Prediction Score for Identification of ESBL Producers in Urinary Infections Overestimates Risk in High-ESBL-Prevalence Setting
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Design and Setting
4.1.1. Variables and Definitions
Microbiology
Variables
Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| UTI | Urinary tract infection |
| ESBL | Extended-spectrum beta-lactamase |
| AUC | Area under the curve |
| ED | Emergency department |
| s | Sensibility |
| e | Specificity |
| TP | True positive |
| TN | True negative |
| FP | False positive |
| FN | False negative |
| PPV | Positive predictive value |
| NPV | Negative predictive value |
| Acc | Accuracy |
References
- Wagenlehner, F.M.E.; Bjerklund Johansen, T.E.; Cai, T.; Koves, B.; Kranz, J.; Pilatz, A.; Tandogdu, Z. Epidemiology, definition and treatment of complicated urinary tract infections. Nat. Rev. Urol. 2020, 17, 586–600. [Google Scholar] [CrossRef]
- De Angelis, G.; Del Giacomo, P.; Posteraro, B.; Sanguinetti, M.; Tumbarello, M. Molecular Mechanisms, Epidemiology, and Clinical Importance of beta-Lactam Resistance in Enterobacteriaceae. Int. J. Mol. Sci. 2020, 21, 5090. [Google Scholar] [CrossRef]
- Gonzalez, L.; Cortes, J.A. Systematic review of antimicrobial resistance in Enterobacteriaceae isolates from Colombian hospitals. Biomedica 2014, 34, 180–197. [Google Scholar] [CrossRef][Green Version]
- Guzman-Blanco, M.; Labarca, J.A.; Villegas, M.V.; Gotuzzo, E.; Latin America Working Group on Bacterial, R. Extended spectrum beta-lactamase producers among nosocomial Enterobacteriaceae in Latin America. Braz. J. Infect. Dis. 2014, 18, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Castrillón Spitia, J.D.; Machado-Alba, J.E.; Gómez Idarraga, S.; Gómez Gutierrez, M.; Remolina León, N.; Ríos Gallego, J.J. Etiología y perfil de resistencia antimicrobiana en pacientes con infección urinaria. Infectio 2018, 23, 45. [Google Scholar] [CrossRef]
- Rada, A.M.; Hernandez-Gomez, C.; Restrepo, E.; Villegas, M.V. Distribution and molecular characterization of beta-lactamases in Gram-negative bacteria in Colombia, 2001–2016. Biomedica 2019, 39, 199–220. [Google Scholar] [CrossRef] [PubMed]
- Doi, Y.; Iovleva, A.; Bonomo, R.A. The ecology of extended-spectrum beta-lactamases (ESBLs) in the developed world. J. Travel Med. 2017, 24, S44–S51. [Google Scholar] [CrossRef]
- Hayer, J.; Salgado-Caxito, M.; Opazo-Capurro, A.; Munoz, P.G.; Millan, J.; Pineiro, A.; Munita, J.M.; Rivas, L.; Benavides, J.A. Multiple clonal transmissions of clinically relevant extended-spectrum beta-lactamase-producing Escherichia coli among livestock, dogs, and wildlife in Chile. J. Glob. Antimicrob. Resist. 2023, 34, 247–252. [Google Scholar] [CrossRef]
- Mohd Sazlly Lim, S.; Wong, P.L.; Sulaiman, H.; Atiya, N.; Hisham Shunmugam, R.; Liew, S.M. Clinical prediction models for ESBL-Enterobacteriaceae colonization or infection: A systematic review. J. Hosp. Infect. 2019, 102, 8–16. [Google Scholar] [CrossRef]
- Tumbarello, M.; Trecarichi, E.M.; Bassetti, M.; De Rosa, F.G.; Spanu, T.; Di Meco, E.; Losito, A.R.; Parisini, A.; Pagani, N.; Cauda, R. Identifying patients harboring extended-spectrum-beta-lactamase-producing Enterobacteriaceae on hospital admission: Derivation and validation of a scoring system. Antimicrob. Agents Chemother. 2011, 55, 3485–3490. [Google Scholar] [CrossRef]
- Colello, R.; Kruger, A.; Conza, J.D.; Rossen, J.W.A.; Friedrich, A.W.; Gutkind, G.; Etcheverria, A.I.; Padola, N.L. Antimicrobial Resistance in Class 1 Integron-Positive Shiga Toxin-Producing Escherichia coli Isolated from Cattle, Pigs, Food and Farm Environment. Microorganisms 2018, 6, 99. [Google Scholar] [CrossRef] [PubMed]
- Overdevest, I.; Willemsen, I.; Rijnsburger, M.; Eustace, A.; Xu, L.; Hawkey, P.; Heck, M.; Savelkoul, P.; Vandenbroucke-Grauls, C.; van der Zwaluw, K.; et al. Extended-spectrum beta-lactamase genes of Escherichia coli in chicken meat and humans, The Netherlands. Emerg. Infect. Dis. 2011, 17, 1216–1222. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, A.M.; García, M.I.; Cienfuegos, A.V.; Vásquez-Jaramillo, L. Isolation of Escherichia coli and Klebsiella pneumoniae strains producing extended spectrum β-lactamases from dog urine of the Metropolitan Area of the Aburrá Valley Antioquia-Colombia. Rev. Fac. Med. Vet. Zootec. 2022, 69, 245–258. [Google Scholar] [CrossRef]
- Castellanos, L.R.; Donado-Godoy, P.; Leon, M.; Clavijo, V.; Arevalo, A.; Bernal, J.F.; Timmerman, A.J.; Mevius, D.J.; Wagenaar, J.A.; Hordijk, J. High Heterogeneity of Escherichia coli Sequence Types Harbouring ESBL/AmpC Genes on IncI1 Plasmids in the Colombian Poultry Chain. PLoS ONE 2017, 12, e0170777. [Google Scholar] [CrossRef]
- Maechler, F.; Schwab, F.; Hansen, S.; Fankhauser, C.; Harbarth, S.; Huttner, B.D.; Diaz-Agero, C.; Lopez, N.; Canton, R.; Ruiz-Garbajosa, P.; et al. Contact isolation versus standard precautions to decrease acquisition of extended-spectrum beta-lactamase-producing Enterobacterales in non-critical care wards: A cluster-randomised crossover trial. Lancet Infect. Dis. 2020, 20, 575–584. [Google Scholar] [CrossRef]
- Lodise, T.P.; Chen, L.H.; Wei, R.; Im, T.M.; Contreras, R.; Bruxvoort, K.J.; Rodriguez, M.; Friedrich, L.; Tartof, S.Y. Clinical Risk Scores to Predict Nonsusceptibility to Trimethoprim-Sulfamethoxazole, Fluoroquinolone, Nitrofurantoin, and Third-Generation Cephalosporin Among Adult Outpatient Episodes of Complicated Urinary Tract Infection. Open Forum Infect. Dis. 2023, 10, ofad319. [Google Scholar] [CrossRef]
- Tsui, K.; Wong, S.S.; Lin, L.C.; Tsai, C.R.; Chen, L.C.; Huang, C.H. Laboratory identification, risk factors, and clinical outcomes of patients with bacteremia due to Escherichia coli and Klebsiella pneumoniae producing extended-spectrum and AmpC type beta-lactamases. J. Microbiol. Immunol. Infect. 2012, 45, 193–199. [Google Scholar] [CrossRef]
- Cruz-Vargas, S.A.; Garcia-Munoz, L.; Cuervo-Maldonado, S.I.; Alvarez-Moreno, C.A.; Saavedra-Trujillo, C.H.; Alvarez-Rodriguez, J.C.; Arango-Gutierrez, A.; Gomez-Rincon, J.C.; Garcia-Guzman, K.; Leal, A.L.; et al. Molecular and Clinical Data of Antimicrobial Resistance in Microorganisms Producing Bacteremia in a Multicentric Cohort of Patients with Cancer in a Latin American Country. Microorganisms 2023, 11, 359. [Google Scholar] [CrossRef]
- Baghdadi, J.D.; Goodman, K.E.; Magder, L.S.; Claeys, K.C.; Sutherland, M.E.; Harris, A.D. Association Between Delayed Broad-Spectrum Gram-negative Antibiotics and Clinical Outcomes: How Much Does Getting It Right With Empiric Antibiotics Matter? Clin. Infect. Dis. 2025, 80, 949–958. [Google Scholar] [CrossRef]
- Kadry, N.; Natarajan, M.; Bein, E.; Kim, P.; Farley, J. Discordant Clinical and Microbiological Outcomes Are Associated With Late Clinical Relapse in Clinical Trials for Complicated Urinary Tract Infections. Clin. Infect. Dis. 2023, 76, 1768–1775. [Google Scholar] [CrossRef]
- Nocua-Baez, L.C.; Reyes, P.; Cortes, J.A. Effect of Inadequate Treatment in Adult Patients with Community-Acquired Acute Pyelonephritis Due to Enterobacterales Under Empirical Management with Cefazolin. Antibiotics 2025, 14, 197. [Google Scholar] [CrossRef]
- Frimodt-Moller, N. Correlation between pharmacokinetic/pharmacodynamic parameters and efficacy for antibiotics in the treatment of urinary tract infection. Int. J. Antimicrob. Agents 2002, 19, 546–553. [Google Scholar] [CrossRef]
- Cortes, J.A.; Sierra, C.R.; Sanchez, R. Effect of Inappropriate Treatment in Hospitalized Patients with Pyelonephritis Treated with Cefuroxime: A Cohort Study. Antibiotics 2024, 13, 274. [Google Scholar] [CrossRef]
- Teshome, B.F.; Park, T.; Arackal, J.; Hampton, N.; Kollef, M.H.; Micek, S.T. Preventing New Gram-negative Resistance Through Beta-lactam De-escalation in Hospitalized Patients With Sepsis: A Retrospective Cohort Study. Clin. Infect. Dis. 2024, 79, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Tello, A.; Gimbernat, H.; Redondo, C.; Meilan, E.; Arana, D.M.; Cacho, J.; Dorado, J.F.; Angulo, J.C. Prediction of infection caused by extended-spectrum beta-lactamase-producing Enterobacteriaceae: Development of a clinical decision-making nomogram. Scand. J. Urol. 2018, 52, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Shields, R.K.; Cheng, W.Y.; Kponee-Shovein, K.; Indacochea, D.; Gao, C.; Kuwer, F.; Joshi, A.V.; Mitrani-Gold, F.S.; Schwab, P.; Ferrinho, D.; et al. Development of Predictive Models to Inform a Novel Risk Categorization Framework for Antibiotic Resistance in Escherichia coli-Caused Uncomplicated Urinary Tract Infection. Clin. Infect. Dis. 2024, 79, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Al-Khlifeh, E.M.; Alkhazi, I.S.; Alrowaily, M.A.; Alghamdi, M.; Alrashidi, M.; Tarawneh, A.S.; Alkhawaldeh, I.M.; Hassanat, A.B. Extended Spectrum beta-Lactamase Bacteria and Multidrug Resistance in Jordan are Predicted Using a New Machine-Learning system. Infect. Drug. Resist. 2024, 17, 3225–3240. [Google Scholar] [CrossRef]
- Lee, H.G.; Seo, Y.; Kim, J.H.; Han, S.B.; Im, J.H.; Jung, C.Y.; Durey, A. Machine learning model for predicting ciprofloxacin resistance and presence of ESBL in patients with UTI in the ED. Sci. Rep. 2023, 13, 3282. [Google Scholar] [CrossRef]
- Arias Ramos, D.; Hoyos Pulgarin, J.A.; Moreno Gomez, G.A.; Alzate, J.A.; Olaya Gomez, J.C.; Cortes Bonilla, I.; Vargas Mosquera, C. Geographic mapping of Enterobacteriaceae with extended-spectrum beta-lactamase (ESBL) phenotype in Pereira, Colombia. BMC Infect. Dis. 2020, 20, 540. [Google Scholar] [CrossRef]
- Antimicrobial Resistance, C. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Leal, A.L.; Cortes, J.A.; Arias, G.; Ovalle, M.V.; Saavedra, S.Y.; Buitrago, G.; Escobar, J.A.; Castro, B.E.; GREBO. Emergence of resistance to third generation cephalosporins by Enterobacteriaceae causing community-onset urinary tract infections in hospitals in Colombia. Enferm. Infecc. Microbiol. Clin. 2013, 31, 298–303. [Google Scholar] [CrossRef]
| Characteristics | Non ESBL | ESBL | Total | p Value |
|---|---|---|---|---|
| Demographical | ||||
| Male, n (%) | 227 (30.8) | 42 (53.2) | 270 (33.0) | <0.001 |
| Age, mean (SD) | 64.0 (19.4) | 65.6 (15.6) | 64.2 (19.1) | 0.492 |
| Older than 70 years, n (%) | 337 (45.7) | 31 (39.2) | 369 (45.2) | 0.326 |
| Past medical information | ||||
| Diabetes, n (%) | 136 (18.5) | 14 (17.7) | 151 (18.5) | 0.995 |
| Charlson, mean (SD) | 4.0 (1.8) | 3.3 (1.8) | 3.9 (1.8) | 0.001 |
| Previous antibiotic use, n (%) | 139 (18.9) | 18 (22.8) | 157 (19.2) | 0.490 |
| Previous beta-lactam use, n (%) | 70 (9.5) | 11 (13.9) | 81 (9.9) | 0.293 |
| Previous quinolone use, n (%) | 24 (3.3) | 5 (6.3) | 29 (3.5) | 0.279 |
| Previously hospitalized, n (%) | 168 (22.8) | 21 (26.6) | 189 (23.1) | 0.537 |
| Previous urinary catheter, n (%) | 19 (2.6) | 7 (8.9) | 26 (3.2) | 0.007 |
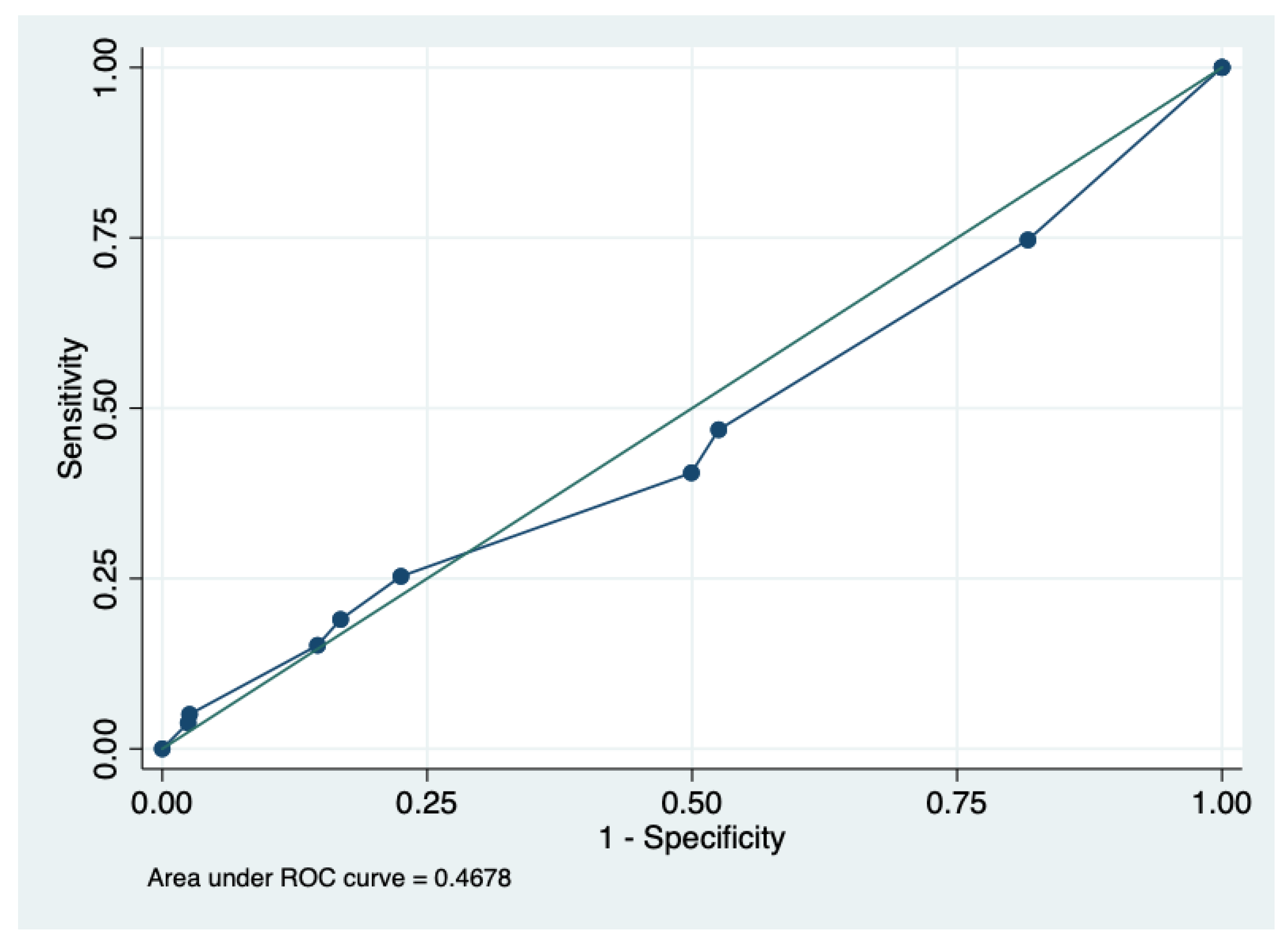
| Prediction of ESBL Phenotype | |||||||||
| Score≥ | TP | FP | TN | FN | Sens (%) | Esp (%) | PPV (%) | NPV (%) | ACC (%) |
| 2 | 59 | 602 | 135 | 20 | 74.7 | 18.3 | 8.9 | 87.1 | 23.8 |
| 3 | 37 | 387 | 350 | 42 | 46.8 | 47.4 | 8.7 | 89.2 | 47.4 |
| 4 | 32 | 368 | 369 | 47 | 40.5 | 50 | 8.0 | 88.7 | 49.1 |
| 5 | 20 | 166 | 571 | 59 | 25.3 | 77.4 | 10.7 | 90.6 | 72.4 |
| 6 | 15 | 124 | 613 | 64 | 18.9 | 83.1 | 10.7 | 90.5 | 76.9 |
| 7 | 12 | 108 | 629 | 67 | 15.1 | 85.3 | 10.0 | 90.3 | 78.5 |
| 8 | 4 | 19 | 718 | 75 | 5.1 | 97.4 | 17.3 | 90.5 | 88.4 |
| 9 | 3 | 18 | 719 | 76 | 3.7 | 97.5 | 14.2 | 90.4 | 88.4 |
| Prediction of Third-Generation Cephalosporin Non-Susceptibility | |||||||||
| Score≥ | TP | FP | TN | FN | Sens (%) | Esp (%) | PPV (%) | NPV (%) | ACC (%) |
| 2 | 83 | 579 | 128 | 27 | 75.4 | 18.1 | 12.5 | 82.5 | 25.8 |
| 3 | 56 | 369 | 338 | 54 | 50.9 | 47.8 | 13.1 | 86.2 | 48.2 |
| 4 | 51 | 350 | 357 | 59 | 46.3 | 50.4 | 12.7 | 85.8 | 49.9 |
| 5 | 27 | 159 | 548 | 83 | 24.5 | 77.5 | 14.5 | 86.8 | 70.3 |
| 6 | 22 | 117 | 590 | 88 | 20.0 | 83.4 | 15.8 | 87.0 | 74.9 |
| 7 | 19 | 101 | 606 | 91 | 17.2 | 85.7 | 15.8 | 86.9 | 76.4 |
| 8 | 6 | 17 | 690 | 104 | 5.4 | 97.5 | 26.0 | 86.9 | 85.1 |
| 9 | 5 | 16 | 691 | 105 | 4.5 | 97.7 | 23.8 | 86.8 | 85.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortés, J.A.; Niño-Godoy, J.A.; Muñoz-Latorre, H.J. Prediction Score for Identification of ESBL Producers in Urinary Infections Overestimates Risk in High-ESBL-Prevalence Setting. Antibiotics 2025, 14, 938. https://doi.org/10.3390/antibiotics14090938
Cortés JA, Niño-Godoy JA, Muñoz-Latorre HJ. Prediction Score for Identification of ESBL Producers in Urinary Infections Overestimates Risk in High-ESBL-Prevalence Setting. Antibiotics. 2025; 14(9):938. https://doi.org/10.3390/antibiotics14090938
Chicago/Turabian StyleCortés, Jorge Alberto, Julián Antonio Niño-Godoy, and Heidi Johanna Muñoz-Latorre. 2025. "Prediction Score for Identification of ESBL Producers in Urinary Infections Overestimates Risk in High-ESBL-Prevalence Setting" Antibiotics 14, no. 9: 938. https://doi.org/10.3390/antibiotics14090938
APA StyleCortés, J. A., Niño-Godoy, J. A., & Muñoz-Latorre, H. J. (2025). Prediction Score for Identification of ESBL Producers in Urinary Infections Overestimates Risk in High-ESBL-Prevalence Setting. Antibiotics, 14(9), 938. https://doi.org/10.3390/antibiotics14090938






