Discrepancy in Vancomycin AUC/MIC Ratio Targeted Attainment Based upon the Susceptibility Testing in Staphylococcus aureus
Abstract
:1. Introduction
2. Results
2.1. Patient Characteristics
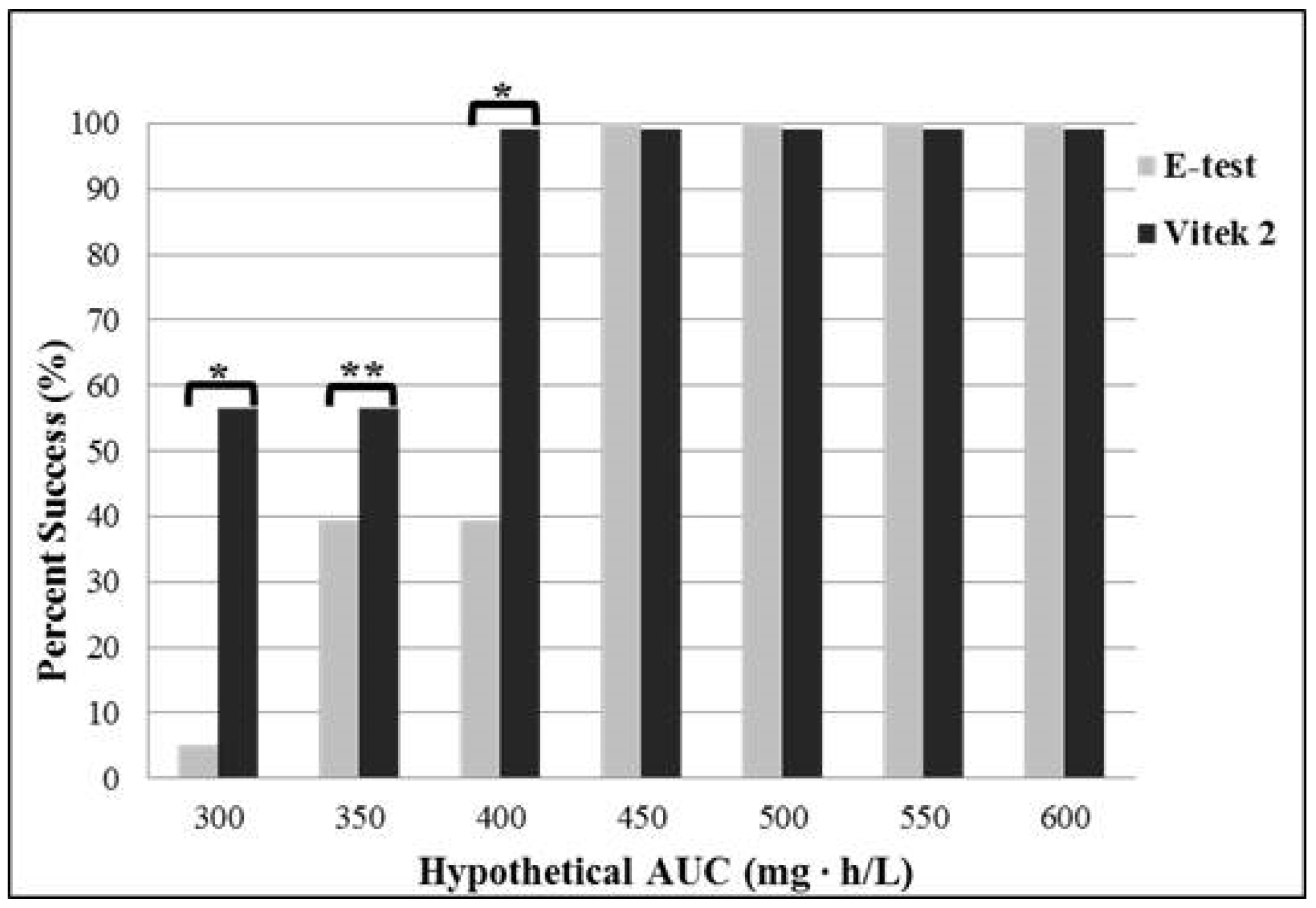
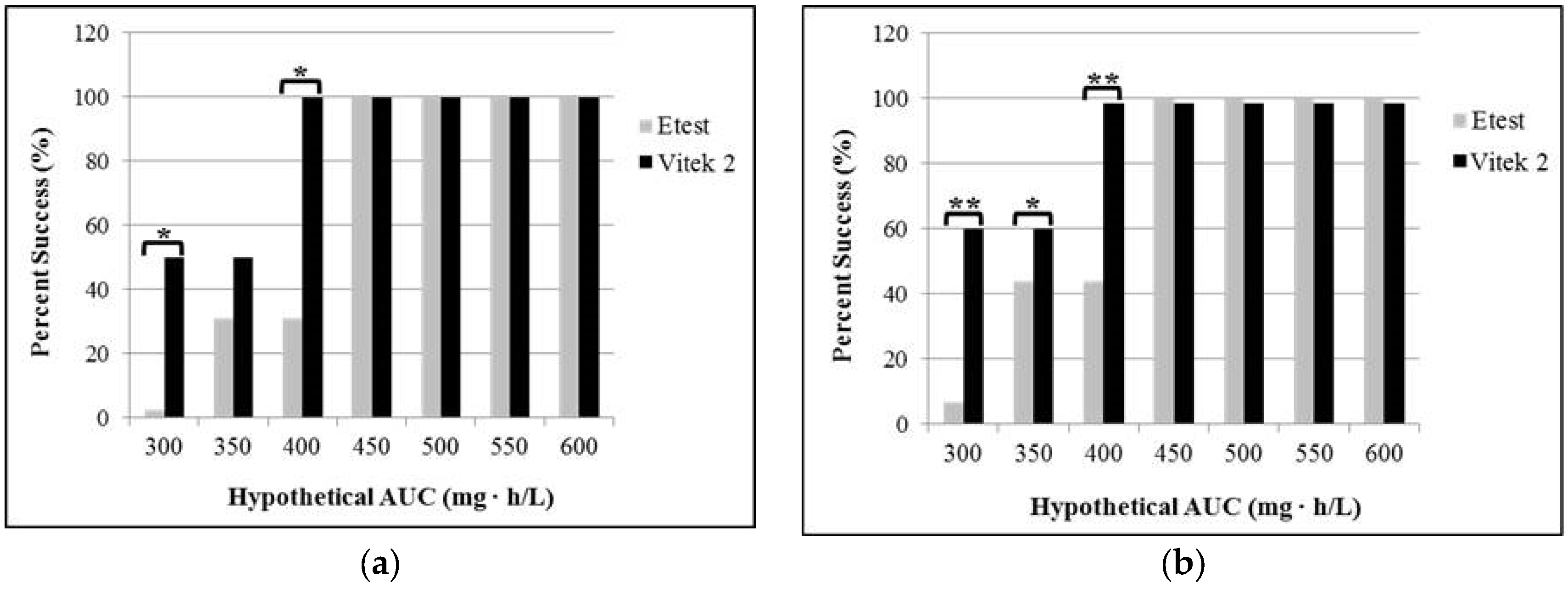
2.2. Effects of Testing Method on Vancomycin MIC
3. Discussion
4. Materials and Methods
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Holmes, N.E.; Turnidge, J.D.; Munckhof, W.J.; Robinson, J.O.; Korman, T.M.; O’Sullivan, M.V.N.; Anderson, T.L.; Roberts, S.A.; Warren, S.J.C.; Gao, W.; et al. Vancomycin AUC/MIC ratio and 30-day mortality in patients with Staphylococcus aureus bacteremia. Antimicrob. Agents Chemother. 2013, 57, 1654–1663. [Google Scholar] [CrossRef] [PubMed]
- Prakash, V.; Lewis, J.S., 2nd; Jorgensen, J.H. Vancomycin MICs for methicillin-resistant Staphylococcus aureus isolates differ based upon the susceptibility test method used. Antimicrob. Agents Chemother. 2008. [Google Scholar] [CrossRef] [PubMed]
- Hsu, D.I.; Hidayat, L.K.; Quist, R.; Hindler, J.; Karlsson, A.; Yusof, A.; Wong-Beringer, A. Comparison of method-specific vancomycin minimum inhibitory concentration values and their predictability for treatment outcome of meticillin-resistant Staphylococcus aureus (MRSA) infections. Int. J. Antimicrob. Agents 2008, 32, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Sader, H.S.; Fey, P.D.; Limaye, A.P.; Madinger, N.; Fish, D.N.; Pankey, G.; Rahal, J.; Rybak, M.J.; Snydman, D.R.; Steed, L.L.; et al. Evaluation of vancomycin and daptomycin potency trends (MIC creep) against methicillin-resistant Staphylococcus aureus isolates collected in nine U.S. medical centers from 2002 to 2006. Antimicrob. Agents Chemother. 2009, 53, 4127–4132. [Google Scholar] [CrossRef] [PubMed]
- Van Hal, S.J.; Barbagiannakos, T.; Jones, M.; Wehrhahn, M.C.; Mercer, J.; Chen, D.; Paterson, D.L.; Gosbell, I.B. Methicillin-resistant Staphylococcus aureus vancomycin susceptibility testing: Methodology correlations, temporal trends and clonal patterns. J. Antimicrob. Chemother. 2011, 66, 2284–2287. [Google Scholar] [CrossRef] [PubMed]
- Lodise, T.P.; Graves, J.; Evans, A.; Graffunder, E.; Helmecke, M.; Lomaestro, B.M.; Stellrecht, K. Relationship between vancomycin MIC and failure among patients with methicillin-resistant Staphylococcus aureus bacteremia treated with vancomycin. Antimicrob. Agents Chemother. 2008, 52, 3315–3320. [Google Scholar] [CrossRef] [PubMed]
- Van Hal, S.J.; Lodise, T.P.; Paterson, D.L. The clinical significance of vancomycin minimum inhibitory concentration in Staphylococcus aureus infections: A systematic review and meta-analysis. Clin. Infect. Dis. 2012, 54, 755–771. [Google Scholar] [CrossRef] [PubMed]
- Rybak, M.; Lomaestro, B.; Rotschafer, J.C.; Moellering, R., Jr.; Craig, W.; Billeter, M.; Dalovisio, J.R.; Levine, D.P. Therapeutic monitoring of vancomycin in adult patients: A consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am. J. Health Syst. Pharm. 2009, 66, 82–98. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.H.; Norris, R.; Barras, M.; Roberts, J.; Morris, R.; Doogue, M.; Jones, G.R. Therapeutic monitoring of vancomycin in adult patients: A consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Clin. Biochem. Rev. 2010, 31, 21–24. [Google Scholar] [PubMed]
- Bland, C.M.; Porr, W.H.; Davis, K.A.; Mansell, K.B. Vancomycin MIC susceptibility testing of methicillin-susceptible and methicillin-resistant Staphylococcus aureus isolates: A comparison between Etest® and an automated testing method. South. Med. J. 2010, 103, 1124–1128. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.L.; Scheetz, M.H.; Bosso, J.A.; Goff, D.A.; Rybak, M.J. Adherence to the 2009 consensus guidelines for vancomycin dosing and monitoring practices: A cross-sectional survey of U.S. hospitals. Pharmacotherapy 2013, 33, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Neely, M.N.; Youn, G.; Jones, B.; Jelliffe, R.W.; Drusano, G.L.; Rodvold, K.A.; Lodise, T.P. Are vancomycin trough concentrations adequate for optimal dosing? Antimicrob. Agents Chemother. 2014, 58, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Moise-Broder, P.A.; Forrest, A.; Birmingham, M.C.; Schentag, J.J. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin. Pharmacokinet. 2004, 43, 925–942. [Google Scholar] [CrossRef] [PubMed]
- Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Third Informational Supplement; CLSI document M100-S23; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2013.
- Jeffres, M.N.; Isakow, W.; Doherty, J.A.; McKinnon, P.S.; Ritchie, D.J.; Micek, S.T.; Kollef, M.H. Predictors of mortality for methicillin-resistant Staphylococcus aureus health-care-associated pneumonia: Specific evaluation of vancomycin pharmacokinetic indices. Chest 2006, 130, 947–955. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eum, S.; Bergsbaken, R.L.; Harvey, C.L.; Warren, J.B.; Rotschafer, J.C. Discrepancy in Vancomycin AUC/MIC Ratio Targeted Attainment Based upon the Susceptibility Testing in Staphylococcus aureus. Antibiotics 2016, 5, 34. https://doi.org/10.3390/antibiotics5040034
Eum S, Bergsbaken RL, Harvey CL, Warren JB, Rotschafer JC. Discrepancy in Vancomycin AUC/MIC Ratio Targeted Attainment Based upon the Susceptibility Testing in Staphylococcus aureus. Antibiotics. 2016; 5(4):34. https://doi.org/10.3390/antibiotics5040034
Chicago/Turabian StyleEum, Seenae, Robert L. Bergsbaken, Craig L. Harvey, J. Bryan Warren, and John C. Rotschafer. 2016. "Discrepancy in Vancomycin AUC/MIC Ratio Targeted Attainment Based upon the Susceptibility Testing in Staphylococcus aureus" Antibiotics 5, no. 4: 34. https://doi.org/10.3390/antibiotics5040034
APA StyleEum, S., Bergsbaken, R. L., Harvey, C. L., Warren, J. B., & Rotschafer, J. C. (2016). Discrepancy in Vancomycin AUC/MIC Ratio Targeted Attainment Based upon the Susceptibility Testing in Staphylococcus aureus. Antibiotics, 5(4), 34. https://doi.org/10.3390/antibiotics5040034




