Increase in Penicillin Non-Susceptibility in Group B Streptococci Alongside Rising Isolation Rates—Based on 24 Years of Clinical Data from a Single University Hospital
Abstract
1. Introduction
2. Results
2.1. Study Isolates and PCN-NS Rates
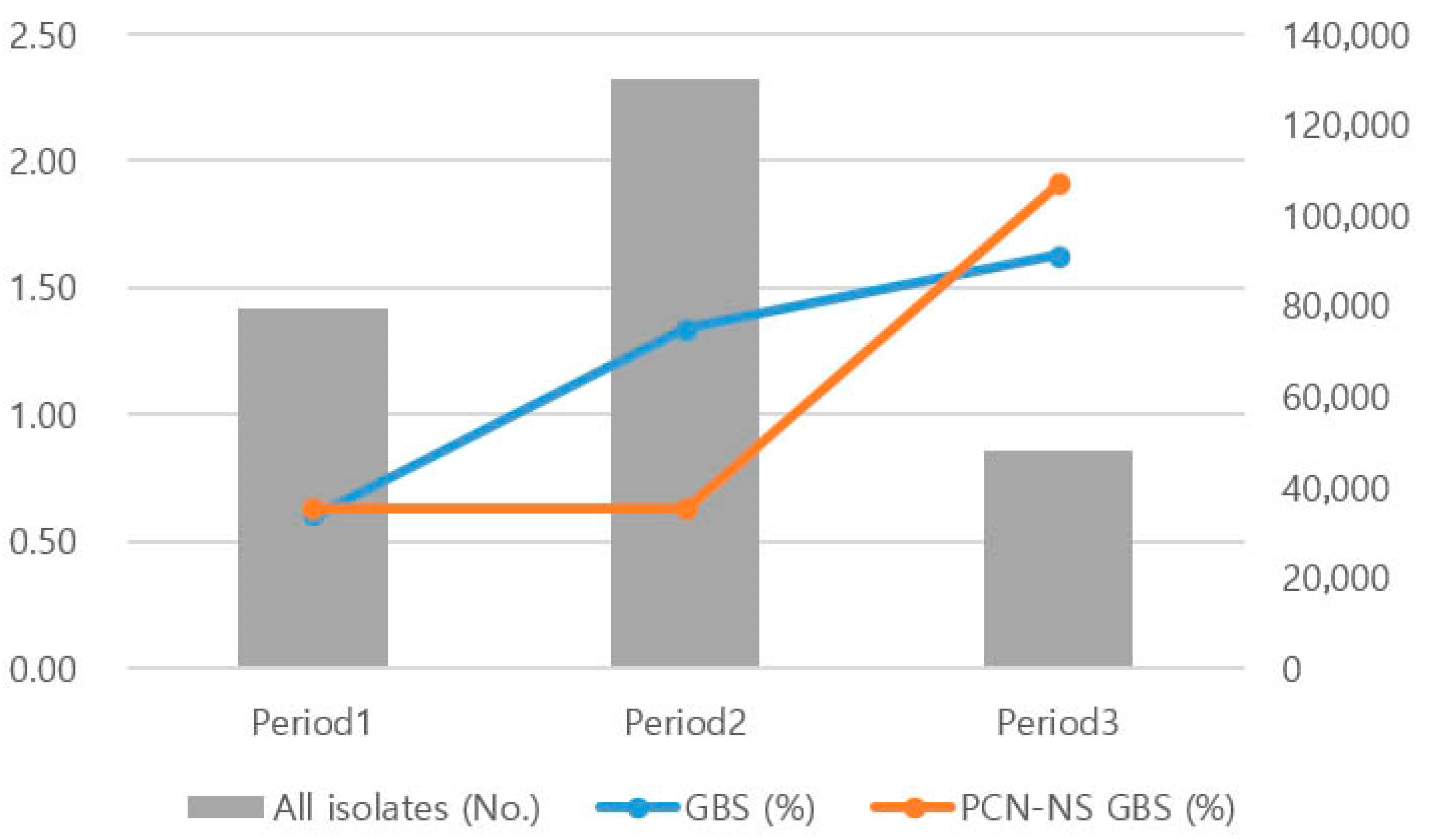
2.2. GBS Isolation Rate and PCN-NS Trends by Period
2.3. Antimicrobial Susceptibility of PCN-NS GBS
2.4. Number of GBS Isolates and Penicillin Non-Susceptibility by Specimen Type
3. Discussion
4. Materials and Methods
4.1. Study Isolates
4.2. Division of the Study Period into Periods 1, 2, and 3
4.3. Comparison of Antimicrobial Resistance Between PCN-NS GBS and Total GBS
4.4. Analysis of Antimicrobial Resistance in PCN-NS GBS Across Different Specimen Types
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GBS | group B streptococcus |
| PCN-NS GBS | penicillin non-susceptible group B streptococcus |
References
- Carroll, K.C.; Pfaller, M.A. Manual of Clinical Microbiology, 12th ed.; ASM Press: Washington, DC, USA, 2019; Volume 1, pp. 399–417. [Google Scholar]
- Lancefield, R.C. A Serological differentiation of human and other groups of hemolytic Streprococci. J. Exp. Med. 1933, 57, 571–595. [Google Scholar] [CrossRef]
- Eickhoff, T.C.; Klein, J.O.; Daly, A.K.; Ingall, D.; Finland, M. Neonatal sepsis and other infections due to group B beta-hemolytic Streptococci. N. Engl. J. Med. 1964, 271, 1221–1228. [Google Scholar] [CrossRef]
- Regan, J.A.; Klebanoff, M.A.; Nugent, R.P. The epidemiology of group B streptococcal colonization in pregnancy. Vaginal infections and prematurity study group. Obstet. Gynecol. 1991, 77, 604–610. [Google Scholar]
- Verani, J.R.; McGee, L.; Schrag, S.J. Prevention of perinatal group B streptococcal disease—Revised guidelines from CDC, 2010. MMWR Recomm. Rep. 2010, 59, 1–32. [Google Scholar]
- Raabe, V.N.; Shane, A.L. Group B Streptococcus (Streptococcus agalactiae). Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Skoff, T.H.; Farley, M.M.; Petit, S.; Craig, A.S.; Schaffner, W.; Gershman, K.; Harrison, L.H.; Lynfield, R.; Mohle-Boetani, J.; Zansky, S.M.; et al. Increasing burden of invasive group B streptococcal disease in nonpregnant adults, 1990–2007. Clin. Infect. Dis. 2009, 49, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Watkins, L.K.; McGee, L.; Schrag, S.J.; Beall, B.; Jain, J.H.; Pondo, T.; Farley, M.M.; Harrison, L.H.; Zansky, S.; Lynfield, R.; et al. Epidemiology of invasive group B streptococcal infections among nonpregnant adults in the United States, 2008–2016. JAMA Intern. Med. 2019, 179, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Torné, A.; Curcio, D.; Moïsi, J.C.; Jodar, L.; Melo-Cristino, J. Burden of invasive group B Streptococcus disease in non-pregnant adults: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0258030. [Google Scholar] [CrossRef]
- Uggen, E.; Olaisen, C.; Lyng, R.V.; Simonsen, G.S.; Bævre-Jensen, R.M.; Gran, F.W.; Åsvold, B.O.; Nilsen, T.I.L.; Damås, J.K.; Afset, J.E. Incidence of invasive infections with Group B streptococcus in adults in Norway 1996–2019: A nationwide registry-based case-control study. Infection 2024, 52, 1745–1752. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Moradkasani, S.; Suliman, M.; Uthirapathy, S.; Zwamel, A.H.; Hjazi, A.; Vashishth, R.; Beig, M. Global patterns of antibiotic resistance in group B Streptococcus: A systematic review and meta-analysis. Front. Microbiol. 2025, 16, 1541524. [Google Scholar] [CrossRef]
- Humphries, R.M.; Lu, J.; Martin, I.; Rauch, C.A.; Wojewoda, C.; McCarter, Y.; Long, T.; Simner, P.J. Detection of penicillin nonsusceptible Streptococcus agalactiae by laboratories that participate in the college of American pathologist’s proficiency testing program. J. Clin. Microbiol. 2023, 61, e0059523. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, M100, 35th ed.; Approved Guideline. M100; CLSI: Wayne, PA, USA, 2025. [Google Scholar]
- Crespo-Ortiz, M.P.; Castillo-Ramírez, C.R.; Recalde-Bolaños, M.; Vélez-Londoño, J.D. Emerging trends in invasive and noninvasive isolates of Streptococcus agalactiae in a Latin American hospital: A 17-year study. BMC Infect. Dis. 2014, 14, 428. [Google Scholar] [CrossRef]
- Betriu, C.; Gomez, M.; Sanchez, A.; Cruceyra, A.; Romero, J.; Picazo, J.J. Antibiotic resistance and penicillin tolerance in clinical isolates of group B streptococci. Antimicrob. Agents Chemother. 1994, 38, 2183–2186. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chu, Y.W.; Tse, C.; Tsang, G.K.; So, D.K.; Fung, J.T.; Lo, J.Y. Invasive group B Streptococcus isolates showing reduced susceptibility to penicillin in Hong Kong. J. Antimicrob. Chemother. 2007, 60, 1407–1409. [Google Scholar] [CrossRef]
- Kimura, K.; Suzuki, S.; Wachino, J.I.; Kurokawa, H.; Yamane, K.; Shibata, N.; Nagano, N.; Kato, H.; Shibayama, K.; Arakawa, Y. First molecular characterization of group B streptococci with reduced penicillin susceptibility. Antimicrob. Agents Chemother. 2008, 52, 2890–2897. [Google Scholar] [CrossRef]
- Li, Y.; Yang, W.; Li, Y.; Hua, K.; Zhao, Y.; Wang, T.; Liu, L.; Liu, Y.; Wang, Y.; Liu, W.; et al. The increasing burden of group B Streptococcus from 2013 to 2023: A retrospective cohort study in Beijing, China. Microbiol. Spectr. 2024, 13, e0226624. [Google Scholar] [CrossRef] [PubMed]
- van der Linden, M.; Mamede, R.; Levina, N.; Helwig, P.; Vila-Cerqueira, P.; Carriço, J.A.; Melo-Cristino, J.; Ramirez, M.; Martins, E.R. Heterogeneity of penicillin-non-susceptible group B streptococci isolated from a single patient in Germany. J. Antimicrob. Chemother. 2020, 75, 296–299. [Google Scholar] [CrossRef]
- Van Du, V.; Dung, P.T.; Toan, N.L.; Mao, C.V.; Bac, N.T.; Tong, H.V.; Son, H.A.; Thuan, N.D.; Viet, N.T. Antimicrobial resistance in colonizing group B Streptococcus among pregnant women from a hospital in Vietnam. Sci. Rep. 2021, 11, 20845. [Google Scholar] [CrossRef] [PubMed]
- Yi, A.; Kim, C.K.; Kimura, K.; Arakawa, Y.; Hur, M.; Yun, Y.M.; Moon, H.W. First case in Korea of group B Streptococcus with reduced penicillin susceptibility harboring amino acid substitutions in penicillin-binding protein 2X. Ann. Lab. Med. 2019, 39, 414–416. [Google Scholar] [CrossRef]
- Park, J.; So, M.K.; Kim, Y.H.; Chung, H.S. Characterization of penicillin-non-susceptible, multidrug-resistant Streptococcus agalactiae using whole-genome sequencing. Curr. Microbiol. 2025, 82, 344. [Google Scholar] [CrossRef]
- Creti, R. Have group A and B streptococcal infections become neglected diseases in Europe? Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1063–1064. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Donders, G.G.; Halperin, S.A.; Devlieger, R.; Baker, S.; Forte, P.; Wittke, F.; Slobod, K.S.; Dull, P.M. Maternal immunization with an investigational trivalent group B streptococcal vaccine: A randomized controlled trial. Obstet. Gynecol. 2016, 127, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Gergova, R.T.; Muhᴛarova, A.; Tsitou, V.M.; Mitov, I. Emergence of multidrug-resistant and -hypervirulent Streptococcus agalactiae in Bulgarian patients. Balk. Med. J. 2021, 38, 143–144. [Google Scholar] [CrossRef]
- Schrag, S.J.; Zell, E.R.; Lynfield, R.; Roome, A.; Arnold, K.E.; Craig, A.S.; Harrison, L.H.; Reingold, A.; Stefonek, K.; Smith, G.; et al. A population-based comparison of strategies to prevent early-onset group B streptococcal disease in neonates. N. Engl. J. Med. 2002, 347, 233–239. [Google Scholar] [CrossRef]
- Guo, H.; Fu, M.; Peng, Q.; Chen, Z.; Liu, J.; Qiu, Y.; Huang, Y. Antimicrobial resistance and molecular characterization of Streptococcus agalactiae from pregnant women in southern China. J. Infect. Dev. Ctries. 2019, 13, 802–809. [Google Scholar] [CrossRef] [PubMed]
| Antibiotics | Susceptibility Rate (%) | p Value | |
|---|---|---|---|
| GBS | PCN-NS GBS | ||
| Ampicillin | 99.39 | 37.93 | <0.0001 |
| Ceftriaxone | 99.63 | 68.18 | <0.0001 |
| Cefotaxime | 99.86 | 90.48 | <0.0001 |
| Levofloxacin | 76.46 | 54.55 | 0.016 |
| Clindamycin | 68.66 | 56.00 | 0.175 |
| Erythromycin | 62.99 | 48.28 | 0.103 |
| Tetracycline | 43.57 | 7.41 | <0.0001 |
| Specimen Type | No. of GBS | No. of PCN-NS GBS | PCN-NS Rate (%) |
|---|---|---|---|
| Blood | 279 | 4 | 1.43 |
| Vaginal swab | 770 | 4 | 0.52 |
| Urine | 1378 | 12 | 0.87 |
| Respiratory specimen | 88 | 3 | 3.41 |
| Others | 488 | 6 | 1.23 |
| Total | 3003 | 29 | 0.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, S.; Whang, D.H.; Um, T.-H.; Cho, C.R.; Chang, J. Increase in Penicillin Non-Susceptibility in Group B Streptococci Alongside Rising Isolation Rates—Based on 24 Years of Clinical Data from a Single University Hospital. Antibiotics 2025, 14, 928. https://doi.org/10.3390/antibiotics14090928
Shin S, Whang DH, Um T-H, Cho CR, Chang J. Increase in Penicillin Non-Susceptibility in Group B Streptococci Alongside Rising Isolation Rates—Based on 24 Years of Clinical Data from a Single University Hospital. Antibiotics. 2025; 14(9):928. https://doi.org/10.3390/antibiotics14090928
Chicago/Turabian StyleShin, Sunghwan, Dong Hee Whang, Tae-Hyun Um, Chong Rae Cho, and Jeonghyun Chang. 2025. "Increase in Penicillin Non-Susceptibility in Group B Streptococci Alongside Rising Isolation Rates—Based on 24 Years of Clinical Data from a Single University Hospital" Antibiotics 14, no. 9: 928. https://doi.org/10.3390/antibiotics14090928
APA StyleShin, S., Whang, D. H., Um, T.-H., Cho, C. R., & Chang, J. (2025). Increase in Penicillin Non-Susceptibility in Group B Streptococci Alongside Rising Isolation Rates—Based on 24 Years of Clinical Data from a Single University Hospital. Antibiotics, 14(9), 928. https://doi.org/10.3390/antibiotics14090928





