Resistance to β-Lactams in Neisseria ssp Due to Chromosomally Encoded Penicillin-Binding Proteins
Abstract
:Acknowledgments
Conflicts of Interest
References
- Cole, M.J.; Unemo, M.; Grigorjev, V.; Quaye, N.; Woodford, N. Genetic diversity of blaTEM alleles, antimicrobial susceptibility and molecular epidemiological characteristics of penicillinase-producing Neisseria gonorrhoeae from England and Wales. J. Antimicrob. Chemother. 2015, 70, 3238–3243. [Google Scholar] [PubMed]
- Muhammad, I.; Golparian, D.; Dillon, J.A.; Johansson, A.; Ohnishi, M.; Sethi, S.; Chen, S.C.; Nakayama, S.; Sundqvist, M.; Bala, M.; et al. Characterisation of blaTEM genes and types of beta-lactamase plasmids in Neisseria gonorrhoeae—The prevalent and conserved blaTEM-135 has not recently evolved and existed in the Toronto plasmid from the origin. BMC Infect. Dis. 2014. [Google Scholar] [CrossRef] [PubMed]
- Pagotto, F.; Aman, A.T.; Ng, L.K.; Yeung, K.H.; Brett, M.; Dillon, J.A. Sequence analysis of the family of penicillinase-producing plasmids of Neisseria gonorrhoeae. Plasmid 2000, 43, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Backman, A.; Orvelid, P.; Vazquez, J.A.; Skold, O.; Olcen, P. Complete sequence of a beta-lactamase-encoding plasmid in Neisseria meningitidis. Antimicrob. Agents Chemother. 2000, 44, 210–212. [Google Scholar] [CrossRef] [PubMed]
- Olesky, M.; Zhao, S.; Rosenberg, R.L.; Nicholas, R.A. Porin-mediated antibiotic resistance in Neisseria gonorrhoeae: Ion, solute, and antibiotic permeation through PIB proteins with penB mutations. J. Bacteriol. 2006, 188, 2300–2308. [Google Scholar] [CrossRef] [PubMed]
- Sauvage, E.; Kerff, F.; Terrak, M.; Ayala, J.A.; Charlier, P. The Penicillin-Binding Proteins: Structure and role in peptidoglycan biosynthesis. FEMS Microbiol. Rev. 2008, 32, 234–258. [Google Scholar] [CrossRef] [PubMed]
- Tipper, D.J.; Strominger, J.L. Mechanism of action of penicillins: A proposal based on their structural similarity to acyl-d-alanyl-d-alanine. Proc. Natl. Acad. Sci. USA 1965, 54, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Stefanova, M.E.; Tomberg, J.; Davies, C.; Nicholas, R.A.; Gutheil, W.G. Overexpression and enzymatic characterization of Neisseria gonorrhoeae Penicillin-Binding Protein 4. Eur. J. Biochem. 2004, 271, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Hagman, M.; Forslin, L.; Moi, H.; Danielsson, D. Neisseria meningitidis in specimens from urogenital sites. Is increased awareness necessary? Sex. Transm. Dis. 1991, 18, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Maiden, M.C. Population genetics of a transformable bacterium: The influence of horizontal genetic exchange on the biology of Neisseria meningitidis. FEMS Microbiol. Lett. 1993, 112, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Harrison, L.H.; Pelton, S.I.; Wilder-Smith, A.; Holst, J.; Safadi, M.A.; Vazquez, J.A.; Taha, M.K.; LaForce, F.M.; von Gottberg, A.; Borrow, R.; et al. The global meningococcal initiative: Recommendations for reducing the global burden of meningococcal disease. Vaccine 2011, 29, 3363–3371. [Google Scholar] [CrossRef] [PubMed]
- Jafri, R.Z.; Ali, A.; Messonnier, N.E.; Tevi-Benissan, C.; Durrheim, D.; Eskola, J.; Fermon, F.; Klugman, K.P.; Ramsay, M.; Sow, S.; et al. Global epidemiology of invasive meningococcal disease. Popul. Health Metr. 2013. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Surveillance of Invasive Bacterial Diseases in Europe, 2012; ECDC: Stockholm, Sweden, 2015. [Google Scholar]
- World Health Organization. Global Incidence and Prevalence of Selected Curable Sexually Transmitted Infections—2008; WHO: Geneva, Switzerland, 2012. [Google Scholar]
- Unemo, M.; Shafer, W.M. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: Past, evolution, and future. Clin. Microbiol. Rev. 2014, 27, 587–613. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, T.J.; Koller, A.E.; Tomasz, A. Penicillin-Binding Proteins of penicillin-susceptible and intrinsically resistant Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 1980, 18, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Mendelman, P.M.; Campos, J.; Chaffin, D.O.; Serfass, D.A.; Smith, A.L.; Saez-Nieto, J.A. Relative penicillin G resistance in Neisseria meningitidis and reduced affinity of Penicillin-Binding Protein 3. Antimicrob. Agents Chemother. 1988, 32, 706–709. [Google Scholar] [CrossRef] [PubMed]
- Antignac, A.; Boneca, I.G.; Rousselle, J.C.; Namane, A.; Carlier, J.P.; Vazquez, J.A.; Fox, A.; Alonso, J.M.; Taha, M.K. Correlation between alterations of the Penicillin-Binding Protein 2 and modifications of the peptidoglycan structure in Neisseria meningitidis with reduced susceptibility to penicillin G. J. Biol. Chem. 2003, 278, 31529–31535. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.K.; Vazquez, J.A.; Hong, E.; Bennett, D.E.; Bertrand, S.; Bukovski, S.; Cafferkey, M.T.; Carion, F.; Christensen, J.J.; Diggle, M.; et al. Target gene sequencing to characterize the penicillin G susceptibility of Neisseria meningitidis. Antimicrob. Agents Chemother. 2007, 51, 2784–2792. [Google Scholar] [CrossRef] [PubMed]
- Thulin, S.; Olcen, P.; Fredlund, H.; Unemo, M. Total variation in the penA gene of Neisseria meningitidis: Correlation between susceptibility to beta-lactam antibiotics and penA gene heterogeneity. Antimicrob. Agents Chemother. 2006, 50, 3317–3324. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Deguchi, T.; Mizutani, K.S.; Yasuda, M.; Yokoi, S.; Ito, S.; Takahashi, Y.; Ishihara, S.; Kawamura, Y.; Ezaki, T. Emergence and spread of Neisseria gonorrhoeae clinical isolates harboring mosaic-like structure of Penicillin-Binding Protein 2 in central Japan. Antimicrob. Agents Chemother. 2005, 49, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Takahata, S.; Senju, N.; Osaki, Y.; Yoshida, T.; Ida, T. Amino acid substitutions in mosaic Penicillin-Binding Protein 2 associated with reduced susceptibility to cefixime in clinical isolates of Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 2006, 50, 3638–3645. [Google Scholar] [CrossRef] [PubMed]
- Whiley, D.M.; Limnios, E.A.; Ray, S.; Sloots, T.P.; Tapsall, J.W. Diversity of penA alterations and subtypes in Neisseria gonorrhoeae strains from Sydney, Australia, that are less susceptible to ceftriaxone. Antimicrob. Agents Chemother. 2007, 51, 3111–3116. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, R.; Fredlund, H.; Nicholas, R.; Unemo, M. Neisseria gonorrhoeae isolates with reduced susceptibility to cefixime and ceftriaxone: Association with genetic polymorphisms in penA, mtrR, porB1b, and ponA. Antimicrob. Agents Chemother. 2007, 51, 2117–2122. [Google Scholar] [CrossRef] [PubMed]
- Pandori, M.; Barry, P.M.; Wu, A.; Ren, A.; Whittington, W.L.; Liska, S.; Klausner, J.D. Mosaic Penicillin-Binding Protein 2 in Neisseria gonorrhoeae isolates collected in 2008 in San Francisco, California. Antimicrob. Agents Chemother. 2009, 53, 4032–4034. [Google Scholar] [CrossRef] [PubMed]
- Allen, V.G.; Farrell, D.J.; Rebbapragada, A.; Tan, J.; Tijet, N.; Perusini, S.J.; Towns, L.; Lo, S.; Low, D.E.; Melano, R.G. Molecular analysis of antimicrobial resistance mechanisms in Neisseria gonorrhoeae isolates from Ontario, Canada. Antimicrob. Agents Chemother. 2011, 55, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Gu, W.M.; Yang, Y.; Dillon, J.A. Analysis of mutations in multiple loci of Neisseria gonorrhoeae isolates reveals effects of pib, pbp2 and mtrR on reduced susceptibility to ceftriaxone. J. Antimicrob. Chemother. 2011, 66, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Camara, J.; Serra, J.; Ayats, J.; Bastida, T.; Carnicer-Pont, D.; Andreu, A.; Ardanuy, C. Molecular characterization of two high-level ceftriaxone-resistant Neisseria gonorrhoeae isolates detected in Catalonia, Spain. J. Antimicrob. Chemother. 2012, 67, 1858–1860. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, S.I.; Shimuta, K.; Furubayashi, K.I.; Kawahata, T.; Unemo, M.; Ohnishi, M. New ceftriaxone- and multidrug-resistant Neisseria gonorrhoeae strain with a novel mosaic penA gene isolated in Japan. Antimicrob. Agents Chemother. 2016. [Google Scholar] [CrossRef] [PubMed]
- Heymans, R.; Bruisten, S.M.; Golparian, D.; Unemo, M.; de Vries, H.J.; van Dam, A.P. Clonally related Neisseria gonorrhoeae isolates with decreased susceptibility to the extended-spectrum cephalosporin cefotaxime in Amsterdam, the Netherlands. Antimicrob. Agents Chemother. 2012, 56, 1516–1522. [Google Scholar] [CrossRef] [PubMed]
- Spratt, B.G. Hybrid Penicillin-Binding Proteins in penicillin-resistant strains of Neisseria gonorrhoeae. Nature 1988, 332, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Brannigan, J.A.; Tirodimos, I.A.; Zhang, Q.Y.; Dowson, C.G.; Spratt, B.G. Insertion of an extra amino acid is the main cause of the low affinity of Penicillin-Binding Protein 2 in penicillin-resistant strains of Neisseria gonorrhoeae. Mol. Microbiol. 1990, 4, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Fedarovich, A.; Cook, E.; Tomberg, J.; Nicholas, R.A.; Davies, C. Structural effect of the Asp345a insertion in Penicillin-Binding Protein 2 from penicillin-resistant strains of Neisseria gonorrhoeae. Biochemistry 2014, 53, 7596–7603. [Google Scholar] [CrossRef] [PubMed]
- Bharat, A.; Demczuk, W.; Martin, I.; Mulvey, M.R. Effect of variants of Penicillin-Binding Protein 2 on cephalo–5006. sporin and carbapenem susceptibilities in Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 2015. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Lai, W.; Liu, M.; Hua, Z.; Sun, Y.; Xu, Q.; Xia, Y.; Zhao, Y.; Xie, X. Novel genes related to ceftriaxone resistance found among ceftriaxone-resistant Neisseria gonorrhoeae strains selected in vitro. Antimicrob. Agents Chemother. 2016, 60, 2043–2051. [Google Scholar] [CrossRef] [PubMed]
- Tomberg, J.; Unemo, M.; Davies, C.; Nicholas, R.A. Molecular and structural analysis of mosaic variants of Penicillin-Binding Protein 2 conferring decreased susceptibility to expanded-spectrum cephalosporins in Neisseria gonorrhoeae: Role of epistatic mutations. Biochemistry 2010, 49, 8062–8070. [Google Scholar] [CrossRef] [PubMed]
- Unemo, M.; Golparian, D.; Nicholas, R.; Ohnishi, M.; Gallay, A.; Sednaoui, P. High-level cefixime- and ceftriaxone-resistant Neisseria gonorrhoeae in France: Novel penA mosaic allele in a successful international clone causes treatment failure. Antimicrob. Agents Chemother. 2012, 56, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.R.; Grad, Y.; Ganakammal, S.R.; Burroughs, M.; Frace, M.; Lipsitch, M.; Weil, R.; Trees, D. In vitro selection of Neisseria gonorrhoeae mutants with elevated MIC values and increased resistance to cephalosporins. Antimicrob. Agents Chemother. 2014, 58, 6986–6989. [Google Scholar] [CrossRef] [PubMed]
- Powell, A.J.; Tomberg, J.; Deacon, A.M.; Nicholas, R.A.; Davies, C. Crystal structures of Penicillin-Binding Protein 2 from penicillin-susceptible and -resistant strains of Neisseria gonorrhoeae reveal an unexpectedly subtle mechanism for antibiotic resistance. J. Biol. Chem. 2009, 284, 1202–1212. [Google Scholar] [CrossRef] [PubMed]
- Whiley, D.M.; Goire, N.; Lambert, S.B.; Ray, S.; Limnios, E.A.; Nissen, M.D.; Sloots, T.P.; Tapsall, J.W. Reduced susceptibility to ceftriaxone in Neisseria gonorrhoeae is associated with mutations G542S, P551S and P551L in the gonococcal Penicillin-Binding Protein 2. J. Antimic. Chemother. 2010, 65, 1615–1618. [Google Scholar] [CrossRef] [PubMed]
- Fussenegger, M.; Rudel, T.; Barten, R.; Ryll, R.; Meyer, T.F. Transformation competence and type-4 pilus biogenesis in Neisseria gonorrhoeae—A review. Gene 1997, 192, 125–134. [Google Scholar] [CrossRef]
- Dowson, C.G.; Hutchison, A.; Brannigan, J.A.; George, R.C.; Hansman, D.; Linares, J.; Tomasz, A.; Smith, J.M.; Spratt, B.G. Horizontal transfer of penicillin-binding protein genes in penicillin-resistant clinical isolates of Streptococcus pneumoniae. Proc. Natl. Acad. Sci. USA 1989, 86, 8842–8846. [Google Scholar] [CrossRef] [PubMed]
- Zapun, A.; Contreras-Martel, C.; Vernet, T. Penicillin-Binding Proteins and beta-lactam resistance. FEMS Microbiol. Rev. 2008, 32, 361–385. [Google Scholar] [CrossRef] [PubMed]
- Spratt, B.G.; Zhang, Q.Y.; Jones, D.M.; Hutchison, A.; Brannigan, J.A.; Dowson, C.G. Recruitment of a Penicillin-Binding Protein gene from Neisseria flavescens during the emergence of penicillin resistance in Neisseria meningitidis. Proc. Natl. Acad. Sci. USA 1989, 86, 8988–8992. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, M.; Watanabe, Y.; Ono, E.; Takahashi, C.; Oya, H.; Kuroki, T.; Shimuta, K.; Okazaki, N.; Nakayama, S.; Watanabe, H. Spread of a chromosomal cefixime-resistant penA gene among different Neisseria gonorrhoeae lineages. Antimicrob. Agents Chemother. 2010, 54, 1060–1067. [Google Scholar] [CrossRef] [PubMed]
- Bowler, L.D.; Zhang, Q.Y.; Riou, J.Y.; Spratt, B.G. Interspecies recombination between the penA genes of Neisseria meningitidis and commensal Neisseria species during the emergence of penicillin resistance in N. meningitidis: Natural events and laboratory simulation. J. Bacteriol. 1994, 176, 333–337. [Google Scholar] [PubMed]
- Spratt, B.G.; Bowler, L.D.; Zhang, Q.Y.; Zhou, J.; Smith, J.M. Role of interspecies transfer of chromosomal genes in the evolution of penicillin resistance in pathogenic and commensal Neisseria species. J. Mol. Evol. 1992, 34, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Ameyama, S.; Onodera, S.; Takahata, M.; Minami, S.; Maki, N.; Endo, K.; Goto, H.; Suzuki, H.; Oishi, Y. Mosaic-like structure of Penicillin-Binding Protein 2 gene (penA) in clinical isolates of Neisseria gonorrhoeae with reduced susceptibility to cefixime. Antimicrob. Agents Chemother. 2002, 46, 3744–3749. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.G.; Lee, H.; Jeong, S.H.; Yong, D.; Chung, G.T.; Lee, Y.S.; Chong, Y.; Lee, K. Various penA mutations together with mtrR, porB and ponA mutations in Neisseria gonorrhoeae isolates with reduced susceptibility to cefixime or ceftriaxone. J. Antimicrob. Chemother. 2010, 65, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Karch, A.; Vogel, U.; Claus, H. Role of penA polymorphisms for penicillin susceptibility in Neisseria lactamica and Neisseria meningitidis. Int. J. Med. Microbiol. 2015, 305, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Tomberg, J.; Temple, B.; Fedarovich, A.; Davies, C.; Nicholas, R.A. A highly conserved interaction involving the middle residue of the SXN active-site motif is crucial for function of class B Penicillin-Binding Proteins: Mutational and computational analysis of PBP2 from N. gonorrhoeae. Biochemistry 2012, 51, 2775–2784. [Google Scholar] [CrossRef] [PubMed]
- Carapito, R.; Chesnel, L.; Vernet, T.; Zapun, A. Pneumococcal beta-lactam resistance due to a conformational change in Penicillin-Binding Protein 2x. J. Biol. Chem. 2006, 281, 1771–1777. [Google Scholar] [CrossRef] [PubMed]
- Osaka, K.; Takakura, T.; Narukawa, K.; Takahata, M.; Endo, K.; Kiyota, H.; Onodera, S. Analysis of amino acid sequences of Penicillin-Binding Protein 2 in clinical isolates of Neisseria gonorrhoeae with reduced susceptibility to cefixime and ceftriaxone. J. Infect. Chemother. 2008, 14, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Chesnel, L.; Pernot, L.; Lemaire, D.; Champelovier, D.; Croize, J.; Dideberg, O.; Vernet, T.; Zapun, A. The structural modifications induced by the M339F substitution in PBP2x from Streptococcus pneumoniae further decreases the susceptibility to beta-lactams of resistant strains. J. Biol. Chem. 2003, 278, 44448–44456. [Google Scholar] [CrossRef] [PubMed]
- Tomberg, J.; Unemo, M.; Ohnishi, M.; Davies, C.; Nicholas, R.A. Identification of amino acids conferring high-level resistance to expanded-spectrum cephalosporins in the penA gene from Neisseria gonorrhoeae strain H041. Antimicrob. Agents Chemother. 2013, 57, 3029–3036. [Google Scholar] [CrossRef] [PubMed]
- Lahra, M.M.; Ryder, N.; Whiley, D.M. A new multidrug-resistant strain of Neisseria gonorrhoeae in Australia. N. Engl. J. Med. 2014, 371, 1850–1851. [Google Scholar] [CrossRef] [PubMed]
- Antignac, A.; Ducos-Galand, M.; Guiyoule, A.; Pires, R.; Alonso, J.M.; Taha, M.K. Neisseria meningitidis strains isolated from invasive infections in France (1999–2002): Phenotypes and antibiotic susceptibility patterns. Clin. Infect. Dis. 2003, 37, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Nabu, S.; Nantasenamat, C.; Owasirikul, W.; Lawung, R.; Isarankura-Na-Ayudhya, C.; Lapins, M.; Wikberg, J.E.; Prachayasittikul, V. Proteochemometric model for predicting the inhibition of Penicillin-Binding Proteins. J. Comput. Aided Mol. Des. 2015, 29, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Lujan, R.; Zhang, Q.Y.; Saez Nieto, J.A.; Jones, D.M.; Spratt, B.G. Penicillin-resistant isolates of Neisseria lactamica produce altered forms of Penicillin-Binding Protein 2 that arose by interspecies horizontal gene transfer. Antimicrob. Agents Chemother. 1991, 35, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Saez-Nieto, J.A.; Lujan, R.; Martinez-Suarez, J.V.; Berron, S.; Vazquez, J.A.; Vinas, M.; Campos, J. Neisseria lactamica and Neisseria polysaccharea as possible sources of meningococcal beta-lactam resistance by genetic transformation. Antimicrob. Agents Chemother. 1990, 34, 2269–2272. [Google Scholar] [CrossRef] [PubMed]
- Maiden, M.C.; Malorny, B.; Achtman, M. A global gene pool in the Neisseriae. Mol. Microbiol. 1996, 21, 1297–1298. [Google Scholar] [CrossRef] [PubMed]
- Reichmann, P.; Konig, A.; Linares, J.; Alcaide, F.; Tenover, F.C.; McDougal, L.; Swidsinski, S.; Hakenbeck, R. A global gene pool for high-level cephalosporin resistance in commensal Streptococcus species and Streptococcus pneumoniae. J. Infect. Dis. 1997, 176, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Ropp, P.A.; Hu, M.; Olesky, M.; Nicholas, R.A. Mutations in ponA, the gene encoding Penicillin-Binding Protein 1, and a novel locus, penC, are required for high-level chromosomally mediated penicillin resistance in Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 2002, 46, 769–777. [Google Scholar] [CrossRef] [PubMed]
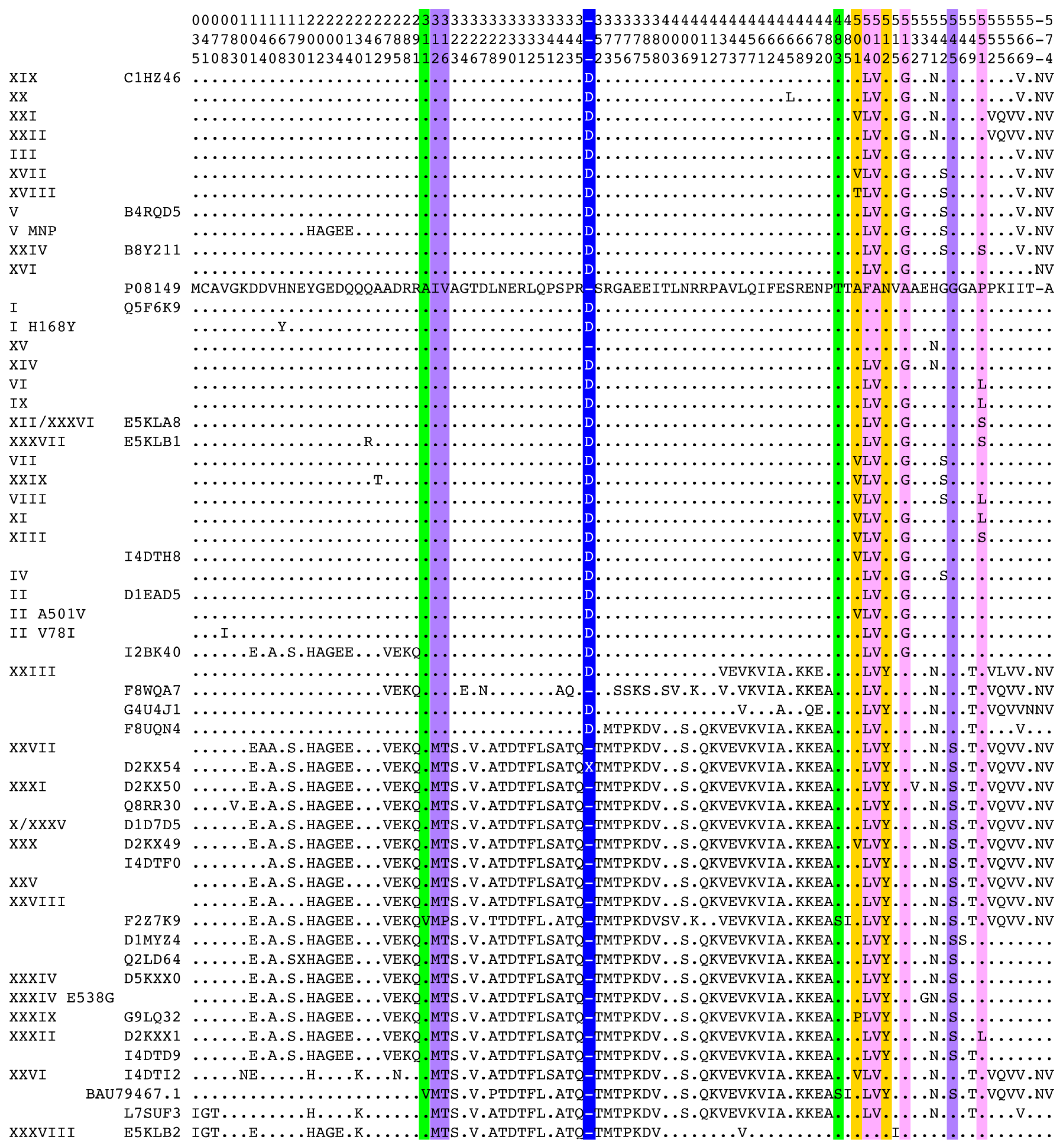
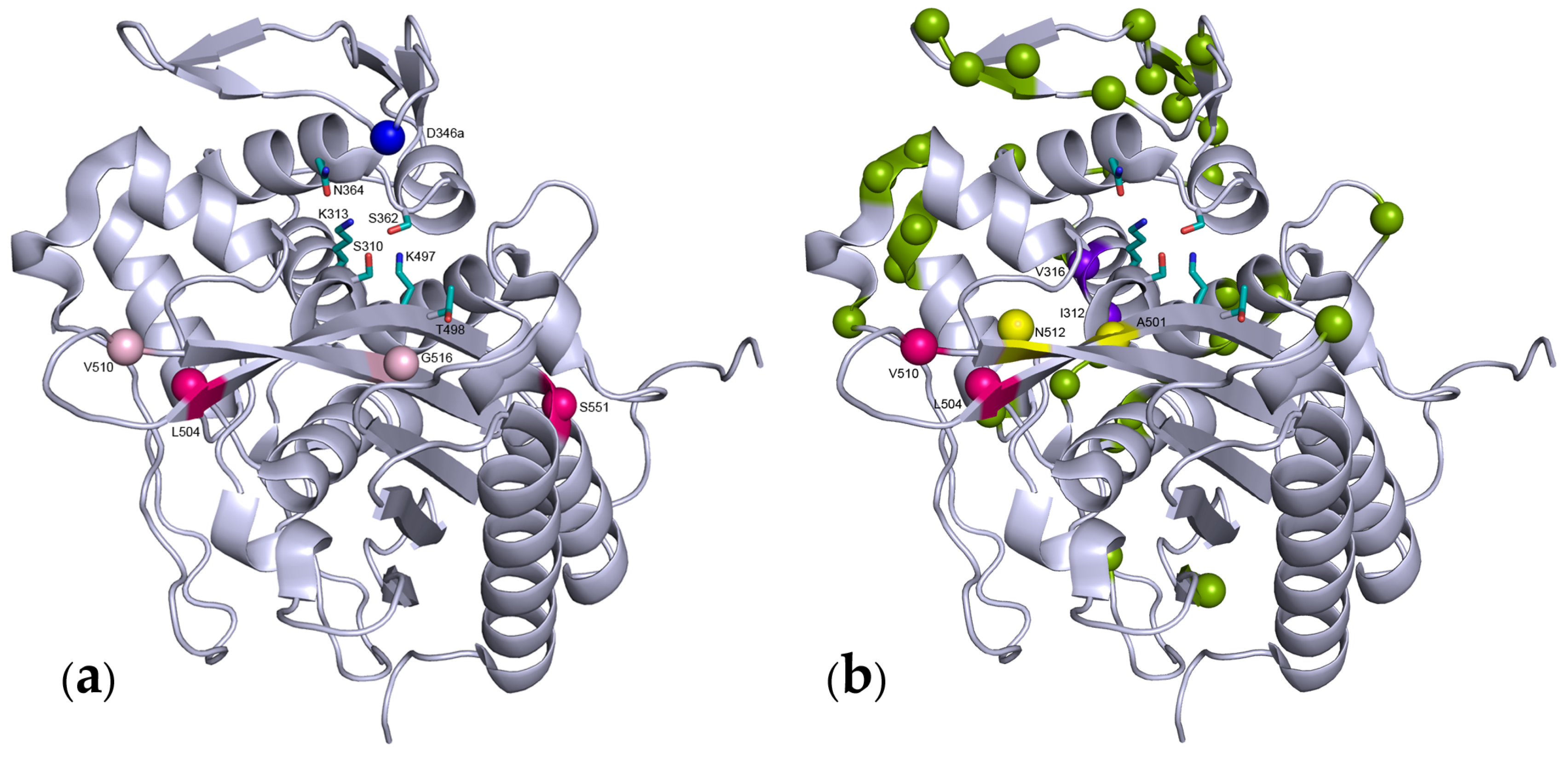
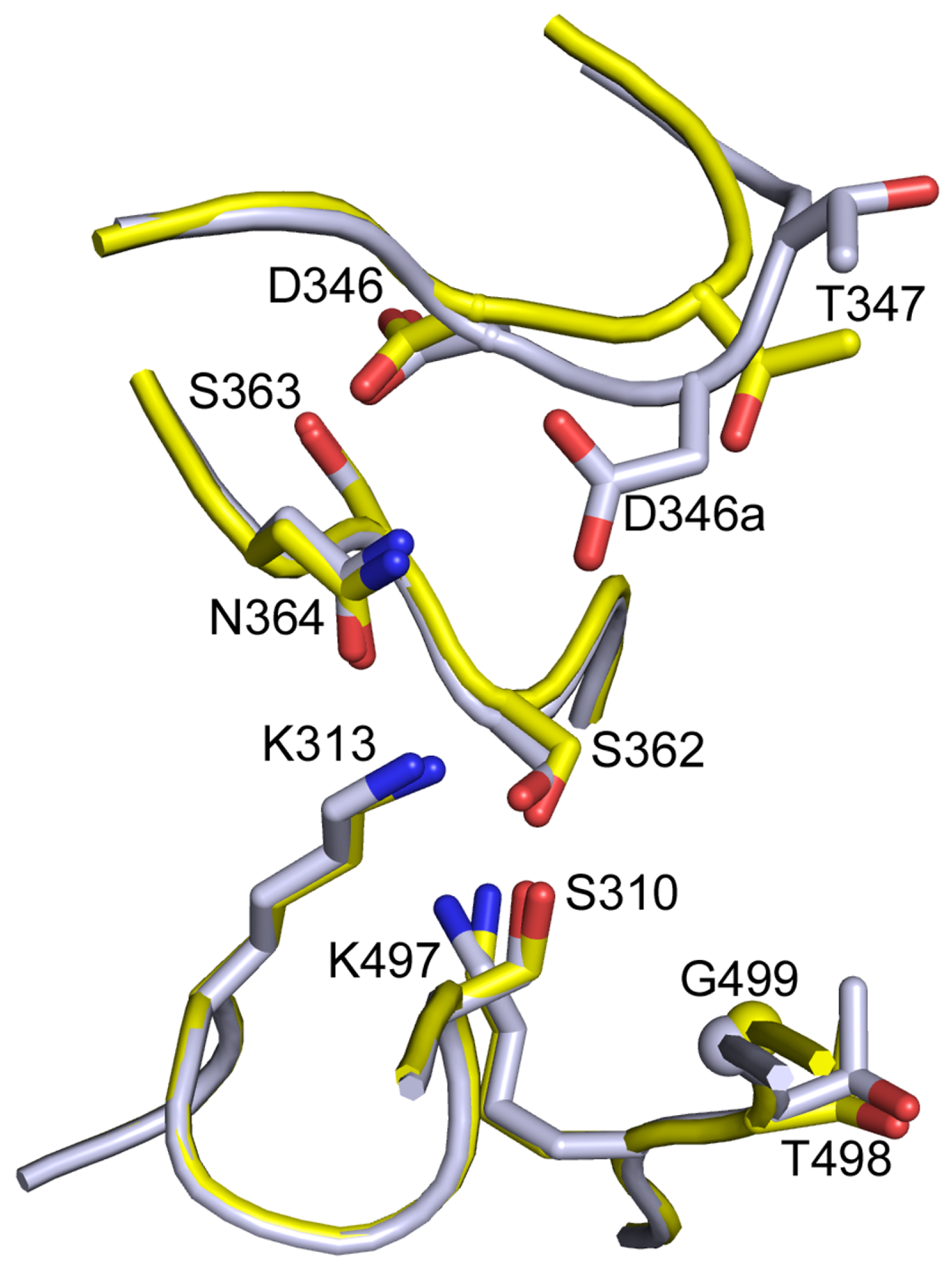
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zapun, A.; Morlot, C.; Taha, M.-K. Resistance to β-Lactams in Neisseria ssp Due to Chromosomally Encoded Penicillin-Binding Proteins. Antibiotics 2016, 5, 35. https://doi.org/10.3390/antibiotics5040035
Zapun A, Morlot C, Taha M-K. Resistance to β-Lactams in Neisseria ssp Due to Chromosomally Encoded Penicillin-Binding Proteins. Antibiotics. 2016; 5(4):35. https://doi.org/10.3390/antibiotics5040035
Chicago/Turabian StyleZapun, André, Cécile Morlot, and Muhamed-Kheir Taha. 2016. "Resistance to β-Lactams in Neisseria ssp Due to Chromosomally Encoded Penicillin-Binding Proteins" Antibiotics 5, no. 4: 35. https://doi.org/10.3390/antibiotics5040035
APA StyleZapun, A., Morlot, C., & Taha, M.-K. (2016). Resistance to β-Lactams in Neisseria ssp Due to Chromosomally Encoded Penicillin-Binding Proteins. Antibiotics, 5(4), 35. https://doi.org/10.3390/antibiotics5040035





