cuong, N.V.; Thwaites, G.; Carrique-Mas, J. Antimicrobial usage and antimicrobial resistance in animal production in Southeast Asia: A review. Antibiotics 2016, 5, 37. [Google Scholar] [CrossRef] [PubMed]
- Adegoke, A.A.; Faleye, A.C.; Singh, G.; Stenstrom, T.A. Antibiotic resistant superbugs: Assessment of the interrelationship of occurrence in clinical settings and environmental niches. Molecules 2017, 22, 29. [Google Scholar] [CrossRef] [PubMed]
- Chellat, M.F.; Raguz, L.; Riedl, R. Targeting antibiotic resistance. Angew. Chem. Int. Ed. 2016, 55, 6600–6626. [Google Scholar] [CrossRef] [PubMed]
- Perez-Jorge, C.; Gomez-Barrena, E.; Horcajada, J.-P.; Puig-Verdie, L.; Esteban, J. Drug treatments for prosthetic joint infections in the era of multidrug resistance. Expert Opin. Pharmacother. 2016, 17, 1233–1246. [Google Scholar] [CrossRef] [PubMed]
- Samy, R.P.; Satyanarayanajois, S.; Franco, O.L.; Stiles, B.G.; Gopalakrishnakone, P. Animal venoms as natural sources of antimicrobials. In Antibiotics: Current Innovations and Future Trends; Sánchez, S., Demain, A.L., Eds.; Caister Academic Press: Haverhill, UK, 2015; pp. 229–247. [Google Scholar] [CrossRef]
- Harvey, A.L. Toxins and drug discovery. Toxicon 2014, 92, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Dang, L.; van Damme, E.J.M. Toxic proteins in plants. Phytochemistry 2015, 117, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Mishra, B.; Lau, K.; Lushnikova, T.; Golla, R.; Wang, X. Antimicrobial peptides in 2014. Pharmaceuticals 2015, 8, 123–150. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P.L.; Abdel-Rahman, M.A.; Miller, K.; Strong, P.N. Antimicrobial peptides from scorpion venoms. Toxicon 2014, 88, 115–137. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.L.H.; Wang, H.; Siaw, T.A.; Chapman, M.R.; Waring, A.J.; Kindt, J.T.; Lee, K.Y.C. Mechanism of structural transformations induced by antimicrobial peptides in lipid membranes. Biochim. Biophys. Acta Biomembr. 2012, 1818, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, M.; Ochiai, A.; Takahashi, K.; Nakamichi, S.-I.; Nomoto, T.; Saitoh, E.; Kato, T.; Tanaka, T. Antimicrobial activity and mechanism of action of a novel cationic alpha-helical octadecapeptide derived from alpha-amylase of rice. Biopolymers 2015, 104, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Yasuda, A.; Naoki, H.; Corzo, G.; Andriantsiferana, M.; Nakajima, T. IsCT, a novel cytotoxic linear peptide from scorpion opisthacanthus madagascariensis. Biochem. Biophys. Res. Commun. 2001, 286, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Belokoneva, O.S.; Villegas, E.; Corzo, G.; Dai, L.; Nakajima, T. The hemolytic activity of six arachnid cationic peptides is affected by the phosphatidylcholine-to-sphingomyelin ratio in lipid bilayers. Biochim. Biophys. Acta Biomembr. 2003, 1617, 22–30. [Google Scholar] [CrossRef]
- Lim, S.S.; Kim, Y.; Park, Y.; Kim, J.I.; Park, I.-S.; Hahm, K.-S.; Shin, S.Y. The role of the central L- or D-pro residue on structure and mode of action of a cell-selective alpha-helical IsCT-derived antimicrobial peptide. Biochem. Biophys. Res. Commun. 2005, 334, 1329–1335. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, J.K.; Kathuria, M.; Kumar, A.; Mitra, K.; Ghosh, J.K. An unprecedented alteration in mode of action of IsCT resulting its translocation into bacterial cytoplasm and inhibition of macromolecular syntheses. Sci. Rep. 2015, 5, 9127. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Shin, S.Y.; Kim, K.; Lim, S.S.; Hahm, K.-S.; Kim, Y. Antibiotic activity and structural analysis of the scorpion-derived antimicrobial peptide IsCT and its analogs. Biochem. Biophys. Res. Commun. 2004, 323, 712–719. [Google Scholar] [CrossRef] [PubMed]
- De la Salud Bea, R.; Petraglia, A.F.; de Johnson, L.E.L. Synthesis, antimicrobial activity and toxicity of analogs of the scorpion venom BmKn peptides. Toxicon 2015, 101, 79–84. [Google Scholar] [CrossRef]
- Lopez-Llano, J.; Campos, L.A.; Sancho, J. Alpha-helix stabilization by alanine relative to glycine: Roles of polar and apolar solvent exposures and of backbone entropy. Proteins Struct. Funct. Bioinform. 2006, 64, 769–778. [Google Scholar] [CrossRef] [PubMed]
- De la Salud Bea, R.; Ascuitto, M.R.; de Johnson, L.E.L. Synthesis of analogs of peptides from Buthus martensii scorpion venom with potential antibiotic activity. Peptides 2015, 68, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; He, L.; Li, G.; Zhai, N.; Jiang, H.; Chen, Y. Role of helicity of alpha-helical antimicrobial peptides to improve specificity. Protein Cell 2014, 5, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Corzo, G.; Naoki, H.; Andriantsiferana, M.; Nakajima, T. Purification, structure-function analysis, and molecular characterization of novel linear peptides from scorpion Opisthacanthus madagascariensis. Biochem. Biophys. Res. Commun. 2002, 293, 1514–1522. [Google Scholar] [CrossRef]

| Peptide | Amino Acid Sequence a | Molecular Mass | HPLC Retention Times | Net Charge | |
|---|---|---|---|---|---|
| Calculated | Observed | (min.) | |||
| IsCT1 | ILGKIWEGIKSLF-NH2 | 1502.9 | 1504.2 | 22.7 | +1 |
| IsCT2 | IFGAIWNGIKSLF-NH2 | 1464.8 | 1466.0 | 20.5 | +1 |
| IsCT1A1 | ILGKAWEGAKSLF-NH2 | 1418.7 | 1419.8 | 17.5 | +1 |
| IsCT1V1 | ILGKVWEGVKSLF-NH2 | 1474.8 | 1476.0 | 19.0 | +1 |
| IsCT1L1 | ILGKLWEGLKSLF-NH2 | 1502.9 | 1503.8 | 20.6 | +1 |
| IsCT1K7 | ILGKIWKGIKSLF-NH2 | 1501.9 | 1502.1 | 20.0 | +3 |
| IsCT1E7 | ILGKIWEGIESLF-NH2 | 1503.8 | 1505.0 | 22.0 | −1 |
| IsCT2A1 | ILGAAWNGAKSLF-NH2 | 1380.6 | 1380.8 | 19.3 | +1 |
| IsCT2V1 | ILGAVWNGVKSLF-NH2 | 1436.7 | 1437.1 | 20.5 | +1 |
| Peptides | Water | 50% TFE | ||
|---|---|---|---|---|
| [θ]222 | % Helix | [θ]222 | % Helix | |
| IsCT1 | −2046.57 | Random | −11,484.85 | 30.2 |
| IsCT2 | −1242.43 | Random | −9006.83 | 22.0 |
| IsCT1A1 | −1408.15 | Random | −6602.05 | 14.1 |
| IsCT1V1 | −1722.88 | Random | −8803.10 | 21.3 |
| IsCT1L1 | −1855.45 | Random | −10,500.37 | 27.0 |
| IsCT1K7 | −1520.41 | Random | −9533.00 | 23.8 |
| IsCT1E7 | −2561.86 | 1.0 | −12,183.56 | 33.0 |
| IsCT2A1 | −89.92 | Random | −3633.66 | 4.3 |
| IsCT2V1 | −793.14 | Random | −6586.81 | 14.0 |
| MIC (μg/mL) | |||||
|---|---|---|---|---|---|
| S. aureus | B. cereus | S. typhimurium | E. aerogenes | E. coli | |
| IsCT1 | 50 | >100 | 100 | >100 | 50 |
| IsCT2 | 50 | 100 | 100 | >100 | 50 |
| IsCT1A1 | >100 | >100 | >100 | >100 | >100 |
| IsCT1V1 | >100 | >100 | >100 | >100 | >100 |
| IsCT1L1 | 50 | >100 | 100 | >100 | 50 |
| IsCT1K7 | 100 | >100 | 100 | >100 | >100 |
| IsCT1E7 | >100 | >100 | >100 | >100 | >100 |
| IsCT2A1 | >100 | >100 | >100 | >100 | >100 |
| IsCT2V1 | >100 | >100 | >100 | >100 | >100 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De la Salud Bea, R.; Petraglia, A.F.; Ascuitto, M.R.; Buck, Q.M. Antibacterial Activity and Toxicity of Analogs of Scorpion Venom IsCT Peptides. Antibiotics 2017, 6, 13. https://doi.org/10.3390/antibiotics6030013
De la Salud Bea R, Petraglia AF, Ascuitto MR, Buck QM. Antibacterial Activity and Toxicity of Analogs of Scorpion Venom IsCT Peptides. Antibiotics. 2017; 6(3):13. https://doi.org/10.3390/antibiotics6030013
Chicago/Turabian StyleDe la Salud Bea, Roberto, Adam F. Petraglia, Michael R. Ascuitto, and Quentin M. Buck. 2017. "Antibacterial Activity and Toxicity of Analogs of Scorpion Venom IsCT Peptides" Antibiotics 6, no. 3: 13. https://doi.org/10.3390/antibiotics6030013
APA StyleDe la Salud Bea, R., Petraglia, A. F., Ascuitto, M. R., & Buck, Q. M. (2017). Antibacterial Activity and Toxicity of Analogs of Scorpion Venom IsCT Peptides. Antibiotics, 6(3), 13. https://doi.org/10.3390/antibiotics6030013





