Host Defense Peptides from Asian Frogs as Potential Clinical Therapies
Abstract
:1. Introduction
2. Diversity of HDPs Found in the Skin Secretion of Asian Frogs
2.1. Amolops
| Peptides isolated from the respective frog species: numbers indicate paralogs of each peptide family | ||||||||
|---|---|---|---|---|---|---|---|---|
| Genus: Amolops | Brevinin-1 | Brevinin-2 | Esculentin-2 | Palustrin-2 | Temporin | Novel family of Peptides | Ref | |
| 1 | A. chunganensis | 5 | 1 | 1 | 1 | 5 | [9,10,11,12,13,14,15,16,17] | |
| 2 | A. hainanensis | 2 | 1 | Amylopin-1: 3 Amylopin-6: 1 Hainanenin 1-5,5 families: 5 | ||||
| 3 | A. jindongenensis | 1 | 2 | 2 | Jindongnenin: 1 | |||
| 4 | A. loloensis | 4 | 2 | 11 | Amylopin 1-2,2 families: 2 Amylopkinin: 1 | |||
| 5 | A. lifanensis | 3 | 1 | |||||
| 6 | A. ricketti | 3 | 2 | |||||
| 7 | A. torrentis | 1 | ||||||
| 8 | A. wuyiensis | Amylopkinin: 2 | ||||||
| Genus: Glandirana | Brevinin-1 | Brevinin-2 | Esculentin-2 | Ref | ||||
| 1 | G. rugosa | 2 (Gaegurin 5–6) | 6 (Gaegurin 1–3 Rugosin A,B,C) | [13,18,19,20] | ||||
| 2 | G. emeljanovi | 2 (Gaegurin 5–6) | 1 (Gaegurin 5–6) | |||||
| Genus: Hylarana | Brevinin-1 | Brevinin-2 | Esculentin-1 | Esculentin-2 | Temporin | Novel family of Peptides | Ref | |
| 1 | H. erythrea | 3 | 2 | 4 | 1 | [8,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36] | ||
| 2 | H. guentheri | 2 | 6 | 6 | Guentherin: 1 Bradykinin BRP: 12 * | |||
| 3 | H. latuouchii | 4 | 3 | 2 | 2 | 6 | Palustrin-2 | |
| 4 | H. luctiosa | 4 | 1 | 1 | 2 | Palustrin-2: 2 | ||
| 5 | H. nigrovittata | 7 (7 Gaegurins) | 9 (9 Rugosins) | 3 | Nigroain: 15 Ranakinin N: 1 Cholycytokinin: 1 | |||
| 6 | H. picturata | 2 | 5 | 1 | ||||
| 7 | H. signata | 5 | 4 | 2 | Palustrin-2 | |||
| 8 | H. spinulosa | 2 | 4 | 1 | 2 | 5 | Spinulosain: 1 Ranatuerin: 1 Nigroain: 5 Odorranain: 1 Ranacyclin: 1 | |
| 9 | H. temporalis | 1 | 2 | 3 | Hylaranakinin: 2 | |||
| Genus: Odorrana | Brevinin-1 | Brevinin-2 | Esculentin-1 | Esculentin-2 | Nigrocin-2 | Novel family of Peptides | Ref | |
| 1 | O. grahamii | 2 | 4 | 2 | 4 | 4 | Takykinin: 2 TrypsinInhibitor: 1, BRP: 3 * BLP: 5 * Odorrnalectin: 1 Palustrin-2: 1 Grahamin 1–2: 2 families: 2 Odorranain: 27 | [14,28,31,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58] |
| 2 | O. hananensis | 2 | 2 | Odorrnain: 2 Temporin: 2 | ||||
| 3 | O. hejiangensis | TrypsinInhibitor: 1 | ||||||
| 4 | O. hossi | 2 | 2 | 1 | 1 | 2 | ||
| 5 | O. ishikawae | 1 | 3 | 2 | 1 | 5 | Ishikawain 1–8, 8 families: 8 Palustrin 2: 3 Odorranain: 2 | |
| 6 | O. jingdongensis | 3 | 1 | 2 | 2 | |||
| 7 | O. livida | Lividin 1–4, 4 families: 4 | ||||||
| 8 | O. schmakeri | 3 | 3 | 1 | 1 | BRP: 7 * | ||
| 9 | O. tiannanensis | 3 | 1 | 2 | 1 | Mararetaein: 2 Pleurain: 1 Tiannenensin: 1 Odorranain: 10 | ||
| 10 | O. versablis | 2 | 2 | 2 | Ranatuerins: 2 Temporin: 1 TrypsinInhibitor: 1 | |||
| Genus: Pelophylax | Brevinin-1 | Brevinin-2 | Esculentin-1 | Esculentin-2 | Novel family of Peptides | Ref | ||
| 1 | P. plancyi | 3 | [13,20,56,59,60] | |||||
| 2 | P. porosus | 1 | ||||||
| 3 | P. chosenicus | 1 | ||||||
| 4 | P. fukienensis | 1 | 1 | 1 | Pelophylaxin 1–4, 4 families Ranakinestatin: 1 | |||
| 5 | P. nigromaculata | 1 (Nigrocin-1) | 2 | Nigocin-2: 1 | ||||
| Genus: Rana | Brevinin-1 | Brevinin-2 | Temporin-1 | Ranatuerin-2 | Novel family of Peptides | Ref | ||
| 1 | R. amurensis | 3 (Amurin 1–3:3 families) | 2 | [20,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80] | ||||
| 2 | R brevipoda porsa | 1 | 1 | |||||
| 3 | R. chaochioensis | Japonicin-2: 4 | ||||||
| 4 | R. chensinensis | 6 | 3 | 11 | RCSK 1–4, 4 families: 4 * Chensinin 1–4, 4 families: 7 Japonicin-1: 1 D-1CDYa: 1 ** | |||
| 5 | R. dybowskii | 18 (9 Dybowskins) | 7 | 3 | Japonicin-1: 1 | |||
| 6 | R. japonica | 1 | Japonicin-1: 1 Japonicin-2: 1 | |||||
| 7 | R. okinavana | 4 | 1 | |||||
| 8 | R. ornativentris | 2 | 7 | 4 | Palustrin: 1 | |||
| 9 | R. pirica | 1 | 5 | 2 | 1 | |||
| 10 | R. pleuradan | Pleurain: 2 | ||||||
| 11 | R. sakurai | 2 | 4 | 1 | MRP: 1 *, BRP: 1 * | |||
| 12 | R. shuchinae | Shuchin 1–5, 5 families: 5 | ||||||
| 13 | R. tagoi | 1 | 1 | MRP: 1 * | ||||
| 14 | R. tagoiokiensis | 2 | 2 | 2 | ||||
| 15 | R. tsushimensis | 1 | 1 | 4 | ||||
| Genus: Clinotarsus | Ref | |||||||
| 1 | C. curtipes | Brevinin-1: 5 | [81] | |||||
| Genus: Fejervaria | ||||||||
| 1 | F. carcrivora | Tigerinin: 2 | [82] | |||||
| Genus: Hoplobatracus | ||||||||
| 1 | H. rugulosus | Tigerinin-1: 1 | [83,84,85] | |||||
| 2 | H. tigerinus | Tigerinin 1–4, 4 families: 4 | ||||||
| Genus: Hyla | Ref | |||||||
| 1 | H. annectans | Annotoxin: 1 | [86] | |||||
| Genus: Limnonectes | ||||||||
| 1 | L. fujianensis | Limnonectins: 2 | [87] | |||||
| Genus: Nanorana | ||||||||
| 1 | N. parkeri | Japonicin: 2 Parkerin: 1 | [88] | |||||
| Genus: Rhacophorus | ||||||||
| 1 | R. duboisi | Polypedarelaxin: 1 Polypedatein: 1 | [89,90] | |||||
| 2 | R. scheglii | Histone 2B | ||||||
| Genus: Sanguirana | ||||||||
| 1 | S. varians | BLP: 1 * | [91] | |||||
| Genus: Euphlyctis | ||||||||
| 1 | E. hexadactylus | Crude skin extract: peptidesnot characterized | [92] | |||||
| MIC and IC50/LD50 values | |||||||
|---|---|---|---|---|---|---|---|
| Genus: Amolops | Brevinin-1 | Brevinin-2 | Esculentin-2 | Palustrin-2 | Temporin | Novel family of Peptides | |
| 1 | A. chunganensis | G+: 0.5–75 µM G−: 4–150 µM F: 4–150 µM LD50: 15–150 µM | G+: 2–75 µM G−: 2–150 µM F: 9 µM LD50: 75 µM | G+: 1–19 µM G−: 75–150 µM F: 4.5 µM LD50: 75 µM | G+: 2–150 µM G−: 4–10 µM F: 4.5 µM LD50: 75 µM | G+: 4–150 µM G−: 75 µM F: 9–150 µM LD50: 150–200 µM | |
| 2 | A. hainanensis | Unknown | Unknown | Amylopins: G+: 37–75 µg/mL G−: no active Hainanenin: G+: 4–40 µM G−: 4–75 µM F: 2–75 µM | |||
| 3 | A. jindongenensis | Unknown | Unknown | G+: 20 µM G−: 13–50 µM F: not active IC50: 57–58 µM (K562 & HT29 cell lines) | Jindongnenin:G+: 17–60 µM G−: 10–40 µM F: 60 µM | ||
| 4 | A. loloensis | G+: 5 µg/mL G−: 2–7 µg/mL F: not active IC50: 58 µg/mL (HepG2 cell line) | G+: 1–8 µg/mL G−: 7–50 µg/mL F: 2–22 µg/mL | G+: 1–75 µg/mL G−: 1–75 µg/mL F: 1–25 µg/mL IC50: 77 µg/mL (HepG2 cell line) | Amylopin: G+: 37–75 µg/mL G−: no active Amylopkinin: Smooth muscle active peptide | ||
| 5 | A. lifanensis | Unknown | Unknown | ||||
| 6 | A. ricketti | G+: 3–25 µg/mL G−: 12.5 µg/mL F: 100–200 µg/mL LD50: 100–200 µg/mL | G+: 1–200 µg/mL G−: 6–15 µg/mL F: 200 µg/mL | ||||
| 7 | A. torrentis | Unknown | |||||
| 8 | A. wuyiensis | Amylopkinin: Smooth muscle active peptide | |||||
| Genus: Glandirana | Brevinin-1 | Brevinin-2 | Esculentin-2 | ||||
| 1 | G. rugosa | Unknown | G+: 6–50 µg/mL G−: 12.5–100 µg/mL | ||||
| 2 | G. emeljanovi | Unknown | Unknown | ||||
| Genus: Hylarana | Brevinin-1 | Brevinin-2 | Esculentin-1 | Esculentin-2 | Temporin | Novel family of Peptides | |
| 1 | H. erythrea | Unknown | G+: 12.5 µM G−: 12.5 µM F: 50 µM LD50: 280 µM | Unknown | Unknown | ||
| 2 | H. guentheri | Unknown | G+: 3–6 µM G−: 2–6 µM F: not active LD50: 280 µM | G+: 30–50 µM G−: not active F: not active | Guentherin G+: 33.5 µg/mL Bradykinin, BRP: smooth muscle active peptide | ||
| 3 | H. latuouchii | G+: 6–10 µg/mL G−: 12.5 µg/mL F: 100–200 µg/mL LD50: 100–200 µg/mL | G+: 0.5–8 µg/mL G−: 0.5–130 µg/mL F: not active LD50: 400–600 µg/mL | G+: 0.6–10 µg/mL G−: 0.6–10 µg/mL F: 80 µg/mL LD50: 500 µg/mL | G+: 30–60 µg/mL G−: 6–15 µg/mL F: not active LD50: 500 µg/mL | G+: 6–10 µg/mL G−: not active F: not active LD50: 40 µg/mL | Palustrin: G+: 1–14 µg/mL G−: not active F: not active LD50: 220 µg/mL |
| 4 | H. luctiosa | Unknown | Unknown | G+: 4 µM G−: 32 µM | G+: 32 µM G−: 128 µM | Palustrin: G+: 1–14 µg/mL G−: not active F: not active LD50: 220 µg/mL | |
| 5 | H. nigrovittata | G+: 1–65 µg/mL G−: 18–40 µg/mL F: 2–5 µg/mL | G+: 4–20 µg/mL G−: 25–100 µg/mL F: 5–20 µg/mL | G+: 3–9 µg/mL G−: 4–15 µg/mL F: 3–9 µg/mL | Nigroain:G+: 9–50 µg/mL G−: 25–110 µg/mL F: 2–4 µg/mL Ranakinin N, Cholycytokinin: smooth muscle active peptides | ||
| 6 | H. picturata | G+: 3 µM G−: 24 µM | G+: 9–18 µM G−: 9–72 µM | Unknown | |||
| 7 | H. signata | Unknown | Unknown | Unknown | Palustrin G+: 1–14 µM | ||
| 8 | H. spinulosa | G+: 3–100 µM G−: 100–400 µM F: 12.5 µM | G+: 3–200 µM G−: 3–400 µM F: 100–400 µM | Unknown | G+: 6–200 µM G−: 12–400 µM F: not active | G+: 6–25 µM G−: not active F: 100–400 µM | Spinulosain,Ranatuerin,Nigroain,Odorranain, Ranacyclin:Unknown |
| 9 | H. temporalis | G+: 100–150 µg/mL G−: 30–150 µg/mL | G+: 40–150 µg/mL G−: 20–150 µg/mL | Unknown | Hylaranakinin:Unknown | ||
| Genus: Odorrana | Brevinin-1 | Brevinin-2 | Esculentin-1 | Esculentin-2 | Nigrocin-2 | Novel family of Peptides | |
| 1 | O. grahamii | Unknown | Unknown | Unknown | Unknown | G+: 9–100 µg/mL G−: 4–100 µg/mL F: 1–10 µg/mL | Takykinin,TrypsinInhibitor, BRP,BLP: smooth muscle active peptides Odorrnalectin: drug targeting Grahamin: G+: 2.5 µg/mL G−: 1–8 µg/mL F: 7.5 µg/mL Palustrin: G+: 12–100 µM G−: 100 µM F: 100 µM Odorranain: G+: 2–90 µg/mL G−: 3–50 µg/mL F: 1–50 µg/mL |
| 2 | O. hananensis | G+: 1–150 µM G−: 9–150 µM F: 1–10 µM LD50: 75 µM | G+: 9–150 µM G−: 9–10 µM F: 19–40 µM LD50: 300 µM | Odorranain: Unknown Temporin: G+: 2–150 µM G−: 30–75 µM F: 9–75 µM LD50: 75–300 µM | |||
| 3 | O. hejiangensis | Trypsin Inhibitor: smooth muscle active peptide | |||||
| 4 | O. hossi | G+: 3 µM G−: 24–50 µM | G+: 18 µM G−: 36 µM | G+: 12 µM G−: 12 µM | G+: 16 µM G−: 32 µM | G+: 25–60 µM G−: 10–30 µM | |
| 5 | O. ishikawae | G+: 6–100 µM G−: not active F: 50 µM | G+: 6–100 µM G−: 12–50 µM F: not active | G+: 3–25 µM G−: 3–12 µM F: 50 µM | G+: 3–25 µM G−: 12.5 µM F: 100 µM | G+: 3–15 µM G−: 25–50 µM F: 50 µM | Ishikawain: Unknown Palustrin-2: G+: 12–100 µM G−: 100 µM F: 100 µM Odorranain: Unknown |
| 6 | O. jingdongensis | G+: 6–15 µM G−: 25–50 µM F: 50µM | G+: 19 µM G−: 38 µM F: 19 µM | G+: 8 µM G−: 34 µM F: not active | G+: 8–16 µM G−: 15–16 µM F: 30–70 µM | ||
| 7 | O. livida | Lividin 1–4: Unknown | |||||
| 8 | O. schmakeri | Unknown | Unknown | Unknown | Unknown | BRP: 7 | |
| 9 | O. tiannanensis | Unknown | Unknown | Unknown | Unknown | Mararetaein, Pleurain, Odorranain: Unknown Tiannenensin: G+: 75 µM F:>100 µM | |
| 10 | O. versablis | Unknown | Unknown | Unknown | Ranatuerins, Temporin, Trypsininhibitor: Unknown | ||
| Genus: Pelophylax | Brevinin-1 | Brevinin-2 | Esculentin-1 | Esculentin-2 | Novel family of Peptides | ||
| 1 | P. plancyi | Unknown | |||||
| 2 | P. porosus | Unknown | |||||
| 3 | P. chosenicus | Unknown | |||||
| 4 | P. fukienensis | Unknown | Unknown | Unknown | Pelophylaxin: Unknown Ranakinestatin: bradykinin antagonist | ||
| 5 | P. nigromaculata | Unknown | Nigocin-2 G+: 2.5 µg/mL G−: 10–100 µg/mL F: 150 µg/mL | ||||
| Genus: Rana | Brevinin-1 | Brevinin-2 | Temporin-1 | Ranatuerin-2 | Novel family of Peptides | ||
| 1 | R. amurensis | Unknown | 2 | ||||
| 2 | R. brevipoda porsa | G+: 8 µg/mL G−: 34 µg/mL | G+: 8 µg/mL G−: 4 µg/mL | ||||
| 3 | R. chaochioensis | JaponicinG+: 25–100 µg/mL G−: 12–100 µg/mL | |||||
| 4 | R. chensinensis | G+: 12.5 µM G−: 25 µM HC50: 180–200 µM | 3 | G+: 100 µM G−: 100 µM IC50: 30–60 µM (Mcf 7 breast cancer cell line) LD50: 100 µM | RCSK 1–4, Chensinin 1–4, Japonicin-1, D-1CDYa: G+: 6–8 µM G−: 3–5 µM HC50: 450 µM | ||
| 5 | R. dybowskii | G+: 12.5 µM G−: 25 µM HC50: 125 µM | G+: 15–30 µM G−: 15–30 µM | G+: 60–100 µM G−: 60–100 µM HC50: 180 µM | Japonicin-1:G+: 100 µM G−: 25 µM HC50: 300 µM | ||
| 6 | R. japonica | G+: >100 µM G−: >100 µM | Japonicin-1:G+: >100 µM G−: 30 µM Japonicin-2: | ||||
| G+: 20 µM G−: 12 µM | |||||||
| 7 | R. okinavana | G+: 12.5 µM G−: 6–12.5 µM F: not active | G+: 50 µM G−: 12.5 µM F: 100 µM | ||||
| 8 | R. ornativentris | Unknown | G+: 200 µM F: 200 µM | Unknown | Palustrin: Unknown | ||
| 9 | R. pirica | G+: 13µM G−: not active F: 100 µM HC50: 7 µM | G+: 25 µM G−: 3–12 µM F: 100 µM HC50: 50 µM | G+: 100 µM G−: not active F: 100 µM HC50: 300 µM | G+: 100 µM G−: not active F: 100 µM HC50: 150 µM | ||
| 10 | R. pleuradan | Pleurain: G+: 15–30 µg/mL G−: 60–120 µg/mL F: 30 µg/mL | |||||
| 11 | R. sakurai | G+: >50 µM G−: 3 µM F: not active | G+: 25 µM G−:>50 µM F: >50 µM | G+: >50 µM G−: 50 µM F: >50 µM | MRP (AR 23), BRP: Smooth muscle active peptides | ||
| 12 | R. shuchinae | Shuchin G+: 6–15 µg/mL G−: 3–50 µg/mL F: 6.25 µg/mL | |||||
| 13 | R. tagoi | Unknown | G+: 10–40 µM | MRP (AR23): G+: 2–20 µM | |||
| 14 | R. tagoiokiensis | G+: 5 µM G−: 20 µM F: 20 µM | G+: 10 µM G−: 160 µM F: 80µM | G+: 160 µM G−: 80 µM F: 160 µM | |||
| 15 | R. tsushimensis | G+: 12–25 µM G−: 25–100 µM F: 50 µM LD50: 12 µM | G+: 5 µM G−: 20 µM F: 20 µM LD50: 100 µM | Unknown | |||
| Genus: Clinotarsus | |||||||
| 1 | C. curtipes | Brevinin-1 G+: 6–100 µg/mL G−: 7–60 µg/mL | |||||
| Genus: Fejervaria | |||||||
| 1 | F. carcrivora | Tigerinin 2G+: 20–80 µg/mL G−: 10–40 µg/mL F: 80–180 µg/mL | |||||
| Genus: Hoplobatracus | |||||||
| 1 | H. rugulosus | Tigerinin 1: Insulin releasing peptide | |||||
| 2 | H. tigerinus | Tigerinin 1: G+: 20–50 µg/mL G−: 20–100 µg/mL | |||||
| Genus : Hyla | |||||||
| 1 | H. annectans | Annotoxin-1: Inhibitor of tetradotoxin sensitive sodium channel | |||||
| Genus: Limnonectes | |||||||
| 1 | L. fujianensis | Limnonectin-2: G+: not active G−: 35–70 µM LD50: 160 µM | |||||
| Genus: Nanorana | |||||||
| 1 | N. parkeri | Japonicin:G+: 9–40 µg/mL G−: >100 µg/mL Parkerin: G+: 37.5 µg/mL G−: 37–100 µg/mL | |||||
| Genus: Rhacophorus | |||||||
| 1 | R. duboisi | Polypedarelaxin: Smooth muscle active peptide Polypedatein: Unknown | |||||
| 2 | R. Scheglii | Histone 2B: Unknown | |||||
| Genus: Sanguirana | |||||||
| 1 | S. varians | BLP: Smooth muscle active peptide | |||||
| Genus: Euphlyctis | |||||||
| E. hexadactylus | Crude skin extract: G+: 120–260 µg/mL G−: 120–520 µg/mL F: 32–64 µg/mL | ||||||
2.2. Clinotarsus
2.3. Euphlyctis
2.4. Fejervarya
2.5. Glandirana
2.6. Hoplobatrachus
2.7. Hyla
2.8. Hylarana
2.9. Limnonectus
2.10. Nanorana
2.11. Odorrana
2.12. Pelophylax
2.13. Rana
2.14. Rhacophorus
2.15. Sanguirana
3. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix
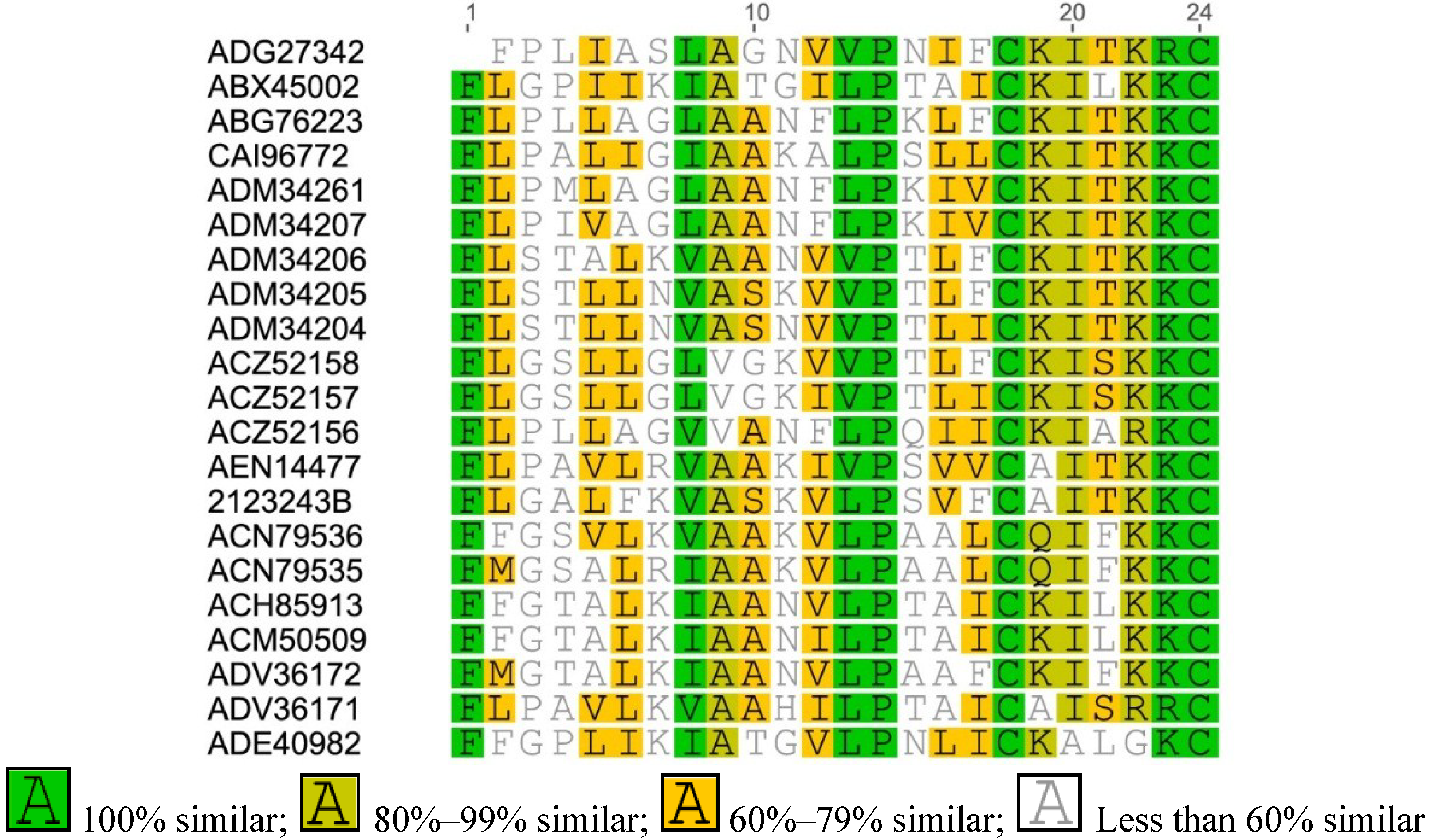
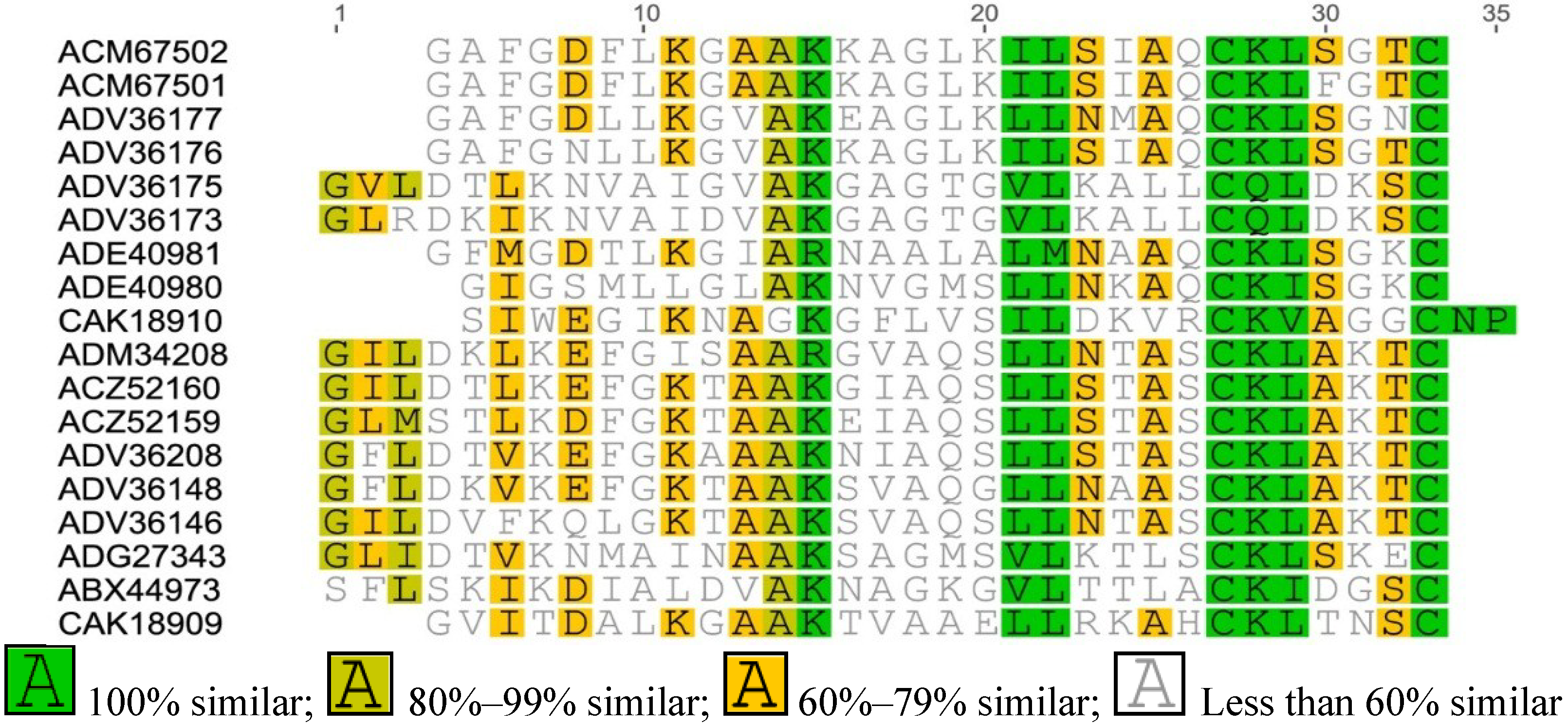

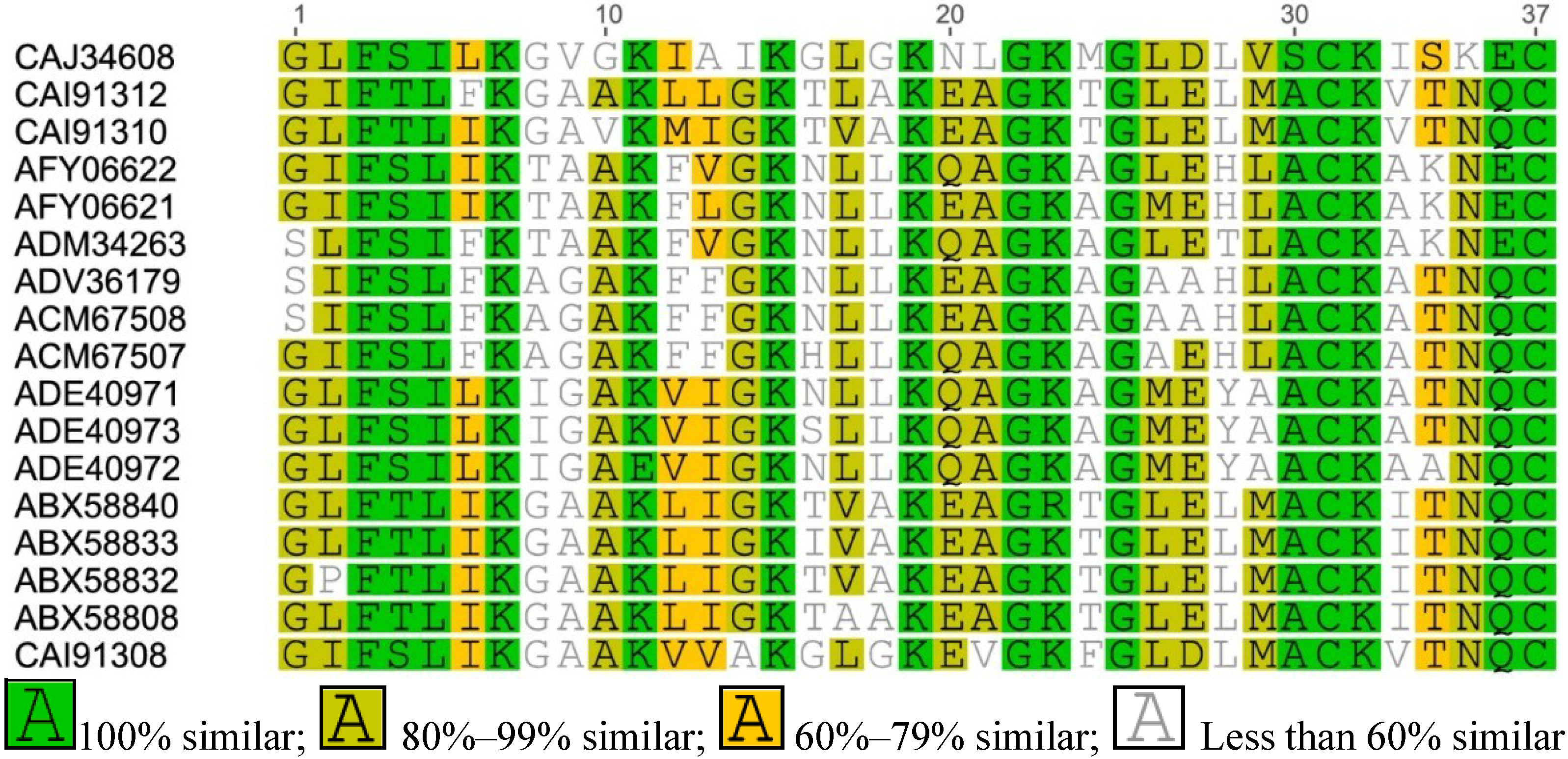
References
- Aoki, W.; Kuroda, K.; Ueda, M. Next generation of antimicrobial peptides as molecular targeted medicines. J. Biosci. Bioeng. 2012, 114, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Erspamer, V. Bioactive secretions of the integument. In Amphibian Biology. The Integument; Heatwole, H., Barthalamus, G., Eds.; Surrey Beatty & Sons: Chipping Norton, Australia, 1994; Volume 1, pp. 179–350. [Google Scholar]
- Nicolas, P.; Vanhoye, D.; Amiche, M. Molecular strategies in biological evolution of antimicrobial peptides. Peptides 2003, 24, 1669–1680. [Google Scholar] [CrossRef] [PubMed]
- Galdiero, S.; Falanga, A.; Cantisani, M.; Vitiello, M.; Morelli, G.; Galdiero, M. Peptide-lipid interactions: Experiments and applications. Int. J. Mol. Sci. 2013, 14, 18758–18789. [Google Scholar] [CrossRef] [PubMed]
- Calderon, L.A.; Stabeli, R.G. Anuran amphibians: A huge and threatened factory of a variety of active peptides with potential nanobiotechnological applications in the face of amphibian decline. Available online: http://cdn.intechopen.com/pdfs-wm/23583.pdf (accessed on 2 December 2014).
- Calderon, L.A.; Soares, A.M.; Stábeli, R.G. Anuran antimicrobial peptides: An alternative for the development of nanotechnological based therapies for multidrug—Resistant infections. Available online: http://signpostejournals.com/ejournals/Portals/5/25-161-1-PB.pdf (accessed on 2 December 2014).
- Conlon, J.M.; Sonnevend, A. Clinical applications of amphibian antimicrobial peptides. J. Med. Sci. 2011, 4, 62–72. [Google Scholar]
- Conlon, J.M.; Mechkarska, M.; Lukic, M.L.; Flatt, P.R. Potential therapeutic applications of multifunctional host-defense peptides from frog skin as anti-cancer, anti-viral, immunomodulatory, and anti-diabetic agents. Peptides 2014, 57, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, J.; Yu, H.; Xu, X.; Liang, J.; Tian, Y.; Mab, D.; Lin, G.; Huang, G.; Lai, R. Two families of antimicrobial peptides with multiple functions from skin of rufous-spotted torrent frog, Amolops loloensis. Peptides 2006, 27, 3085–3091. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Guo, H.; Shi, F.; Wang, H.; Li, L.; Jiao, X.; Wang, Y.; Yu, H. Hainanenins: A novel family of antimicrobial peptides with strong activity from Hainan cascade-frog, Amolops hainanensis. Peptides 2012, 33, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yang, X.; Liu, Z.; Zeng, L.; Lee, W.; Zhang, Y. Two novel families of antimicrobial peptides from skin secretions of the Chinese torrent frog, Amolops jingdongensis. Biochimie 2012, 94, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, Y.; Wang, A.; Song, Y.; Ma, D.; Yang, H.; Ma, Y.; Lai, R. Five novel antimicrobial peptides from skin secretions of the frog, Amolops loloensis. Comp. Biochem. Physiol. 2010, 155, 72–76. [Google Scholar] [CrossRef]
- Yang, X.; Xia, J.; Yu, Z.; Hu, Y.; Li, F.; Meng, H.; Yang, S.; Liu, J.; Wang, H. Characterization of diverse antimicrobial peptides in skin secretions of Chungan torrent frog Amolops chunganensis. Peptides 2012, 38, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ran, R.; Yu, H.; Yu, Z.; Hu, Y.; Zheng, H.; Wang, D.; Yang, F.; Liu, R.; Liu, J. Identification and characterization of antimicrobial peptides from skin of Amolops ricketti (Anura: Ranidae). Peptides 2012, 33, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Wang, J.; Hong, J.; Feng, H.; Yang, H.; Yu, X.; Ma, Y.; Lai, R. A novel family of antimicrobial peptides from the skin of Amolops loloensis. Biochimie 2008, 90, 863–867. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Han, Y.; Li, J.; Xu, X.; Rees, H.; Lai, R. A novel bradykinin-like peptide from skin secretions of rufous-spotted torrent frog, Amolops loloensis. Peptides 2006, 27, 2683–2687. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, L.; Zhou, M.; Chen, T.; Ding, A.; Rao, P.; Walker, B.; Shaw, C. Amolopkinins W1 and W2—Novel bradykinin-related peptides (BRPs) from the skin of the Chinese torrent frog, Amolops wuyiensis: Antagonists of bradykinin-induced smooth muscle contraction of the rat ileum. Peptides 2009, 30, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Okobo, T.; Kakegawa, T.; Tatemoto, K. Isolation and characterization of antimicrobial peptides, rugosins A, B, and C, from the skin of the frog, Rana rugosa. Biochem. Biophys. Res. Commun. 1995, 212, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Won, H.; Kang, S.; Lee, B. Action mechanism and structural requirements of the antimicrobial peptides, gaegurins. Biochim. Biophys. Acta 2009, 1788, 1620–1629. [Google Scholar] [CrossRef] [PubMed]
- Conlon, J.M. Reflections on a systematic nomenclature for antimicrobial peptides from the skins of frogs of the family Ranidae. Peptides 2008, 29, 1815–1819. [Google Scholar] [CrossRef]
- Al-Ghaferi, N.; Kolodziejek, J.; Nowotny, N.; Coquet, L.; Jouenne, T.; Leprince, J.; Vaudry, H.; King, J.D.; Conlon, J.M. Antimicrobial peptides from the skin secretions of the South-East Asian frog Hylarana erythraea (Ranidae). Peptides 2009, 31, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lu, Y.; Zhang, X.; Hu, Y.; Yu, H.; Liu, J.; Sun, J. The novel antimicrobial peptides from skin of Chinese broad-folded frog, Hylarana latouchii (Anura: Ranidae). Peptides 2009, 30, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yan, X.; Yu, H.; Hu, Y.; Yu, Z.; Zheng, H.; Chen, Z.; Zhang, Z.; Liu, J. Isolation, characterization and molecular cloning of new antimicrobial peptides belonging to the brevinin-1 and temporin families from the skin of Hylarana latouchii (Anura: Ranidae). Biochimie 2009, 91, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yu, Z.; Hu, Y.; Yu, H.; Ran, R.; Xia, J.; Wang, D.; Yang, S.; Yang, X.; Liu, J. Molecular cloning and characterization of antimicrobial peptides from skin of the broad-folded frog, Hylarana latouchii. Biochimie 2012, 94, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Conlon, J.M.; Power, G.J.; Abdel-Wahab, Y.H.A.; Flatt, P.R.; Jiansheng, H.; Coquet, L.; Leprince, J.; Jouenne, T.; Vaudry, H. A potent, non-toxic insulin-releasing peptide isolated from an extract of the skin of the Asian frog, Hylarana guntheri (Anura: Ranidae). Regul. Pept. 2008, 29, 151–153. [Google Scholar]
- Zhou, J.; McClean, S.; Thompson, A.; Zhang, Y.; Shaw, C.; Rao, P.; Bjourson, A.J. Purification and characterization of novel antimicrobial peptides from the skin secretion of Hylarana guentheri. Peptides 2006, 27, 3077–3084. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Liu, C.; Liu, X.; Wu, J.; Yang, H.; Wang, Y.; Li, J.; Yu, H.; Lai, R. Peptidomics and genomics analysis of novel antimicrobial peptides from the frog, Rana nigrovittata. Genomics 2010, 95, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Conlon, J.M.; Kolodziejek, J.; Nowotny, N.; Leprince, J.; Vaudry, H.; Coquet, L.; Jouenne, T.; King, J.D. Characterization of antimicrobial peptides from the skin secretions of the Malaysian frogs, Odorrana hosii and Hylarana picturata (Anura:Ranidae). Toxicon 2008, 52, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Conlon, J.M.; Kolodziejek, J.; Mechkarska, M.; Coquet, L.; Leprince, J.; Jouenne, T.; Vaudry, H.; Nielsen, P.F.; Nowotny, N.; King, J.D. Host defense peptides from Lithobates forreri, Hylarana luctuosa, and Hylarana signata (Ranidae): Phylogenetic relationships inferred from primary structures of ranatuerin-2 and brevinin-2 peptides. Comp. Biochem. Physiol. 2014, D9, 49–57. [Google Scholar]
- Yang, X.; Hu, Y.; Xu, S.; Hu, Y.; Meng, H.; Guo, C.; Liu, Y.; Liu, J.; Yu, Z.; Wang, H. Identification of multiple antimicrobial peptides from the skin of fine-spined frog, Hylarana spinulosa (Ranidae). Biochimie 2013, 95, 2429–2436. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Y.; Cheng, L.; Song, Y.; Lai, R. Isolation and cDNA cloning of cholecystokinin from the skin of Rana nigrovittata. Peptides 2007, 28, 1540–1544. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; You, D.; Chen, L.; Wang, X.; Zhang, K.; Lai, R. A novel bradykinin-like peptide from skin secretions of the frog, Rana nigrovittata. J. Pept. Sci. 2008, 14, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; B jourson, A.J.; Coulter, D.J.M.; Chen, T.; Shaw, C.; Rourke, M.O.; Hirst, D.G.; Zhang, Y.; Rao, P.; McClean, S. Bradykinin- related peptides, including a novel structural variant, (Val1)-bradykinin, from the skin secretion of Guenther’s frog, Hylarana guentheri and their molecular precursors. Peptides 2007, 28, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Reshmy, V.; Preeji, V.; Parvin, A.; Santhoshkumar, K.; George, S. Three novel antimicrobial peptides from the skin of the Indian bronzed frog Hylarana temporalis (Anura: Ranidae). J. Pept. Sci. 2011, 17, 342–347. [Google Scholar] [CrossRef]
- Reshmy, V.; Santhosh Kumar, K.; George, S. Full length cDNA derived novel peptides belonging to Esculentin Family from skin of Indian Bronzed Frog Hylarana temporalis. Res. J. Biotech. 2011, 6, 71–74. [Google Scholar]
- Reshmy, V.; Preeji, V.; Parvin, A.; Santhosh Kumar, K.; George, S. Molecular cloning of a novel Bradykinin- related peptide from the skin of Indian bronzed frog Hylarana temporalis. J. Genomics Insights. 2010, 3, 23–28. [Google Scholar]
- Li, J.; Yu, H.; Xu, X.; Wang, X.; Liu, D.; Lai, R. Multiple bombesin-like peptides with opposite functions from skin of Odorrana grahami. Genomics 2007, 89, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Conlon, J.M.; Leprince, J.; Vaudry, H.; Jiansheng, H.; Nielsen, P.F. A family of antimicrobial peptides related to japonicin-2 isolated from the skin of the chaochiao brown frog Rana chaochiaoensis. Comp. Biochem. Physiol. 2006, 144, 101–105. [Google Scholar] [CrossRef]
- Wang, H.; Yu, Z.; Hu, Y.; Li, F.; Liu, L.; Zhen, H.; Meng, H.; Yang, S.; Yang, X.; Liu, J. Novel antimicrobial peptides isolated from the skin secretions of Hainan odorous frog, Odorrana hainanensis. Peptides 2012, 35, 285–290. [Google Scholar]
- Iwakoshi-Ukena, E.; Ukena, K.; Okimoto, A.; Soga, M.; Okada, G.; Sano, N.; Fujii, T.; Sugawara, Y.; Sumida, M. Identification and characterization of antimicrobial peptides from the skin of the endangered frog Odorrana ishikawae. Peptides 2011, 32, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Iwakoshi-Ukena, E.; Soga, M.; Okada, G.; Fujii, T.; Sumida, M.; Ukena, K. Characterization of novel antimicrobial peptides from the skin of the endangered frog Odorrana ishikawae by shotgun cDNA cloning. Biochem. Biophys. Res. Commun. 2011, 412, 673–677. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Feng, F.; Huang, Y.; Guo, H.; Zhang, S.; Zheng, Li.; Liu, J.; Wang, Y.; Yu, H. Host defense peptides in skin secretions of Odorrana tiannanensis: Proof for other survival strategy of the frog than merely anti-microbial. Biochimie 2012, 94, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Li, L.; Zhou, M.; Rao, P.; Walker, B.; Shaw, C. Amphibian skin peptides and their corresponding cDNAs from single lyophilized secretion samples: Identification of novel brevinins from three species of Chinese frogs. Peptides 2006, 27, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Zhou, M.; Chen, W.; Lorimer, J.; Rao, P.; Walker, B.; Shaw, C. Cloning from tissue surrogates: Antimicrobial peptide (esculentin) cDNAs from the defensive skin secretions of Chinese ranid frogs. Genomics 2006, 87, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Zhou, M.; Rao, P.; Walker, B.; Shaw, C. The Chinese bamboo leaf odorous frog (Rana (Odorrana) versabilis) and North American Rana frogs share the same families of skin antimicrobial peptides. Peptides 2006, 27, 1738–1744. [Google Scholar] [CrossRef] [PubMed]
- Quan, Z.; Zhou, M.; Chen, W.; Chen, T.; Walker, B.; Shaw, C. Novel brevinins from Chinese piebald odorous frog (Huia schmackeri) skin deduced from cloned biosynthetic precursors. Peptides 2008, 29, 1456–1460. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Chen, T.; Walker, B.; Shaw, C. Lividins: Novel antimicrobial peptide homologs from the skin secretion of the Chinese Large Odorous frog, Rana (Odorrana) livida—Identification by “shotgun” cDNA cloning and sequence analysis. Peptides 2008, 27, 2118–2123. [Google Scholar] [CrossRef]
- Che, Q.; Zhou, Y.; Yang, H.; Li, J.; Xu, X.; Lai, R. A novel antimicrobial peptide from amphibian skin secretions of Odorrana grahami. Peptides 2008, 29, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, Y.; Li, J.; Xu, X.; Lai, R.; Zou, Q. An antimicrobial peptide with antimicrobial activity against Helicobacter pylori. Peptides 2007, 28, 1527–1531. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, T.; Xu, X.; Wang, X.; Wu, M.; Yang, H.; Lai, R. Amphibian tachykinin precursor. Biochem. Biophys. Res. Commun. 2006, 350, 983–986. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, H.; Hong, J.; Xu, X.; Yang, H.; Wu, B.X.; Wang, Y.P.; Zhu, J.; Lai, R.; Jiang, X.; et al. Odorranalectin is a small peptide lectin with potential for drug delivery and targeting. PLOS ONE 2008, 3. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, X.; Xu, C.; Zhou, W.; Zhang, K.; Yu, H.; Zhang, Y.; Zheng, Y.; Rees, H.H.; Lai, R.; et al. Anti-infection peptidomics of amphibian skin. Mol. Cell. Proteomics 2007, 6, 882–894. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Yan, Z.; Hu, K.; Pang, Z.; Cheng, X.; Guo, L.R.; Zhang, Q.; Jiang, X.; Fang, L.; Lai, R. Odorranalectin-conjugated nanoparticles: Preparation, brain delivery and pharmacodynamic study on Parkinson’s disease following intranasal administration. J. Control. Release 2011, 151, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, J.; Wang, Y.; Xu, X.; Liu, T.; Lai, R.; Zhu, H. A small trypsin inhibitor from the frog of Odorrana grahami. Biochimie 2008, 90, 1356–1361. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, L.; Chen, T.; Walker, B.; Zhou, M.; Sui, D.; Conlon, J. M.; Shaw, C. Identification and molecular cloning of a novel amphibian Bowman Birk-type trypsin inhibitor from the skin of the Hejiang Odorous Frog; Odorrana hejiangensis. Peptides 2012, 33, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Chen, T.; Walker, B.; Shaw, C. Pelophylaxins: Novel antimicrobial peptide homologs from the skin secretion of the Fukien gold-striped pond frog, Pelophylax plancyi fukienensis Identification by “shotgun” cDNA cloning and sequence analysis. Peptides 2006, 27, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Che, Q.; Wang, X.; Li, J.; Yang, H.; Li, D.; Zhang, K.; Lai, R. Cloning and characterization of the first amphibian bradykinin gene. Biochimie 2010, 92, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Bjourson, A. J.; He, J.; Cai, G.; Rao, P.; Shaw, C. Bradykinins and their cDNA from piebald odorous frog, Odorrana schmackeri, skin. Peptides 2003, 24, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Park, S.; Ahn, H.; Kim, S.; Kim, S.S.; Lee, B.J.; Lee, B. Structural study of novel antimicrobial peptides, nigrocins, isolated from Rana nigromaculata. FEBS Lett. 2001, 507, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Luo, Y.; Ge, L.; Wang, L.; Zhou, M.; Zhang, Y.; Duan, J.; Chen, T.; Shaw, C. Ranakinestatin-PPF from the skin secretion of the Fukien Gold-Striped Pond Frog, Pelophylax plancyi fukienensis: A Prototype of a novel class of Bradykinin B2 receptor antagonist peptide from ranid frogs. Sci. World J. 2014. [Google Scholar] [CrossRef]
- Conlon, J.M.; Al-Ghaferi, N.; Abraham, B.; Sonnevend, A.; Coquet, L.; Leprince, J.; Jouenne, T.; Vaudry, H.; Iwamuro, S. Antimicrobial peptides from the skin of the Tsushima brown frog Rana tsushimensis. Comp. Biochem. Physiol. 2006, 143, 42–49. [Google Scholar] [CrossRef]
- Jin, L.L.; Li, Q.; Song, S.; Feng, K.; Zhang, D.; Wang, Q.; Chen, Y. Characterization of antimicrobial peptides isolated from the skin of the Chinese frog, Rana dybowskii. Comp. Biochem. Physiol. 2009, 154, 174–178. [Google Scholar] [CrossRef]
- Conlon, J.M.; Kolodziejek, J.; Nowotny, N.; Leprincec, J.; Vaudry, H.; Coquet, L.; Jouenne, T.; Iwamuro, S. Cytolytic peptides belonging to the brevinin-1 and brevinin-2 families isolated from the skin of the Japanese brown frog, Rana dybowskii. Toxicon 2007, 50, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.L.; Song, S.S.; Li, Q.; Chen, Y.H.; Wang, Q.Y.; Hou, S.T. Identification and characterisation of a novel antimicrobial polypeptide from the skin secretion of a Chinese frog (Rana chensinensis). Int. J. Antimicrob. Agents 2009, 33, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Isaacson, T.; Soto, A.; Iwamuro, S.; Knoop, F.C.; Conlon, J.M. Antimicrobial peptides with atypical structural features from the skin of the Japanese brown frog Rana japonica. Peptides 2002, 23, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Conlon, J.M.; Sonnevend, A.; Jouenne, T.; Coquet, L.; Cosquer, D.; Vaudry, H.; Iwamuro, S. A family of acyclic brevinin-1 peptides from the skin of the Ryukyu brown frog Rana okinavana. Peptides 2005, 26, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Ohnuma, A.; Conlon, J.M.; Yamaguchi, K.; Kawasaki, H.; Coquet, L.; Leprince, J.; Jouenne, T.; Vaudry, H.; Iwamuro, S. Antimicrobial peptides from the skin of the Japanese mountain brown frog Rana ornativentris: Evidence for polymorphism among preprotemporin mRNAs. Peptides 2007, 24, 524–532. [Google Scholar] [CrossRef]
- Conlon, J.M.; Sonnevend, A.; Patel, M.; Al-Dhaheri, K.; Nielsen, P.F.; Kolodziejek, J.; Nowotny, N.; Iwamuro, S.; Pal, T. A family of brevinin-2 peptides with potent activity against Pseudomonas aeruginosa from the skin of the Hokkaido frog, Rana pirica. Regul. Pept. 2004, 118, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Iwamuro, S.; Ohnuma, A.; Coquet, L.; Leprince, J.; Jouenne, T.; Vaudry, H.; Taylor, C.K.; Abel, P.W.; Conlon, J.M. Expression of genes encoding antimicrobial and bradykinin-related peptides in skin of the stream brown frog Rana sakuraii. Peptides 2007, 28, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Conlon, J.M.; Sonnevend, A.; Patel, M.; Camasamudram, V.; Nowotny, N.; Zilahi, E.; Iwamuro, S.; Nielsen, P.F.; Pal, T. A melittin-related peptide from the skin of the Japanese frog, Rana tagoi, with antimicrobial and cytolytic properties. Biochem. Biophys. Res. Commun. 2003, 306, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Iwamuro, S.; Nakamura, M.; Ohnuma, A.; Conlon, J.M. Molecular cloning and sequence analyses of preprotemporin mRNAs containing premature stop codons from extradermal tissues of Rana tagoi. Peptides 2006, 27, 2124–2128. [Google Scholar] [CrossRef] [PubMed]
- Tazato, S.; Conlon, M.J.; Iwamuro, S. Cloning and expression of genes enocoding antimicrobial peptides and bradykinin from the skin and brain of Oki Tago’s brown frog, Rana tagoi okiensis. Peptides 2010, 31, 1480–1487. [Google Scholar] [CrossRef] [PubMed]
- Conlon, J.M.; Coquet, L.; Jouenne, T.; Leprince, J.; Vaudry, H.; Iwamuro, S. Evidence from the primary structures of dermal antimicrobial peptides that Rana tagoi okiensis and Rana tagoi tagoi (Ranidae) are not conspecific subspecies. Toxicon 2010, 55, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Conlon, J.M.; Al-Ghaferi, N.; Abraham, B.; Jiansheng, H.; Cosette, P.; Leprince, J.; Jouenne, T.; Vaudry, H. Antimicrobial peptides from diverse families isolated from the skin of the Asian frog, Rana grahami. Peptides 2006, 27, 2111–2117. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, N.; Hagiwari, K.; Nakajima, T. Brevinin 1 and 2, unique antimicrobial peptides from skin of the frog Rana brevipoda porsa. Biochem. Biophys. Res. Commun. 1992, 189, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Conlon, J.M.; Iwamuro, S. Evidence that the genes encoding the melittin-related peptides in the skins of the Japanese frogs Rana sakuraii and Rana tagoi are not orthologous to bee venom melittin genes: Developmental- and tissue-dependent gene expression. Peptides 2007, 28, 2061–2068. [Google Scholar] [CrossRef] [PubMed]
- Sin, Y.; Zhou, M.; Chen, W.; Wang, L.; Chen, T.; Walker, B.; Shaw, C. Skin bradykinin-related peptides (BRPs) and their biosynthetic precursors (kininogens): Comparisons between various taxa of Chinese and North American ranid frogs. Peptides 2008, 29, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Yao, B.; Yu, H.; Wang, H.; Bian, J.; Feng, F. Novel family of antimicrobial peptides from the skin of Rana shuchinae. Peptides 2010, 31, 1674–1677. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Zhao, G.; Wang, B.; Wang, H. Three novel antimicrobial peptides from the skin of Rana shuchinae. Gene 2013, 521, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Song, Y.; Li, J.; Liu, H.; Xu, X.; Lai, R.; Zhang, K. A new family of antimicrobial peptides from skin secretions of Rana pleuraden. Peptides 2009, 28, 2069–2074. [Google Scholar] [CrossRef]
- Abraham, P.; George, S.K.; Santhosh, K. Novel antibacterial peptides from the skin secretion of the Indian bicoloured frog Clinotarsus curtipes. Biochimie 2013, 97, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Lub, Y.; Wang, L.; Yang, H.; Zhang, K.; Lai, R. Purification, characterization and cloning of two novel tigerinin-like peptides from skin secretions of Fejervarya cancrivora. Peptides 2009, 30, 1228–1232. [Google Scholar] [CrossRef] [PubMed]
- Sai, K.P.; Jagannadham, M.V.; Vairamani, M.; Raju, N.P.; Devi, A.S.; Nagaraj, R.; Sitaram, N. Tigerinins: Novel antimicrobial peptides from the Indian frog Rana tigerina. J. Biol. Chem. 2001, 276, 2701–2707. [Google Scholar] [CrossRef] [PubMed]
- Ojo, O.O.; Abdel-Wahab, Y.H.A.; Flatt, P.R.; Mechkarska, M.; Conlon, J.M. Tigerinin-1R: A potent, non-toxic insulin-releasing peptide isolated from the skin of the Asian frog, Hoplobatrachus rugulosus. Diabetes Obe. Metab. 2011, 13, 1114–1122. [Google Scholar] [CrossRef]
- Srinivasan, D.; Ojo, O.O.; Abdel-Wahab, Y.H.A.; Flatt, P.R.; Guilhaudis, L.; Conlon, J.M. Insulin-releasing and cytotoxic properties of the frog skin peptide, tigerinin-1R: A structure-activity study. Peptides 2014, 55, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Dong, L.; Zhao, T.; You, D.; Liu, R.; Liu, H.; Yang, H.; Lai, R. Analgesic and anti-inflammatory effects of the amphibian neurotoxin, anntoxin. Biochimie 2011, 93, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, L.; Zhou, M.; Ma, C.; Chen, X.; Bai, B.; Chen, T.; Shaw, C. Limnonectins: A new class of antimicrobial peptides from the skin secretion of the Fujian large-headed frog (Limnonectes fujianensis). Biochimie 2011, 93, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Zhai, L.; Wang, H.; Che, Q.; Wang, D.; Feng, F.; Zhao, Z.; Yu, H. Novel families of antimicrobial peptides with multiple functions from skin of Xizang plateau frog, Nanorana parkeri. Biochimie 2010, 92, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Meng, P.; Wei, L.; Yang, S.; Liu, H.; Liu, R.; Lai, R. A novel frog skin peptide containing function to induce muscle relaxation. Biochimie 2012, 94, 2508–2513. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, H.; Isaacson, T.; Iwamuro, S.; Conlon, J.M. A protein with antimicrobial activity in the skin of Schlegel’s green tree frog Rhacophorus schlegelii (Rhacophoridae) identified as histone H2B. Biochem. Biophys. Res. Commun. 2003, 312, 1082–1086. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Li, W.; Duan, L.; Xiao, Y. A bombesin-like peptide from skin of Sanguirana varians. Comp. Biochem. Physiol. 2010, 155, 106–109. [Google Scholar] [CrossRef]
- Ramesh, B.; Vadivelu, P.; Kavitha, K.; Suresh, G.; Ravichandran, N.; Siva, G.V. Antimicrobial peptide from Euphlyctis hexadactylus and its efficacy against plant pathogens. Int. J. Curr. Res 2010, 6, 14–17. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, V.T.V.; Holthausen, D.; Jacob, J.; George, S. Host Defense Peptides from Asian Frogs as Potential Clinical Therapies. Antibiotics 2015, 4, 136-159. https://doi.org/10.3390/antibiotics4020136
Kumar VTV, Holthausen D, Jacob J, George S. Host Defense Peptides from Asian Frogs as Potential Clinical Therapies. Antibiotics. 2015; 4(2):136-159. https://doi.org/10.3390/antibiotics4020136
Chicago/Turabian StyleKumar, Vineeth T.V., David Holthausen, Joshy Jacob, and Sanil George. 2015. "Host Defense Peptides from Asian Frogs as Potential Clinical Therapies" Antibiotics 4, no. 2: 136-159. https://doi.org/10.3390/antibiotics4020136
APA StyleKumar, V. T. V., Holthausen, D., Jacob, J., & George, S. (2015). Host Defense Peptides from Asian Frogs as Potential Clinical Therapies. Antibiotics, 4(2), 136-159. https://doi.org/10.3390/antibiotics4020136





