Probiotics for the Primary and Secondary Prevention of C. difficile Infections: A Meta-analysis and Systematic Review
Abstract
:1. Introduction
2. Results
2.1. Initial Screening of Data Search
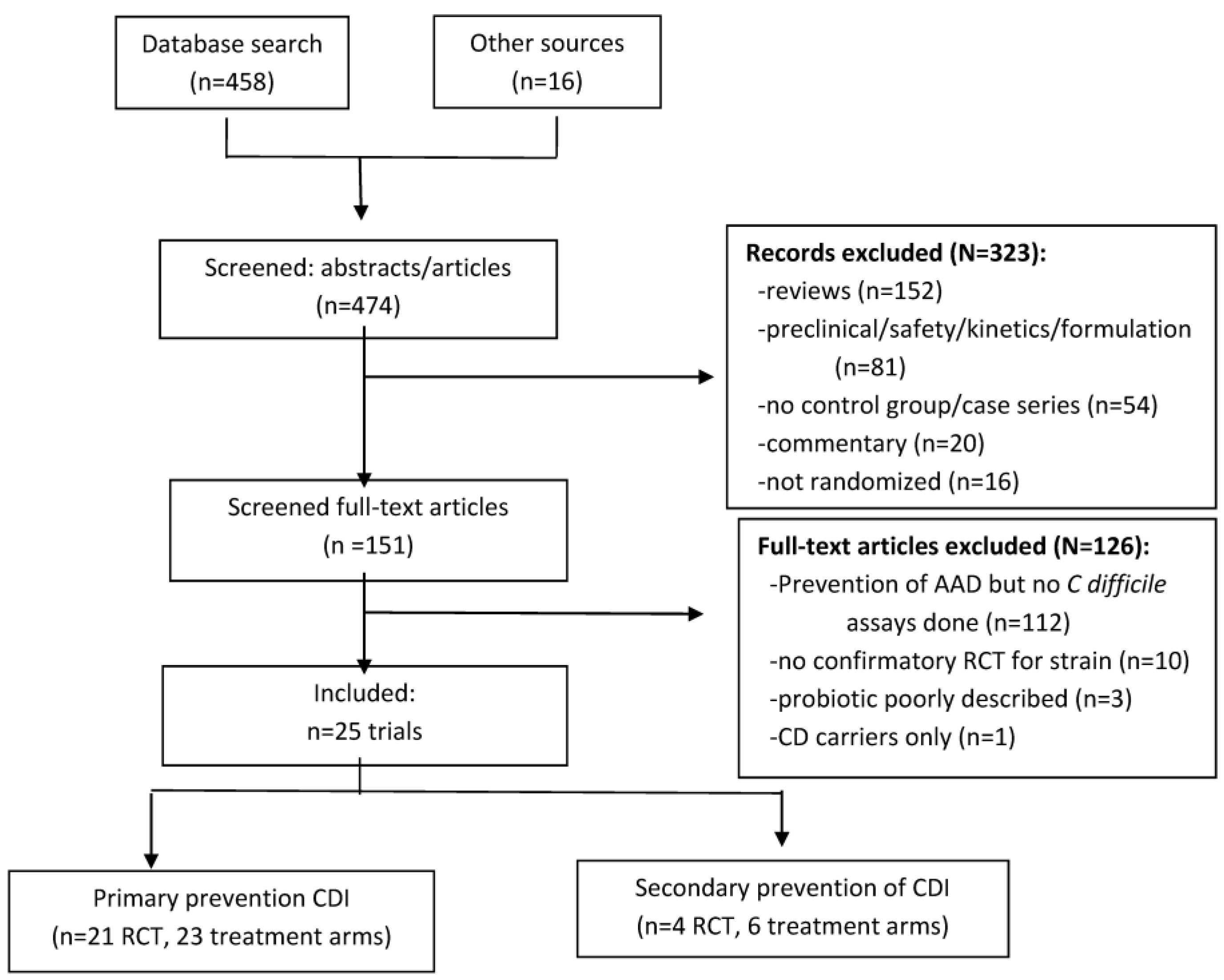
2.2. Secondary Screening of Full Articles
2.3. Included Trials
2.4. Study Design
| Probiotic | Eligible Antibiotic Exposures | Daily Dose of Probiotic (cfu/day) | Duration of Probiotic Treatment | Duration Follow-up | CDI in Probiotic Group (%) | CDI in Control Group (%) | Reference |
|---|---|---|---|---|---|---|---|
| Primary prevention of CDI | |||||||
| L. casei Shirota | nr | 6.5 × 109 | duration + 1 week | 4 weeks | 9/76 (0%) ns | 1/82 (1.2%) | Wong 2014 [7] |
| L. acidophilus | mixed, 77% beta-lactams | 6 × 1010 | 2 weeks | 0 | 0/23 (0%) ns | 1/16 (6.2%) | Safdar 2008 [8] |
| L. plantarum 299v | mixed | 1 × 1010 | duration + 1 week | 1 week | 1/74 (1.3%) ns | 0/76 (0%) | Lonnermark 2010 [9] |
| Bacillus clausii | mixed, beta-lactams | 4 × 109 | duration | 6 weeks | 0/162 (0%) ns | 1/160 (0.6%) | Destura 2008 [10] |
| C. butyricum 588 | mixed, 87% beta-lactams | 1–4 × 107 | 6 days | 0 | 0/83 (0%) ns | 0/27 (0%) | Seki 2003 [11] |
| L rhamnosus (strains E/N, Oxy, Pen) | mixed, mostly pen and ceph | 4 × 1010 | duration (x = 8 day) | 2 weeks | 3/120 (2.5%) ns | 7/120 (5.8%) | Ruszczynski 2008 [12] |
| L. rhamnosus GG +L. acido. La5 + Bifido. lactis Bb12 | mixed, nr types | 5 × 1010 | 2 weeks | 0 | 0/34 (0%) ns | 1/29 (3.4%) | Wenus 2008 [13] |
| L. acidophilus (CUL 60 and CUL 21) + Bifido. bifidum CUL20 +Bifido. lactis CUL34 | mixed, 21% single, 70% pen | 6 × 1010 | 3 weeks | 10 weeks | 12/1470 (0.8%) ns | 17/1471 (1.2%) | Allen 2013 [14] |
| VSL#3 | mixed, 75% pen | 9 × 1011 | duration + 1 week | 3 weeks | 0/117 (0%) ns | 0/112 (0%) | Selinger 2013 [15] |
| Secondary prevention of CDI | |||||||
| L. plantarum 299v | mixed | 5 × 1010 | 5.4 weeks | 4.5 weeks | 4/11 (36%) recurred | 6/9 (67%) | Wullt 2003 [16] |
| Enrolled population | % Attrition | Single or Multiple Types of Inciting Antibiotics | Most Common Type of Antibiotic | Type(s) of Infections | Reference |
|---|---|---|---|---|---|
| adults, I | 43 | 59% multiple | 36% cepha | mixed, nr | Surawicz 1989 [17] |
| adults, I | 38 | 82% multiple | beta-lactams | mixed, nr | McFarland 1995 [18] |
| elderly, I | 4.2 | nr | nr | nr | Lewis 1998 [19] |
| adult, O | 3.3 | 100% multiple | amox and clarithromycin | H. pylori infections | Duman 2005 [20] |
| pediatric, I&O | 8.5 | nr | 41% cepha | 68% resp, 29% otitis media | Kotowska 2005 [21] |
| adults, I | 0 | nr | 83% beta-lactams | nr | Can 2006 [22] |
| adults, O | 4.6 | 100% single | 100% amox | 88% resp | Bravo 2008 [23] |
| adults, I | 26 | 69% single | mixed, nr | nr | Pozzoni 2012 [24] |
| pediatric, I | 15 | nr | 52% cepha | resp | Shan 2014 [25] |
| pediatric, O | 28.7 | nr | 66% amox | 74% otitis media, 26% resp | Arvola 1999 [26] |
| adults, I | 11.6 | nr | 69% beta-lactams | nr | Thomas 2001 [27] |
| adults, I | 0 | nr | cepha | nr | Miller 2008a [28] |
| adults, I | 0 | 69% single | 50% cepha | nr | Miller 2008b [28] |
| adults, I | 5.5 | only 34% with VAP on abx | nr | pneumonia | Morrow 2010 [29] |
| adults, I | 19 | 61% single | 66% amox or cepha | 49% resp | Hickson 2007 [30] |
| adults, I | 0 | nr | 60% amp or cepha | 80% resp or GU | Dietrich 2014 [31] |
| elderly, I | 8 | nr | nr | nr | Plummer 2004 [32] |
| adults, I | 0 | nr | mixed | nr | Rafiq 2007 [33] |
| adults, I | 0 | nr | 48% ceph | nr | Stein 2007 [34] |
| adults, I | 0 | nr | 59% quinolones | 92% resp | Beausoleil 2007 [35] |
| adults, I&O | 7.4 | nr | 78% beta-lactams | 39% resp | Sampalis 2010 [36] |
| adults, I | 9 | nr | 41% cepha | 47% resp | Gao 2010a [37] |
| adults, I | 7 | nr | 37% cepha | 47% resp | Gao 2010b [37] |
2.5. Patient Population
2.6. Antibiotic Exposure
2.7. Interventions
| Probiotic | Daily Dose (cfu/d) | Formulation | Duration Treatment | Follow-up (weeks) | CDI in Probiotic | CDI in Controls | Power | Reference |
|---|---|---|---|---|---|---|---|---|
| S. boulardii | 2 × 1010 | capsules | duration + 2 weeks | 0 | 3 (2.6%) | 5 (7.8%) | 26.5% | Surawicz 1989 [17] |
| S. boulardii | 3 × 1010 | capsules | duration + 3 days | 7 | 3 (3.1%) | 4 (4.2%) | 2.6% | McFarland 1995 [18] |
| S. boulardii | 4.5 × 109 | capsules | duration (x = 7 days) | 0 | 5 (15%) | 3 (8.3%) | 7.2% | Lewis 1998 [19] |
| S. boulardii | 1 × 1010 | capsules | duration (x = 2 weeks) | 4 days | 0 (0%) | 1 (0.5%) | 3.3% | Duman 2005 [20] |
| S. boulardii | 1 × 1010 | wafers | duration (x = 1 week) | 0 | 3 (2.5%) | 10 (7.9%) | 35.6% | Kotowska 2005 [21] |
| S. boulardii | 1 × 1010 | capsules | duration | 4 | 0 (0%) | 2 (2.6%) | 9.1% | Can 2006 [22] |
| S. boulardii | 1 × 1010 | capsules | 12 days | 9 days | 0 (0%) | 0 (0%) | -- | Bravo 2008 [23] |
| S. boulardii | 1 × 1010 | capsules | duration + 7 days | 12 | 3 (2.8%) | 2 (2%) | 3% | Pozzoni 2012 [24] |
| S. boulardii | 1 × 1010 | powder | duration (x = 2 weeks) | 2 | 1 (0.7%) | 8 (5.6%) | 51.9% | Shan 2014 [25] |
| L. rhamnosus GG | 4 × 1010 | capsules | duration (x = 7–10 day) | 12 | 1 (1.6%) | 1 (1.7%) | 10% | Arvola 1999 [26] |
| L. rhamnosus GG | 2 × 1010 | capsules | 2 weeks | 1 | 2 (1.5%) | 3 (2.2%) | 2.7% | Thomas 2001 [27] |
| L. rhamnosus GG | 4 × 1010 | capsules | duration (x = 2 weeks) | 4 | 4 (4.2%) | 7 (7.4%) | 9.2% | Miller 2008a [28] |
| L. rhamnosus GG | 1.2 × 1011 | capsules | duration (x = 2 weeks) | 4 | 2 (1.3%) | 0 | 11.2% | Miller 2008b [28] |
| L. rhamnosus GG | 4 × 109 | capsules | duration (x = 15 day) | 0 | 4 (5.8%) | 13 (18.6%) | 52.9% | Morrow 2010 [29] |
| L. casei DN 114001 | 2 × 1010 | drink | duration + 1 week | 4 | 0 (0%) | 9 (17%) | 81% | Hickson 2007 [30] |
| L. casei DN 114001 | 2 × 1010 | drink | duration (x = 6 days) | 0 | 0 (0%) | 3 (10%) | 21.3% | Dietrich 2014 [31] |
| L acidophilus +Bifido. bifidum | 2 × 1010 | capsules | 20 d | 0 | 2 (2.9%) | 5 (7.2%) | 11.5% | Plummer 2004 [32] |
| L acidophilus +Bifido. bifidum | cfu nr (3g/day) | capsules | duration or LOS | 0 | 5 (11%) | 22 (40%) | 88.0% | Rafiq 2007 [33] |
| L acidophilus +Bifido. bifidum | 6 × 109 | capsules | 3 weeks | 0 | 3 (14.3%) | 1 (4.8%) | 7.2% | Stein 2007 [34] |
| L. acidophilus CL1285 + L. casei LBC80R + L. rhamnosus CLR2 | 5 × 1010 | milk | duration (x = 7–8 day) | 3 | 1 (2.3%) | 7 (15.6%) | 44.2% | Beausoleil 2007 [35] |
| L. acidophilus CL1285 + L. casei LBC80R + L. rhamnosus CLR2 | 5 × 1010 | milk | duration + 5 days | 3 | 1 (0.5%) | 4 (1.8%) | 12.5% | Sampalis 2010 [36] |
| L. acidophilus CL1285 + L. casei LBC80R + L. rhamnosus CLR2 | 5 × 1010 | capsules | duration + 5 days | 3 | 8 (9.4%) | 20 (23.8%) | 64% | Gao 2010a [37] |
| L. acidophilus CL1285 + L. casei LBC80R + L. rhamnosus CLR2 | 1 × 1011 | capsules | duration + 5 days | 3 | 1 (1.2%) | 20 (23.8%) | 99.2% | Gao 2010b [37] |
| History of CDI | Pop-ulation | Type of controls | Adjunctive therapy (daily dose) | Probiotic | Probiotic daily dose (cfu/day) | Duration treated (follow-up) | Frequency CDI recurrences in probiotic | Frequency CDI recurrences in controls | Power (%) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| I/R | 124 adults, In & Out | placebo | V or M (varied) | S. boulardii | 3 × 1010 | 4 weeks (4 weeks) | 15/57 (26.3%)* | 30/67 (44.8%) | 49.5 | McFarland 1994 [38] |
| R | 83 adults, In & Out | placebo | V (500 mg) | S. boulardii | 2 × 1010 | 4 weeks (4 weeks) | 23/45 (51%) | 17/38 (44.7%) | 5.3 | Surawicz 2000a [39] |
| R | 32 adults, In & Out | placebo | V (2 g) | S. boulardii | 2 × 1010 | 4 weeks (4 weeks) | 3/18 (17%)* | 7/14 (50%) | 35.9 | Surawicz 2000b [39] |
| R | 53 adults, In & Out | placebo | M (1g) | S. boulardii | 2 × 1010 | 4 weeks (4 weeks) | 13/27 (48%) | 13/26 (50%) | 3.3 | Surawicz 2000c [39] |
| I/R | 25 adults, In & Out | placebo | V (nr) M (nr) | L rhamnosus GG | nr | 3 weeks (4 weeks) | 4/11 (36.4%) | 5/14 (35.7%) | 5.7 | Pochapin 2000 [40] |
| R | 15 adults | placebo | 20% V (nr) 80% M (nr) | L rhamnosus GG + inulin | 3 × 1011 | duration abx + 21 days (8.6) | 3/8 (37.5%) | 1/7 (14.3%) | 5.3 | Lawrence 2005 [41] |
2.8. Pooled Efficacy of Probiotics for Primary CDI Prevention
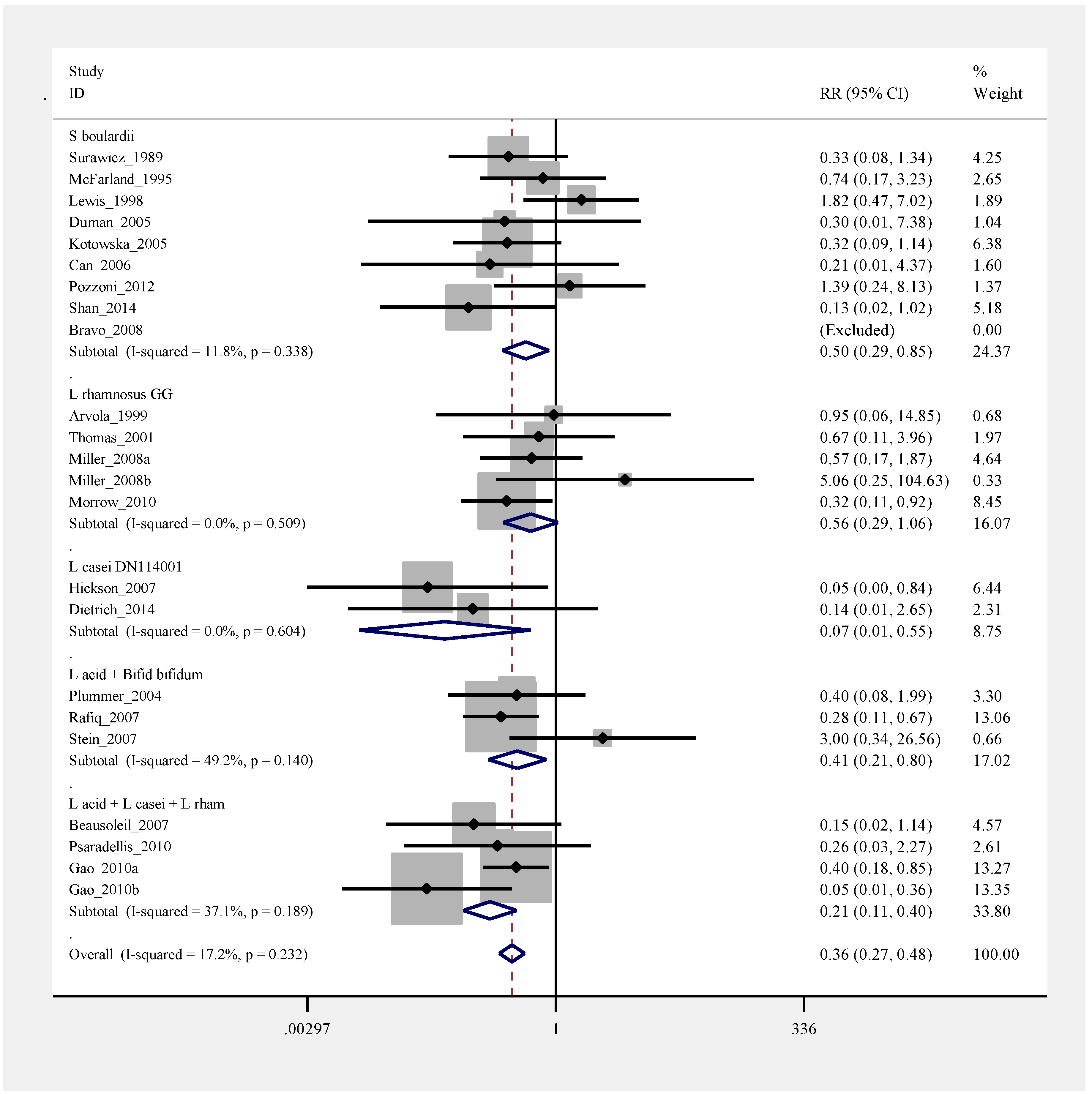
2.9. Pooled Efficacy of Probiotics for Secondary CDI Prevention
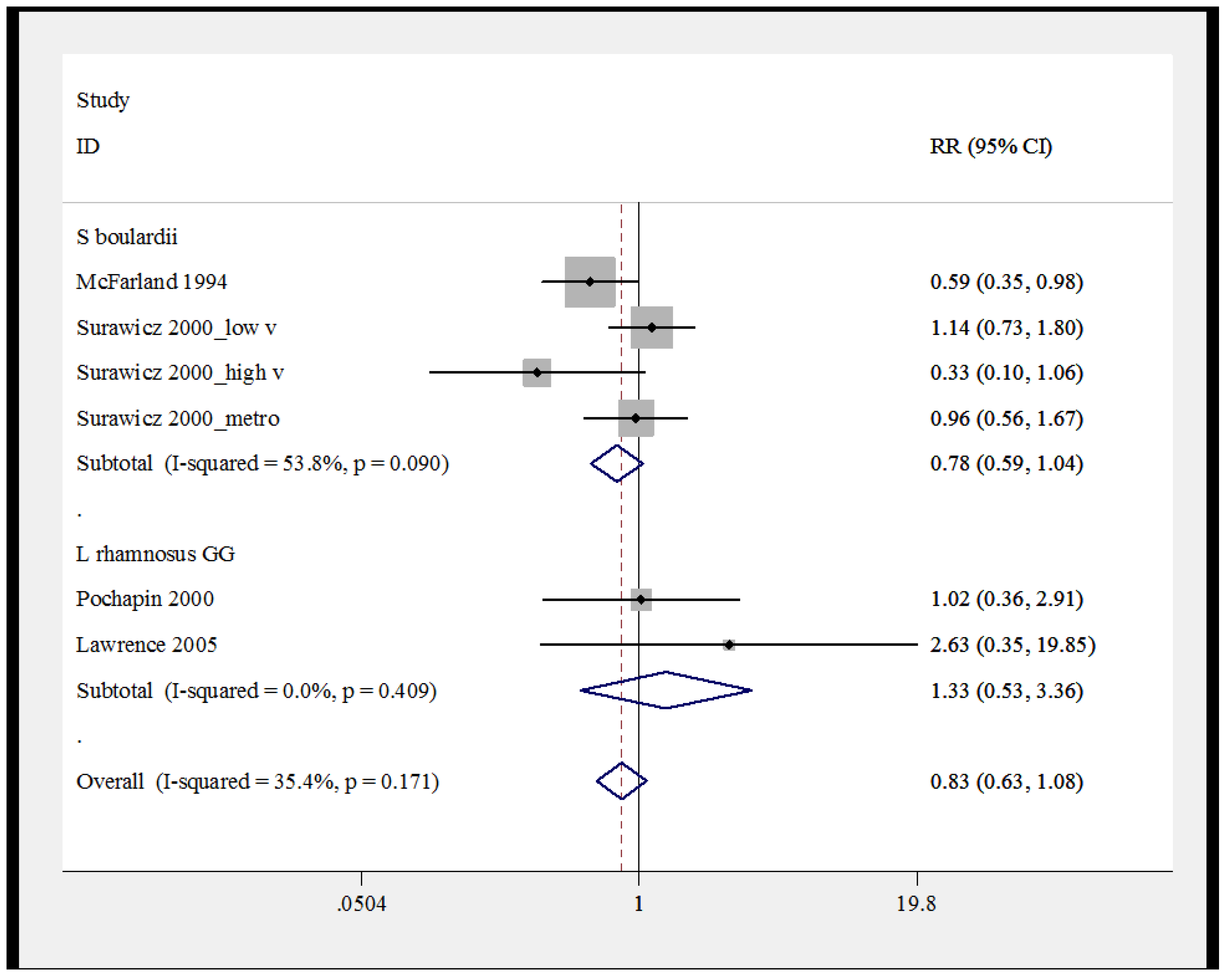
3. Discussion
4. Methods
4.1. Aims
4.2. Search Strategy
4.3. Inclusion and Exclusion Criteria
4.4. Data Extraction
4.5. Interventions
4.6. Statistical Analysis
4.7. Publication Bias
5. Conclusions
Acknowledgements
Conflicts of Interest
References
- Lessa, F.C.; Mu, Y.; Winston, L.G.; Dumyati, G.K.; Farley, M.M.; Beldavs, Z.G.; Kast, K.; Holzbauer, S.M.; Meek, J.I.; Cohen, J.; et al. Burden of Clostridium difficile infection in the United States. N. Engl. J. Med. 2015, 372, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Surawicz, C.M.; Brandt, L.J.; Binion, D.G.; Ananthakrishnan, A.N.; Curry, S.R.; Gilligan, P.H.; McFarland, L.V.; Mellow, M.; Zuckerbraun, B.S. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am. J. Gastroenterol. 2013, 108, 478–498. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Zingg, W.; Knoll, E.; Wilson, C.; Dettenkofer, M. National European guidelines for the prevention of Clostridium difficile infection: A systematic qualitative review. J. Hosp. Infect. 2014, 87, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Maziade, P.J.; Andriessen, J.A.; Pereira, P.; Currie, B.; Goldstein, E.J.C. Impact of adding prophylactic probiotics to a bundle of standard preventative measures for Clostridium difficile infections: Enhanced and sustained decrease in the incidence and severity of infection at a community hospital. Curr. Med. Res. Opin. 2013, 29, 1341–1347. [Google Scholar] [CrossRef] [PubMed]
- McFarland, L.V. Meta-analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile disease. Am. J. Gastroenterol. 2006, 101, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.; Maziade, P.J.; McFarland, L.V.; Trick, W.; Donskey, C.; Currie, B.; Low, D.E.; Goldstein, E.J. Is primary prevention of Clostridium difficile infection possible with specific probiotics? Int. J. Infect. Dis. 2012, 16, e786–e792. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.; Jamous, A.; O’Driscoll, J.; Sekhar, R.; Weldon, M.; Yau, C.Y.; Hirani, S.P.; Grimble, G.; Forbes, A. A Lactobacillus casei Shirota probiotic drink reduces antibiotic-associated diarrhoea in patients with spinal cord injuries: A randomised controlled trial. Br. J. Nutr. 2014, 111, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Safdar, N.; Barigala, R.; Said, A.; McKinley, L. Feasibility and tolerability of probiotics for prevention of antibiotic-associated diarrhea in hospitalized US military veterans. J. Clin. Pharm. Ther. 2008, 33, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Lönnermark, E.; Friman, V.; Lappas, G.; Sandberg, T.; Berggren, A.; Adlerberth, I. Intake of Lactobacillus plantarum reduces certain gastrointestinal symptoms during treatment with antibiotics. J. Clin. Gastroenterol. 2010, 44, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Destura, R.V. Bacillus clausii in preventing antibiotic-associated diarrhea among Filipino infants and children: A multi-center, randomized, open-label clinical trial of efficacy and safety. Available online: http://en.sanofi.com/img/content/study/ENTER_L_01125_summary.pdf (accessed on 3 June 2013).
- Seki, H.; Shiohara, M.; Matsumura, T.; Miyagawa, N.; Tanaka, M.; Komiyama, A.; Kurata, S. Prevention of antibiotic-associated diarrhea in children by Clostridium butyricum MIYAIRI. Ped. Interl. 2003, 45, 86–90. [Google Scholar] [CrossRef]
- Ruszczyński, M.; Radzikowski, A.; Szajewska, H. Clinical trial: Effectiveness of Lactobacillus rhamnosus (strains E/N, Oxy and Pen) in the prevention of antibiotic-associated diarrhoea in children. Aliment. Pharmacol. Ther. 2008, 28, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Wenus, C.; Goll, R.; Loken, E.B.; Biong, A.S.; Halvorsen, D.S.; Florholmen, J. Prevention of antibiotic-associated diarrhoea by a fermented probiotic milk drink. Eur. J. Clin. Nutr. 2008, 62, 299–301. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.J.; Wareham, K.; Wang, D.; Bradley, C.; Sewell, B.; Hutchings, H.; Harris, W.; Dhar, A.; Brown, H.; Foden, A.; et al. A high-dose preparation of lactobacilli and bifidobacteria in the prevention of antibiotic-associated and Clostridium difficile diarrhoea in older people admitted to hospital: A multicentre, randomised, double-blind, placebo-controlled, parallel arm trial (PLACIDE). Health Technol. Assess. 2013, 17, 1–140. [Google Scholar] [CrossRef] [PubMed]
- Selinger, C.P.; Bell, A.; Cairns, A.; Lockett, M.; Sebastian, S.; Haslam, N. Probiotic VSL#3 prevents antibiotic-associated diarrhoea in a double-blind, randomized, placebo-controlled clinical trial. J. Hosp. Infect. 2013, 84, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Wullt, M.; Hagslatt, M.J.; Odenholt, I. Lactobacillus plantarum 299v for the treatment of recurrent Clostridium difficile-associated diarrhoea: A double-blind, placebo-controlled trial. Scand. J. Infect. Dis. 2003, 35, 365–367. [Google Scholar] [CrossRef] [PubMed]
- Surawicz, C.M.; McFarland, L.V.; Elmer, G.; Chinn, J. Treatment of recurrent Clostridium difficile colitis with vancomycin and Saccharomyces boulardii. Am. J. Gastroenterol. 1989, 84, 1285–1287. [Google Scholar] [PubMed]
- McFarland, L.V.; Surawicz, C.M.; Greenberg, R.N.; Elmer, G.W.; Moyer, K.A.; Melcher, S.A.; Bowen, K.E.; Cox, J.L. Prevention of β-lactam-associated diarrhea by Saccharomyces boulardii compared to placebo. Am. J. Gastroenterol. 1995, 90, 439–448. [Google Scholar] [PubMed]
- Lewis, S.J.; Potts, L.F.; Barry, R.E. The lack of therapeutic effect of Saccharomyces boulardii in the prevention of antibiotic-related diarrhoea in elderly patients. J. Infect. 1998, 36, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Duman, D.G.; Bor, S.; Ozütemiz, O.; Sahin, T.; Oğuz, D.; Iştan, F.; Vural, T.; Sandkci, M.; Işksal, F.; Simşek, I.; et al. Efficacy and safety of Saccharomyces boulardii in prevention of antibiotic-associated diarrhoea due to Helicobacterpylori eradication. Eur. J. Gastroenterol. Hepatol. 2005, 17, 1357–1361. [Google Scholar] [CrossRef] [PubMed]
- Kotowska, M.; Albrecht, P.; Szajewska, H. Saccharomyces boulardii in the prevention of antibiotic-associated diarrhoea in children: A randomized double-blind placebo-controlled trial. Aliment. Pharmacol. Ther. 2005, 21, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Can, M.; Beşirbellioglu, B.A.; Avci, I.Y.; Beker, C.M.; Pahsa, A. Prophylactic Saccharomyces boulardii in the prevention of antibiotic-associated diarrhea: A prospective study. Med. Sci. Monit. 2006, 12, PI19–PI22. [Google Scholar] [PubMed]
- Bravo, M.V.; Bunout, D.; Leiva, L.; de la Maza, M.P.; Barrera, G.; de la Maza, J.; Hirsch, S. Effect of probiotic Saccharomyces boulardii on prevention of AAD in adult outpatients with amoxicillin treatment. Rev. Med. Chile. 2008, 136, 981–988. [Google Scholar] [PubMed]
- Pozzoni, P.; Riva, A.; Bellatorre, A.G.; Amigoni, M.; Redaelli, E.; Ronchetti, A.; Stefani, M.; Tironi, R.; Molteni, E.E.; Conte, D.; et al. Saccharomyces boulardii for the prevention of antibiotic-associated diarrhea in adult hospitalized patients: A single-center, randomized, double-blind, placebo-controlled trial. Am. J. Gastroenterol. 2012, 107, 922–931. [Google Scholar]
- Shan, L.; Hou, P.; Wang, Z.; Liu, F.R.; Chen, N.; Shu, L.H.; Zhang, H.; Han, X.H.; Han, X.X.; Cai, X.X.; et al. Prevention and treatment of diarrhea with Saccharomyces boulardii in children with acute lower respiratory tract infections. Benef. Microbes 2013, 4, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Arvola, T.; Laiho, K.; Torkkeli, S.; Mykkanen, H.; Salminen, S.; Maunula, L.; Isolauri, E. Prophylactic Lactobacillus GG reduces antibiotic-associated diarrhea in children with respiratory infections: A randomized study. Pediatrics 1999, 104, e64. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.R.; Litin, S.C.; Osmon, D.R.; Corr, A.P.; Weaver, A.L.; Lohse, C.M. Lack of effect of Lactobacillus GG on antibiotic-associated diarrhea: A randomized, placebo-controlled trial. Mayo Clin. Proc. 2001, 76, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.; Gravel, D.; Mulvey, M.; Taylor, G.; Gardam, M.; McGeer, A.; Hutchinson, J.; Moore, D.; Kelly, S.; Boyd, D.; et al. Health care-associated Clostridium difficile infection in Canada: Patient age and infecting strain type are highly predictive of severe outcome and mortality. Clin. Infect. Dis. 2010, 50, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Morrow, L.E.; Kollef, M.H.; Casale, T.B. Probiotic prophylaxis of ventilator-associated pneumonia: A blinded, randomized, controlled trial. Am. J. Respir. Crit. Care Med. 2010, 182, 1058–1064. [Google Scholar] [CrossRef] [PubMed]
- Hickson, M.; D’Souza, A.L.; Muthu, N.; Rogers, T.R.; Want, S.; Rajkumar, C.; Bulpitt, C.J. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: Randomised double blind placebo controlled trial. Br. Med. J. 2007, 335, 80–83. [Google Scholar] [CrossRef]
- Dietrich, C.G.; Kottmann, T.; Alavi, M. Commercially available probiotic drinks containing Lactobacillus casei DN-114001 reduce antibiotic-associated diarrhea. World J. Gastrol. 2014, 20, 15837–15844. [Google Scholar] [CrossRef]
- Plummer, S.; Weaver, M.A.; Harris, J.C.; Dee, P.; Hunter, J. Clostridium difficile pilot study: Effects of probiotic supplementation on the incidence of C. difficile diarrhoea. Int. Microbiol. 2004, 7, 59–62. [Google Scholar] [PubMed]
- Rafiq, R. Prevention of Clostridium difficile (C. difficile) diarrhea with probiotic in hospitalized patients treated with antibiotics. Gastroenterology 2007, 132, A187. [Google Scholar]
- Stein, G.Y.; Nanim, R.; Karniel, E.; Moskowitz, I.; Zeidman, A. Probiotics as prophylactic agents against antibiotic-associated diarrhea in hospitalized patients. Harefuah 2007, 146, 520–522, 575. [Google Scholar] [PubMed]
- Beausoleil, M.; Fortier, N.; Guénette, S.; L'ecuyer, A.; Savoie, M.; Franco, M.; Lachaine, J.; Weiss, K. Effect of a fermented milk combining Lactobacillus acidophilus Cl1285 and Lactobacillus casei in the prevention of antibiotic-associated diarrhea: A randomized, double-blind, placebo-controlled trial. Can. J. Gastroenterol. 2007, 21, 732–736. [Google Scholar] [PubMed]
- Sampalis, J.; Psaradellis, E.; Rampakakis, E. Efficacy of BIO K+ CL1285® in the reduction of antibiotic-associated diarrhea—A placebo controlled double-blind randomized, multi-center study. Arch. Med. Sci. 2010, 6, 56–64. [Google Scholar] [PubMed]
- Gao, X.W.; Mubasher, M.; Fang, C.Y.; Reifer, C.; Miller, L.E. Dose-Response Efficacy of a Proprietary Probiotic Formula of Lactobacillus acidophilus CL1285 and Lactobacillus casei LBC80R for Antibiotic-Associated Diarrhea and Clostridium difficile-Associated Diarrhea Prophylaxis in Adult Patients. Am. J. Gastroenterol. 2010, 105, 1636–1641. [Google Scholar] [CrossRef] [PubMed]
- McFarland, L.V.; Surawicz, C.M.; Greenberg, R.N.; Fekety, R.; Elmer, G.W.; Moyer, K.A.; Melcher, S.A.; Bowen, K.E.; Cox, J.L.; Noorani, Z.; et al. A randomized placebo-controlled trial of Saccharomyces boulardii in combination with standard antibiotics for Clostridium difficile disease. JAMA 1994, 271, 1913–1918. [Google Scholar] [CrossRef] [PubMed]
- Surawicz, C.M.; McFarland, L.V.; Greenberg, R.N.; Fekety, R.; Elmer, G.W.; Moyer, K.A.; Melcher, S.A.; Bowen, K.E.; Cox, J.L.; Noorani, Z.; et al. The search for a better treatment for recurrent Clostridium difficile disease: Use of high-dose vancomycin combined with Saccharomyces boulardii. Clin. Infect. Dis. 2000, 31, 1012–1017. [Google Scholar] [CrossRef] [PubMed]
- Pochapin, M. The effect of probiotics on Clostridium difficile diarrhea. Am. J. Gastroenterol. 2000, 95, S11–S13. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, S.J.; Korzenik, J.R.; Mundy, L.M. Probiotics for recurrent Clostridium difficile disease. J. Med. Microbiol. 2005, 54, 905–906. [Google Scholar] [CrossRef] [PubMed]
- Dinleyici, E.C.; Kara, A.; Ozen, M.; Vandenplas, Y. Saccharomyces boulardii CNCM I-745 in different clinical conditions. Expert Opin. Biol. Ther. 2014, 14, 1593–1609. [Google Scholar] [CrossRef] [PubMed]
- Auclair, J.; Frappier, M.; Millette, M. Lactobacillus acidophilus C1285, L. casei LBC80R and L. rhamnosus CLR2 (Bio-K+): Characterization, manufacture, mechanisms of action and quality control of a specific probiotic combination for primary prevention of Clostridium difficile infections. Clin. Infect. Dis 2015, in press. [Google Scholar]
- Guarner, F.; Khan, A.G.; Garisch, J.; Eliakim, R.; Gangl, A.; Thomson, A.; Krabshuis, J.; Lemair, T.; Kaufmann, P.; de Paula, J.A.; et al. World Gastroenterology Organisation Global Guidelines: Probiotics and prebiotics October 2011. J. Clin. Gastroenterol. 2012, 46, 468–481. [Google Scholar] [CrossRef] [PubMed]
- Avadhani, A.; Miley, H. Probiotics for prevention of antibiotic-associated diarrhea and Clostridium difficile-associated disease in hospitalized adults—A meta-analysis. J. Am. Acad. Nurse Pract. 2011, 23, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, J.Z.; Ma, S.S.; Saxton, J.D.; Martzen, M.R.; Vandvik, P.O.; Thorlund, K.; Guyatt, G.H.; Johnston, B.C. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst. Rev. 2013, 5, CD006095. [Google Scholar] [PubMed]
- Bermudez-Brito, M.; Plaza-Díaz, J.; Muñoz-Quezada, S.; Gómez-Llorente, C.; Gil, A. Probiotic mechanisms of action. Ann. Nutr. Metab. 2012, 61, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Pattani, R.; Palda, V.A.; Hwang, S.W.; Shah, P.S. Probiotics for the prevention of antibiotic-associated diarrhea and C. difficile infection among hospitalized patients: Systematic review and meta-analysis. Open Med. 2013, 7, e56–e67. [Google Scholar] [PubMed]
- McFarland, L.V.; Goh, S. Preventing Pediatric Antibiotic-Associated Diarrhea and Clostridium difficile Infections with Probiotics: A meta-analysis. World J. Meta-Anal. 2013, 1, 102–120. [Google Scholar] [CrossRef]
- Johnston, B.C.; Ma, S.S.; Goldenberg, J.Z.; Thorlund, K.; Vandvik, P.O.; Loeb, M.; Guyatt, G.H. Probiotics for the prevention of Clostridium difficile-associated diarrhea: A systematic review and meta-analysis. Ann. Intern. Med. 2012, 157, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. Br. Med. J. 2009, 339, b2700. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. Br. Med. J. 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gøtzsche, P.C.; Devereaux, P.J.; Elbourne, D.; Egger, M.; Altman, D.G. Consolidated Standards of Reporting Trials Group. CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomized trials. J. Clin. Epidemiol. 2010, 63, e1–e37. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. Br. Med. J. 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.L.; Sutton, A.J.; Jones, D.R.; Abrams, K.R.; Rushton, L. Comparison of two methods to detect publication bias in meta-analysis. JAMA 2006, 295, 676–680. [Google Scholar] [CrossRef] [PubMed]
- McFarland, L.V.; Elmer, G.W.; Surawicz, C.M. Breaking the cycle: Treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am. J. Gastroenterol. 2002, 97, 1769–1775. [Google Scholar] [CrossRef] [PubMed]
- McFarland, L.V. Systematic review and meta-analysis of Saccharomyces boulardii in adult patients. World J. Gastroenterol. 2010, 16, 2202–2222. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McFarland, L.V. Probiotics for the Primary and Secondary Prevention of C. difficile Infections: A Meta-analysis and Systematic Review. Antibiotics 2015, 4, 160-178. https://doi.org/10.3390/antibiotics4020160
McFarland LV. Probiotics for the Primary and Secondary Prevention of C. difficile Infections: A Meta-analysis and Systematic Review. Antibiotics. 2015; 4(2):160-178. https://doi.org/10.3390/antibiotics4020160
Chicago/Turabian StyleMcFarland, Lynne V. 2015. "Probiotics for the Primary and Secondary Prevention of C. difficile Infections: A Meta-analysis and Systematic Review" Antibiotics 4, no. 2: 160-178. https://doi.org/10.3390/antibiotics4020160
APA StyleMcFarland, L. V. (2015). Probiotics for the Primary and Secondary Prevention of C. difficile Infections: A Meta-analysis and Systematic Review. Antibiotics, 4(2), 160-178. https://doi.org/10.3390/antibiotics4020160





