The Antimicrobial Stewardship Approach to Combating Clostridium Difficile
Abstract
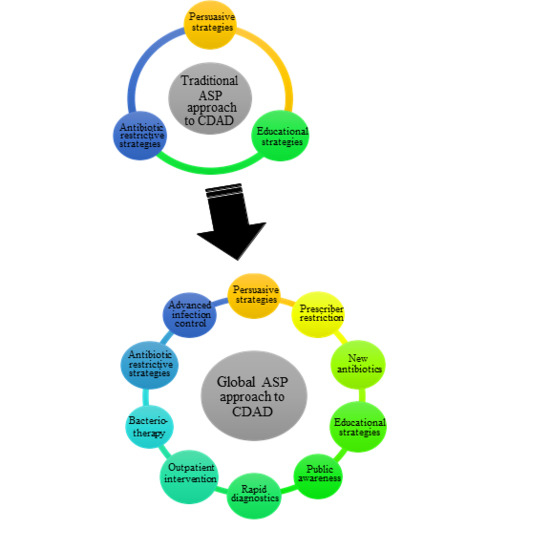
:1. Introduction
1.1. Antibiotic Restrictive Approaches
1.2. Non-Restrictive Approaches
2. Future Directions
2.1. Prescriber Restriction
2.2. Rapid Diagnostics
2.3. Treatment Paradigm
2.4. Infection Control
2.5. Public Awareness
2.6. Outpatient ASP
3. Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States. 2013. Available online: http://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf (accessed on 26 January 2015). [Google Scholar]
- Lessa, F.C.; Mu, Y.; Bamberg, W.M.; Beldavs, Z.G.; Dumyati, G.K.; Dunn, J.R.; Farley, M.M.; Holzbauer, S.M.; Meek, J.I.; Phipps, E.C.; et al. Burden of Clostridium difficile infection in the United States. N. Engl. J. Med. 2015, 372, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Rupnik, M.; Wilcox, M.H.; Gerding, D.N. Clostridium difficile infection: New developments in epidemiology and pathogenesis. Nat. Rev. Microbiol. 2009, 7, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Gerding, D.N. Clindamycin, cephalosporins, fluoroquinolones, and Clostridium difficile-associated diarrhea: This is an antimicrobial resistance problem. Clin. Infect. Dis. 2004, 38, 646–648. [Google Scholar] [CrossRef] [PubMed]
- Braykov, N.P.; Morgan, D.J.; Schweizer, M.L.; Uslan, D.Z.; Kelesidis, T.; Weisenberg, S.A.; Johannsson, B.; Young, H.; Cantey, J.; Srinivasan, A.; et al. Assessment of empirical antibiotic therapy optimisation in six hospitals: An observational cohort study. Lancet Infect. Dis. 2014, 14, 1220–1227. [Google Scholar] [CrossRef]
- Dellit, T.H.; Owens, R.C.; McGowan, J.E., Jr.; Gerding, D.N.; Weinstein, R.A.; Burke, J.P.; Huskins, W.C.; Paterson, D.L.; Fishman, N.O.; Carpenter, C.F.; et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin. Infect. Dis. 2007, 44, 159–177. [Google Scholar] [CrossRef] [PubMed]
- Feazel, L.M.; Malhotra, A.; Perencevich, E.N.; Kaboli, P.; Diekema, D.J.; Schweizer, M.L. Effect of antibiotic stewardship programmes on Clostridium difficile incidence: A systematic review and meta-analysis. J. Antimicrob. Chemother. 2014, 69, 1748–1754. [Google Scholar] [CrossRef] [PubMed]
- Dancer, S.J.; Kirkpatrick, P.; Corcoran, D.S.; Christison, F.; Farmer, D.; Robertson, C. Approaching zero: Temporal effects of a restrictive antibiotic policy on hospital-acquired Clostridium difficile, extended-spectrum beta-lactamase-producing coliforms and meticillin-resistant Staphylococcus aureus. Int. J. Antimicrob. Agents 2013, 41, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Saint, S.; Fowler, K.E.; Krein, S.L.; Ratz, D.; Flanders, S.A.; Dubberke, E.R.; Greene, M.T. Clostridium difficile Infection in the United States: A National Study Assessing Preventive Practices Used and Perceptions of Practice Evidence. Infect. Control Hosp. Epidemiol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Lucado, J.; Gould, C.; Elixhauser, A. Clostridium difficile Infections (CDI) in Hospital Stays, 2009. HCUP Statistical Brief #124; US Agency for Healthcare Research and Quality: Rockville, MD, USA, 2012.
- Aldeyab, M.A.; Kearney, M.P.; Scott, M.G.; Aldiab, M.A.; Alahmadi, Y.M.; Darwish Elhajji, F.W.; Magee, F.A.; McElnay, J.C. An evaluation of the impact of antibiotic stewardship on reducing the use of high-risk antibiotics and its effect on the incidence of Clostridium difficile infection in hospital settings. J. Antimicrob. Chemother. 2012, 67, 2988–2996. [Google Scholar] [CrossRef] [PubMed]
- Climo, M.W.; Israel, D.S.; Wong, E.S.; Williams, D.; Coudron, P.; Markowitz, S.M. Hospital-wide restriction of clindamycin: Effect on the incidence of Clostridium difficile-associated diarrhea and cost. Ann. Intern. Med. 1998, 128, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Cheesbrough, J. Impact of changes in antibiotic policy on Clostridium difficile-associated diarrhoea (CDAD) over a five-year period in a district general hospital. J. Hosp. Infect. 2003, 54, 104–108. [Google Scholar] [CrossRef]
- Pear, S.M.; Williamson, T.H.; Bettin, K.M.; Gerding, D.N.; Galgiani, J.N. Decrease in nosocomial Clostridium difficile-associated diarrhea by restricting clindamycin use. Ann. Intern. Med. 1994, 120, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Riley, T.V. Restriction of third generation cephalosporin use reduces the incidence of Clostridium difficile-associated diarrhoea in hospitalised patients. Commun. Dis. Intell. Q. Rep. 2003, 27, S28–S31. [Google Scholar] [PubMed]
- Valiquette, L.; Cossette, B.; Garant, M.P.; Diab, H.; Pepin, J. Impact of a reduction in the use of high-risk antibiotics on the course of an epidemic of Clostridium difficile-associated disease caused by the hypervirulent NAP1/027 strain. Clin. Infect. Dis. 2007, 45, S112–S121. [Google Scholar] [CrossRef] [PubMed]
- Muto, C.A.; Blank, M.K.; Marsh, J.W.; Vergis, E.N.; O’Leary, M.M.; Shutt, K.A.; Pasculle, A.W.; Pokrywka, M.; Garcia, J.G.; Posey, K.; et al. Control of an outbreak of infection with the hypervirulent Clostridium difficile BI strain in a university hospital using a comprehensive “bundle” approach. Clin. Infect. Dis. 2007, 45, 1266–1273. [Google Scholar] [CrossRef] [PubMed]
- Cook, P.P.; Gooch, M. Long-term effects of an antimicrobial stewardship programme at a tertiary-care teaching hospital. Int. J. Antimicrob. Agents 2015, 45, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.; Homann, S.R.; Bettin, K.M.; Quick, J.N.; Clabots, C.R.; Peterson, L.R.; Gerding, D.N. Treatment of asymptomatic Clostridium difficile carriers (fecal excretors) with vancomycin or metronidazole. A randomized, placebo-controlled trial. Ann. Intern. Med. 1992, 117, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Delmee, M.; Vandercam, B.; Avesani, V.; Michaux, J.L. Epidemiology and prevention of Clostridium difficile infections in a leukemia unit. Eur. J. Clin. Microbiol. 1987, 6, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Wenzler, E.; Rodvold, K.A.; Danziger, L.H. Editorial commentary: Improving prescribers to advance antimicrobial stewardship. Clin. Infect. Dis. 2015, 60, 1259–1261. [Google Scholar] [CrossRef] [PubMed]
- Goff, D.A.; Mendelson, M. Is it time for an antibiotic prenuptial agreement? Lancet Infect. Dis. 2014, 14, 1168–1169. [Google Scholar] [CrossRef]
- Aguado, J.M.; Anttila, V.J.; Galperine, T.; Goldenberg, S.D.; Gwynn, S.; Jenkins, D.; Norén, T.; Petrosillo, N.; Seifert, H.; Stallmach, A.; et al. Highlighting clinical needs in Clostridium difficile infection: The views of European healthcare professionals at the front line. J. Hosp. Infect. 2015, 90, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Ashiru-Oredope, D.; Cookson, B.; Fry, C.; Cookson, B.; Ashiru-Oredope, D.; Avery, T.; Baudouin, S.; Brown, J.; Cooke, J.; Cooper, T.; et al. Developing the first national antimicrobial prescribing and stewardship competences. J. Antimicrob. Chemother. 2014, 69, 2886–2888. [Google Scholar] [CrossRef] [PubMed]
- Bauer, K.A.; Perez, K.K.; Forrest, G.N.; Goff, D.A. Review of rapid diagnostic tests used by antimicrobial stewardship programs. Clin. Infect. Dis. 2014, 59, S134–S145. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Villa, G.; Pecori, D.; Arzese, A.; Wilcox, M. Epidemiology, diagnosis and treatment of Clostridium difficile infection. Expert Rev. Anti Infect. Ther. 2012, 10, 1405–1423. [Google Scholar] [CrossRef] [PubMed]
- Collins, D.A.; Elliott, B.; Riley, T.V. Molecular methods for detecting and typing of Clostridium difficile. Pathology 2015, 47, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Babady, N.E.; Stiles, J.; Ruggiero, P.; Khosa, P.; Huang, D.; Shuptar, S.; Kamboj, M.; Kiehn, T.E. Evaluation of the Cepheid Xpert Clostridium difficile Epi assay for diagnosis of Clostridium difficile infection and typing of the NAP1 strain at a cancer hospital. J. Clin. Microbiol. 2010, 48, 4519–4524. [Google Scholar] [CrossRef] [PubMed]
- Rao, K. Measuring the impact of Clostridium difficile Infection with the NAP1 strain on severity and mortality. Clin. Infect. Dis. 2014, 59, 1193–1194. [Google Scholar] [CrossRef] [PubMed]
- Louie, T.J.; Miller, M.A.; Mullane, K.M.; Weiss, K.; Lentnek, A.; Golan, Y.; Gorbach, S.; Sears, P.; Shue, Y.K. Fidaxomicin versus vancomycin for Clostridium difficile infection. N. Engl. J. Med. 2011, 364, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Cornely, O.A.; Crook, D.W.; Esposito, R.; Poirier, A.; Somero, M.S.; Weiss, K.; Sears, P.; Gorbach, S. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: A double-blind, non-inferiority, randomised controlled trial. Lancet Infect. Dis. 2012, 12, 281–289. [Google Scholar] [CrossRef]
- Scardina, T.; Labuszewski, L.; Pacheco, S.M.; Adams, W.; Schreckenberger, P.; Johnson, S. Clostridium difficile infection (CDI) severity and outcome among patients infected with the NAP1/BI/027 strain in a non-epidemic setting. Infect. Control Hosp. Epidemiol. 2015, 36, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Crawford, T.; Huesgen, E.; Danziger, L. Fidaxomicin: A novel macrocyclic antibiotic for the treatment of Clostridium difficile infection. Am. J. Health Syst. Pharm. 2012, 69, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Kallen, A.J.; Thompson, A.; Ristaino, P.; Chapman, L.; Nicholson, A.; Sim, B.T.; Lessa, F.; Sharapov, U.; Fadden, E.; Boehler, R.; et al. Complete restriction of fluoroquinolone use to control an outbreak of Clostridium difficile infection at a community hospital. Infect. Control Hosp. Epidemiol. 2009, 30, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Aldeyab, M.A.; Devine, M.J.; Flanagan, P.; Mannion, M.; Craig, A.; Scott, M.G.; Harbarth, S.; Vernaz, N.; Davies, E.; Brazier, J.S.; et al. Multihospital outbreak of Clostridium difficile ribotype 027 infection: Epidemiology and analysis of control measures. Infect. Control Hosp. Epidemiol. 2011, 32, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Bomers, M.K.; Menke, F.P.; Savage, R.S.; Vandenbroucke-Grauls, C.M.; van Agtmael, M.A.; Covington, J.A.; Smulders, Y.M. Rapid, Accurate, and On-Site Detection of C. difficile in Stool Samples. Am. J. Gastroenterol. 2015, 110, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Murad, Y.M.; Perez, J.; Nokhbeh, R.; Ybazeta, G.; Dewar, B.; Lefebvre, S.; Diaz-Mitoma, F. Impact of polymerase chain reaction testing on Clostridium difficile infection rates in an acute health care facility. Am. J. Infect. Control 2015, 43, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Herzig, C.T.; Reagan, J.; Pogorzelska-Maziarz, M.; Srinath, D.; Stone, P.W. State-Mandated Reporting of Health Care-Associated Infections in the United States: Trends Over Time. Am. J. Med. Qual. 2014. [Google Scholar] [CrossRef] [PubMed]
- Gerding, D.N. Metronidazole for Clostridium difficile-associated disease: Is it okay for Mom? Clin. Infect. Dis. 2005, 40, 1598–1600. [Google Scholar] [CrossRef] [PubMed]
- Gorbach, S.L. Drugs for your mother-in-law, not your mother. Infect. Dis. Clin. Pract. 1992, 1, 46. [Google Scholar]
- Wilcox, M.H. Editorial Commentary: The trials and tribulations of treating Clostridium difficile infection-one step backward, one step forward, but still progress. Clin. Infect. Dis. 2014, 59, 355–357. [Google Scholar] [CrossRef] [PubMed]
- Louie, T.J.; Emery, J.; Krulicki, W.; Byrne, B.; Mah, M. OPT-80 eliminates Clostridium difficile and is sparing of bacteroides species during treatment of C. difficile infection. Antimicrob. Agents Chemother. 2009, 53, 261–263. [Google Scholar] [CrossRef] [PubMed]
- Louie, T.J.; Cannon, K.; Byrne, B.; Emery, J.; Ward, L.; Eyben, M.; Krulicki, W. Fidaxomicin preserves the intestinal microbiome during and after treatment of Clostridium difficile infection (CDI) and reduces both toxin reexpression and recurrence of CDI. Clin. Infect. Dis. 2012, 55, S132–S142. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.; Louie, T.J.; Gerding, D.N.; Cornely, O.A.; Chasan-Taber, S.; Fitts, D.; Gelone, S.P.; Broom, C.; Davidson, D.M. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: Results from two multinational, randomized, controlled trials. Clin. Infect. Dis. 2014, 59, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, A.; Anand, G.; Friedenberg, F. Factors associated with failure of metronidazole in Clostridium difficile-associated disease. J. Clin. Gastroenterol. 2004, 38, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Belmares, J.; Gerding, D.N.; Parada, J.P.; Miskevics, S.; Weaver, F.; Johnson, S. Outcome of metronidazole therapy for Clostridium difficile disease and correlation with a scoring system. J. Infect. 2007, 55, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Soriano, M.M.; Johnson, S. Treatment of Clostridium difficile infections. Infect. Dis. Clin. N. Am. 2015, 29, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.H.; Gerding, D.N.; Johnson, S.; Kelly, C.P.; Loo, V.G.; McDonald, L.C.; Pepin, J.; Wilcox, M.H. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect. Control Hosp. Epidemiol. 2010, 31, 431–455. [Google Scholar] [CrossRef] [PubMed]
- Surawicz, C.M.; Brandt, L.J.; Binion, D.G.; Ananthakrishnan, A.N.; Curry, S.R.; Gilligan, P.H.; McFarland, L.V.; Mellow, M.; Zuckerbraun, B.S. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am. J. Gastroenterol. 2013, 108, 478–498; quiz 499. [Google Scholar] [CrossRef] [PubMed]
- Debast, S.B.; Bauer, M.P.; Kuijper, E.J. European Society of Clinical Microbiology and Infectious Diseases: Update of the treatment guidance document for Clostridium difficile infection. Clin. Microbiol. Infect. 2014, 20, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Vargo, C.A.; Bauer, K.A.; Mangino, J.E.; Johnston, J.E.; Goff, D.A. An antimicrobial stewardship program’s real-world experience with fidaxomicin for treatment of Clostridium difficile infection: A case series. Pharmacotherapy 2014, 34, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Soriano, M.M.; Danziger, L.H.; Gerding, D.N.; Johnson, S. Novel Fidaxomicin Treatment Regimens for Patients With Multiple Clostridium difficile Infection Recurrences That Are Refractory to Standard Therapies. Open Forum Infect. Dis. 2014, 1, ofu069. [Google Scholar] [CrossRef] [PubMed]
- Esmaily-Fard, A.; Tverdek, F.P.; Crowther, D.M.; Ghantoji, S.S.; Adachi, J.A.; Chemaly, R.F. The use of fidaxomicin for treatment of relapsed Clostridium difficile infections in patients with cancer. Pharmacotherapy 2014, 34, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- Horton, H.A.; Dezfoli, S.; Berel, D.; Hirsch, J.; Ippoliti, A.; McGovern, D.; Kaur, M.; Shih, D.; Dubinsky, M.; Targan, S.R.; et al. Antibiotics for Treatment of Clostridium difficile Infection in Hospitalized Patients with Inflammatory Bowel Disease. Antimicrob. Agents Chemother. 2014, 58, 5054–5059. [Google Scholar] [CrossRef] [PubMed]
- Nathwani, D.; Cornely, O.A.; van Engen, A.K.; Odufowora-Sita, O.; Retsa, P.; Odeyemi, I.A. Cost-effectiveness analysis of fidaxomicin versus vancomycin in Clostridium difficile infection. J. Antimicrob. Chemother. 2014, 69, 2901–2912. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, S.M.; Umscheid, C.A.; Fishman, N.; Lee, B.Y. Is fidaxomicin worth the cost? An economic analysis. Clin. Infect. Dis. 2013, 57, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Konijeti, G.G.; Sauk, J.; Shrime, M.G.; Gupta, M.; Ananthakrishnan, A.N. Cost-effectiveness of competing strategies for management of recurrent Clostridium difficile infection: A decision analysis. Clin. Infect. Dis. 2014, 58, 1507–1514. [Google Scholar] [CrossRef] [PubMed]
- Jarrad, A.M.; Karoli, T.; Blaskovich, M.A.; Lyras, D.; Cooper, M.A. Clostridium difficile Drug Pipeline: Challenges in Discovery and Development of New Agents. J. Med. Chem. 2015. [Google Scholar] [CrossRef]
- Locher, H.H.; Seiler, P.; Chen, X.; Schroeder, S.; Pfaff, P.; Enderlin, M.; Klenk, A.; Fournier, E.; Hubschwerlen, C.; Ritz, D.; et al. In vitro and in vivo antibacterial evaluation of cadazolid, a new antibiotic for treatment of Clostridium difficile infections. Antimicrob. Agents Chemother. 2014, 58, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Mascio, C.T.; Mortin, L.I.; Howland, K.T.; van Praagh, A.D.; Zhang, S.; Arya, A.; Chuong, C.L.; Kang, C.; Li, T.; Silverman, J.A. In vitro and in vivo characterization of CB-183,315, a novel lipopeptide antibiotic for treatment of Clostridium difficile. Antimicrob. Agents Chemother. 2012, 56, 5023–5030. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, E.J.; Citron, D.M.; Tyrrell, K.L. Comparative in vitro activities of SMT19969, a new antimicrobial agent, against 162 strains from 35 less frequently recovered intestinal Clostridium species: Implications for Clostridium difficile recurrence. Antimicrob. Agents Chemother. 2014, 58, 1187–1191. [Google Scholar] [CrossRef] [PubMed]
- Gough, E.; Shaikh, H.; Manges, A.R. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin. Infect. Dis. 2011, 53, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- Youngster, I.; Sauk, J.; Pindar, C.; Wilson, R.G.; Kaplan, J.L.; Smith, M.B.; Alm, E.J.; Gevers, D.; Russell, G.H.; Hohmann, E.L. Fecal microbiota transplant for relapsing Clostridium difficile infection using a frozen inoculum from unrelated donors: A randomized, open-label, controlled pilot study. Clin. Infect. Dis. 2014, 58, 1515–1522. [Google Scholar] [CrossRef] [PubMed]
- Youngster, I.; Russell, G.H.; Pindar, C.; Ziv-Baran, T.; Sauk, J.; Hohmann, E.L. Oral, capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficile infection. JAMA 2014, 312, 1772–1778. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; DuPont, H.L. New Approaches for Bacteriotherapy: Prebiotics, New-Generation Probiotics, and Synbiotics. Clin. Infect. Dis. 2015, 60, S108–S121. [Google Scholar] [CrossRef] [PubMed]
- McFarland, L.V. Probiotics for the Primary and Secondary Prevention of C. difficile Infections: A Meta-analysis and Systematic Review. Antibiotics 2015, 4, 160–178. [Google Scholar] [CrossRef]
- Van Hise, N.W.; Bryant, A.M.; Crannage, A.J.; Hennessey, E.K.; Khoury, J.A. Evaluation of Secondary Prophylaxis with Oral Vancomycin on the Incidence of Recurrent Clostridium difficile Infections in High Risk Patients [abstractK-367]. In Proceedings of the 54th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, DC, USA, 5–9 September 2014.
- Nagel, J.L.; Stevenson, J.G.; Eiland, E.H., 3rd; Kaye, K.S. Demonstrating the value of antimicrobial stewardship programs to hospital administrators. Clin. Infect. Dis. 2014, 59, S146–S153. [Google Scholar] [CrossRef] [PubMed]
- Samore, M.H.; Venkataraman, L.; DeGirolami, P.C.; Arbeit, R.D.; Karchmer, A.W. Clinical and molecular epidemiology of sporadic and clustered cases of nosocomial Clostridium difficile diarrhea. Am. J. Med. 1996, 100, 32–40. [Google Scholar] [CrossRef]
- Shaughnessy, M.K.; Micielli, R.L.; DePestel, D.D.; Arndt, J.; Strachan, C.L.; Welch, K.B.; Chenoweth, C.E. Evaluation of hospital room assignment and acquisition of Clostridium difficile infection. Infect. Control Hosp. Epidemiol. 2011, 32, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Boyce, J.M.; Ligi, C.; Kohan, C.; Dumigan, D.; Havill, N.L. Lack of association between the increased incidence of Clostridium difficile-associated disease and the increasing use of alcohol-based hand rubs. Infect. Control Hosp. Epidemiol. 2006, 27, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Fawley, W.N.; Underwood, S.; Freeman, J.; Baines, S.D.; Saxton, K.; Stephenson, K.; Owens, R.C., Jr.; Wilcox, M.H. Efficacy of hospital cleaning agents and germicides against epidemic Clostridium difficile strains. Infect. Control Hosp. Epidemiol. 2007, 28, 920–925. [Google Scholar] [CrossRef] [PubMed]
- Larson, E. A causal link between handwashing and risk of infection? Examination of the evidence. Infect. Control Hosp. Epidemiol. 1988, 9, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.; Abad, C.; Dinh, M.; Safdar, N. Prevention of endemic healthcare-associated Clostridium difficile infection: Reviewing the evidence. Am. J. Gastroenterol. 2010, 105, 2327–2339; quiz 2340. [Google Scholar] [CrossRef] [PubMed]
- Magill, S.S.; Edwards, J.R.; Bamberg, W.; Beldavs, Z.G.; Dumyati, G.; Kainer, M.A.; Lynfield, R.; Maloney, M.; McAllister-Hollod, L.; Nadle, J.; et al. Multistate point-prevalence survey of health care-associated infections. N. Engl. J. Med. 2014, 370, 1198–1208. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.T.; Safdar, N. Current Trends in the Epidemiology and Outcomes of Clostridium difficile Infection. Clin. Infect. Dis. 2015, 60, S66–S71. [Google Scholar] [CrossRef] [PubMed]
- Jinadatha, C.; Quezada, R.; Huber, T.W.; Williams, J.B.; Zeber, J.E.; Copeland, L.A. Evaluation of a pulsed-xenon ultraviolet room disinfection device for impact on contamination levels of methicillin-resistant Staphylococcus aureus. BMC Infect. Dis. 2014, 14, e187. [Google Scholar] [CrossRef] [PubMed]
- Levin, J.; Riley, L.S.; Parrish, C.; English, D.; Ahn, S. The effect of portable pulsed xenon ultraviolet light after terminal cleaning on hospital-associated Clostridium difficile infection in a community hospital. Am. J. Infect. Control 2013, 41, 746–748. [Google Scholar] [CrossRef] [PubMed]
- Haas, J.P.; Menz, J.; Dusza, S.; Montecalvo, M.A. Implementation and impact of ultraviolet environmental disinfection in an acute care setting. Am. J. Infect. Control 2014, 42, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Nerandzic, M.M.; Thota, P.; Sankar, C.T.; Jencson, A.; Cadnum, J.L.; Ray, A.J.; Salata, R.A.; Watkins, R.R.; Donskey, C.J. Evaluation of a Pulsed Xenon Ultraviolet Disinfection System for Reduction of Healthcare-Associated Pathogens in Hospital Rooms. Infect. Control Hosp. Epidemiol. 2015, 36, 192–197. [Google Scholar] [CrossRef] [PubMed]
- HJ-30i. Altapure LLC. 2015. Available online: http://altapure.com/products/hj-30i-disinfector/ (accessed on 17 April 2015).
- Maki, D.G.; Duster, M. The Promise of Simple and Total Disinfection of Hospital Surfaces by Aerosolization of Peroxyacetic Acid [K-2105]. In Proceedings of the 49th Interscience Conference on Antmicrobial Agents and Chemotherapy, San Fransisco, CA, USA, 12–15 September 2009.
- HRMS. Arcalux, American Green Technology. 2014. Available online: http://www.arcaluxhrms.com/ (accessed on 17 April 2015).
- Kowalski, W.J. Report on the Performance of the Arcalux Health Risk Management System. December 2011. Available online: http://www.competitive-edgeservices.com/wp-content/uploads/2015/01/Performance_rpt.pdf (accessed on 14 April 2015).
- See, I.; Bagchi, S.; Booth, S.; Scholz, D.; Geller, A.I.; Anderson, L.; Moulton-Meissner, H.; Finks, J.L.; Kelley, K.; Gould, C.V.; et al. Outbreak of Clostridium difficile Infections at an Outpatient Hemodialysis Facility-Michigan, 2012–2013. Infect. Control Hosp. Epidemiol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Aberdein, J.; Chapman, A.L. Clostridium difficile infection following outpatient parenteral antimicrobial therapy. J. Hosp. Infect. 2015, 90, 171–172. [Google Scholar] [CrossRef] [PubMed]
- Drekonja, D.M.; Filice, G.A.; Greer, N.; Olson, A.; MacDonald, R.; Rutks, I.; Wilt, T.J. Antimicrobial stewardship in outpatient settings: A systematic review. Infect. Control Hosp. Epidemiol. 2015, 36, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, J.L.; Johnson, E.S.; Raebel, M.A.; Platt, R.W.; Petrik, A.F.; Yang, X.; Thorp, M.L.; Spindel, S.J.; Neil, N.; Smith, D.H. Predicting the risk of Clostridium difficile infection following an outpatient visit: Development and external validation of a pragmatic, prognostic risk score. Clin. Microbiol. Infect. 2015, 21, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, J.L.; Polgreen, P.M. The importance of considering different healthcare settings when estimating the burden of Clostridium difficile. Clin. Infect. Dis. 2015, 60, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Jury, L.A.; Sitzlar, B.; Kundrapu, S.; Cadnum, J.L.; Summers, K.M.; Muganda, C.P.; Deshpande, A.; Sethi, A.K.; Donskey, C.J. Outpatient healthcare settings and transmission of Clostridium difficile. PLoS ONE 2013, 8, e70175. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, J.L.; Johnson, E.S.; Raebel, M.A.; Petrik, A.F.; Yang, X.; Thorp, M.L.; Spindel, S.J.; Neil, N.; Smith, D.H. Epidemiology and healthcare costs of incident Clostridium difficile infections identified in the outpatient healthcare setting. Infect. Control Hosp. Epidemiol. 2012, 33, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- MacDougall, C.; Polk, R.E. Antimicrobial stewardship programs in health care systems. Clin. Microbiol. Rev. 2005, 18, 638–656. [Google Scholar] [CrossRef] [PubMed]
- Davey, P.; Brown, E.; Fenelon, L.; Finch, R.; Gould, I.; Hartman, G.; Holmes, A.; Ramsay, C.; Taylor, E.; Wilcox, M.; et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst. Rev. 2005. [Google Scholar] [CrossRef]
- Goldstein, E.J.; Johnson, S.; Maziade, P.J.; McFarland, L.V.; Trick, W.; Dresser, L.; Millette, M.; Mazloum, H.; Low, D.E. Pathway to Prevention of Nosocomial Clostridium difficile Infection. Clin. Infect. Dis. 2015, 60, S148–S158. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wenzler, E.; Mulugeta, S.G.; Danziger, L.H. The Antimicrobial Stewardship Approach to Combating Clostridium Difficile. Antibiotics 2015, 4, 198-215. https://doi.org/10.3390/antibiotics4020198
Wenzler E, Mulugeta SG, Danziger LH. The Antimicrobial Stewardship Approach to Combating Clostridium Difficile. Antibiotics. 2015; 4(2):198-215. https://doi.org/10.3390/antibiotics4020198
Chicago/Turabian StyleWenzler, Eric, Surafel G. Mulugeta, and Larry H. Danziger. 2015. "The Antimicrobial Stewardship Approach to Combating Clostridium Difficile" Antibiotics 4, no. 2: 198-215. https://doi.org/10.3390/antibiotics4020198
APA StyleWenzler, E., Mulugeta, S. G., & Danziger, L. H. (2015). The Antimicrobial Stewardship Approach to Combating Clostridium Difficile. Antibiotics, 4(2), 198-215. https://doi.org/10.3390/antibiotics4020198