Serratia marcescens Isolates from Bovine Mastitic Milk: Antimicrobial Resistance and Virulence Features
Abstract
1. Introduction
2. Results
2.1. Phenotypic Identification and Antimicrobial Susceptibility Testing
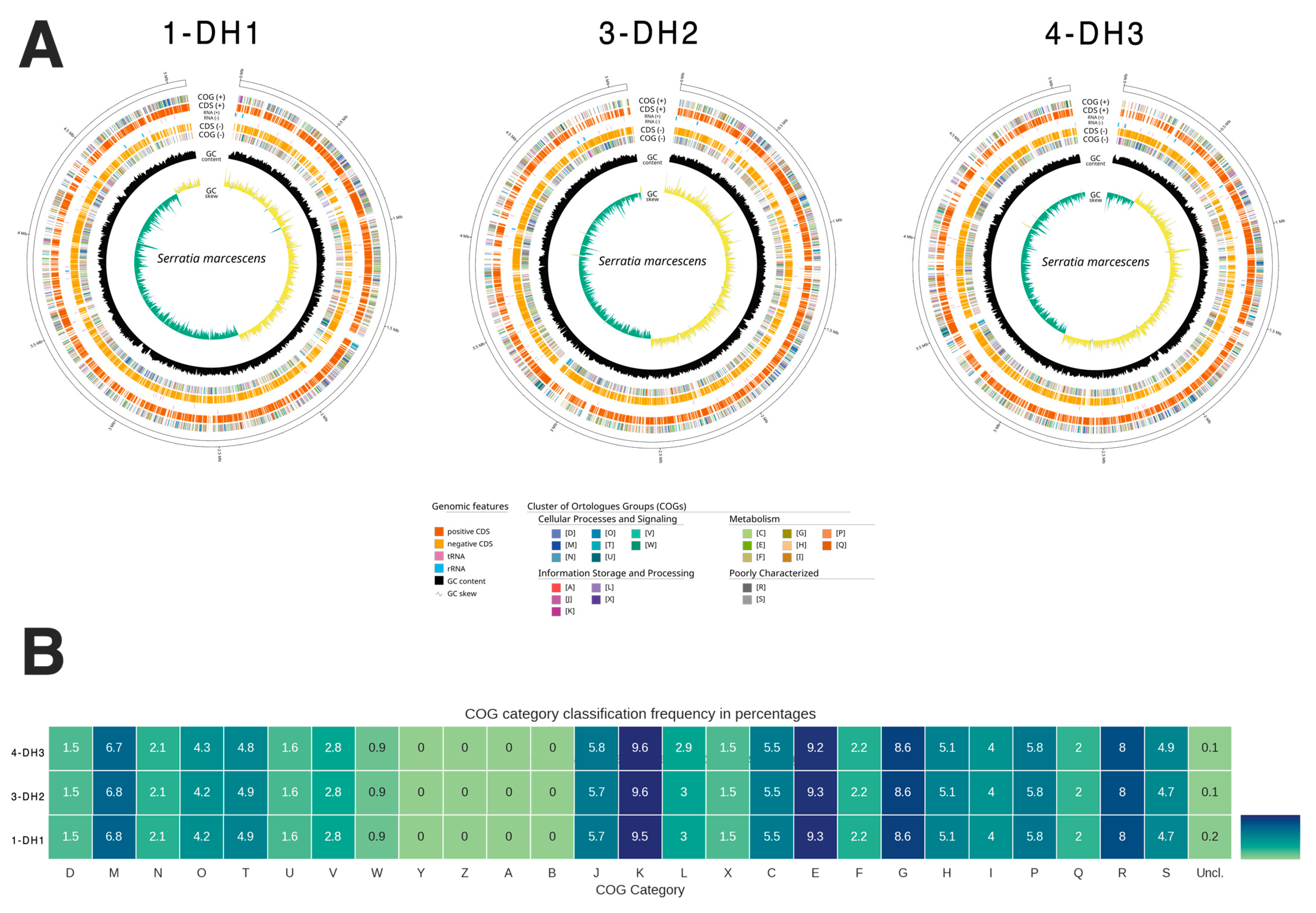
2.2. Genome Characteristics and Bacteria Classification
2.3. Genomic Analysis of Antimicrobial Resistance Genes
2.4. Genomic Analysis of Virulence Factors Genes
2.5. Genomic Analysis of Plasmids
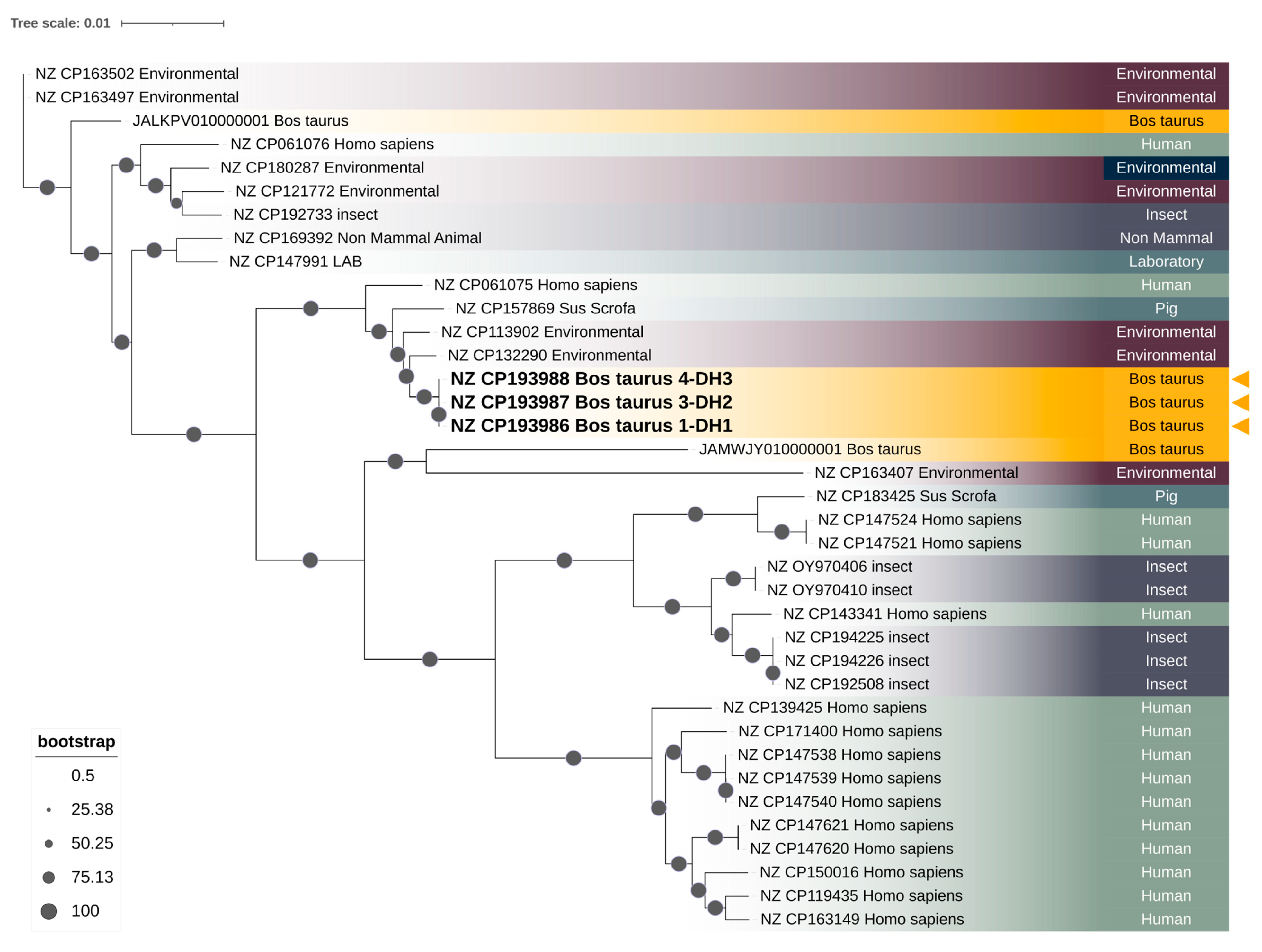
2.6. Phylogenomic Analysis
3. Discussion
4. Materials and Methods
4.1. Samples
4.2. Isolate Identification and Antimicrobial Susceptibility Testing
4.3. Isolates DNA Extraction
4.4. Whole-Genome Sequencing
4.5. Whole-Genome Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sugrue, I.; Tobin, C.; Ross, R.P.; Stanton, C.; Hill, C. Chapter 12-Foodborne Pathogens and Zoonotic Diseases. In Raw Milk; Academic Press: San Diego, CA, USA, 2018; pp. 259–272. [Google Scholar] [CrossRef]
- Barlow, J. Letter to the Editor: Comments on “Mammary Microbial Dysbiosis Leads to the Zoonosis of Bovine Mastitis: A One-Health Perspective” by Maity and Ambatipudi. FEMS Microbiol. Ecol. 2021, 97, fiab078. [Google Scholar] [CrossRef]
- Naranjo-Lucena, A.; Slowey, R. Invited Review: Antimicrobial Resistance in Bovine Mastitis Pathogens: A Review of Genetic Determinants and Prevalence of Resistance in European Countries. J. Dairy Sci. 2023, 106, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Zoonotic Disease: Emerging Public Health Threats in The Region. Available online: http://www.emro.who.int/fr/about-who/rc61/zoonotic-diseases.html (accessed on 30 June 2025).
- Sharun, K.; Dhama, K.; Tiwari, R.; Gugjoo, M.B.; Iqbal Yatoo, M.; Patel, S.K.; Pathak, M.; Karthik, K.; Khurana, S.K.; Singh, R.; et al. Advances in Therapeutic and Managemental Approaches of Bovine Mastitis: A Comprehensive Review. Vet. Q. 2021, 41, 107–136. [Google Scholar] [CrossRef] [PubMed]
- Azooz, M.F.; El-Wakeel, S.A.; Yousef, H.M. Financial and Economic Analyses of the Impact of Cattle Mastitis on the Profitability of Egyptian Dairy Farms. Vet. World 2020, 13, 1750–1759. [Google Scholar] [CrossRef]
- Tong, X.; Barkema, H.W.; Nobrega, D.B.; Xu, C.; Han, B.; Zhang, C.; Yang, J.; Li, X.; Gao, J. Virulence of Bacteria Causing Mastitis in Dairy Cows: A Literature Review. Microorganisms 2025, 13, 167. [Google Scholar] [CrossRef]
- Cheng, W.N.; Han, S.G. Bovine Mastitis: Risk Factors, Therapeutic Strategies, and Alternative Treatments—A Review. Asian-Australas. J. Anim. Sci. 2020, 33, 1699–1713. [Google Scholar] [CrossRef]
- Zhao, X.; Lacasse, P. Mammary Tissue Damage during Bovine Mastitis: Causes and Control. J. Anim. Sci. 2008, 86, 57–65. [Google Scholar] [CrossRef]
- Romero, J.; Benavides, E.; Meza, C. Assessing Financial Impacts of Subclinical Mastitis on Colombian Dairy Farms. Front. Vet. Sci. 2018, 5, 273. [Google Scholar] [CrossRef]
- Cervinkova, D.; Vlkova, H.; Borodacova, I.; Makovcova, J.; Babak, V.; Lorencova, A.; Vrtkova, I.; Marosevic, D.; Jaglic, Z. Prevalence of Mastitis Pathogens in Milk from Clinically Healthy Cows. Veterinární Medicína 2013, 58, 567–575. [Google Scholar] [CrossRef]
- Bogni, C.; Odierno, L.; Raspanti, C.; Giraudo, J.; Larriestra, A.; Reinoso, E.; Lasagno, M.; Ferrari, M.; Ducrós, E.; Frigerio, C. War against mastitis: Current concepts on controlling bovine mastitis pathogens. In Science Against Microbial Pathogens: Communicafing Current Research and Technological Advances; Mendez-Vilas, A., Ed.; World Scientific: Singapore, 2011; pp. 483–494. [Google Scholar]
- Tarazona-Manrique, L.E.; Villate-Hernández, J.R.; Andrade-Becerra, R.J.; Tarazona-Manrique, L.E.; Villate-Hernández, J.R.; Andrade-Becerra, R.J. Bacterial and Fungal Infectious Etiology Causing Mastitis in Dairy Cows in the Highlands of Boyacá (Colombia). Rev. Fac. Med. Vet. Zootec. 2019, 66, 208–218. [Google Scholar] [CrossRef]
- Friman, M.J.; Eklund, M.H.; Pitkälä, A.H.; Rajala-Schultz, P.J.; Rantala, M.H.J. Description of Two Serratia Marcescens Associated Mastitis Outbreaks in Finnish Dairy Farms and a Review of Literature. Acta Vet. Scand. 2019, 61, 54. [Google Scholar] [CrossRef]
- Pinzón-Sánchez, C.; Ruegg, P.L. Risk Factors Associated with Short-Term Post-Treatment Outcomes of Clinical Mastitis. J. Dairy Sci. 2011, 94, 3397–3410. [Google Scholar] [CrossRef] [PubMed]
- Khanna, A.; Khanna, M.; Aggarwal, A. Serratia Marcescens—A Rare Opportunistic Nosocomial Pathogen and Measures to Limit Its Spread in Hospitalized Patients. J. Clin. Diagn. Res. JCDR 2013, 7, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Blair, J.M.A.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J.V. Molecular Mechanisms of Antibiotic Resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, B.; Mawad, A.M.M.; Saleh, M.; Kelley, W.G.; Harrington, P.J.; Lovestad, C.W.; Amezcua, J.; Sarhan, M.M.; El Zowalaty, M.E.; Ramadan, H.; et al. Salmonellosis: An Overview of Epidemiology, Pathogenesis, and Innovative Approaches to Mitigate the Antimicrobial Resistant Infections. Antibiotics 2024, 13, 76. [Google Scholar] [CrossRef]
- Deekshit, V.K.; Srikumar, S. ‘To Be, or Not to Be’—The Dilemma of ‘Silent’ Antimicrobial Resistance Genes in Bacteria. J. Appl. Microbiol. 2022, 133, 2902–2914. [Google Scholar] [CrossRef]
- Morales-Ubaldo, A.L.; Rivero-Perez, N.; Valladares-Carranza, B.; Velázquez-Ordoñez, V.; Delgadillo-Ruiz, L.; Zaragoza-Bastida, A. Bovine Mastitis, a Worldwide Impact Disease: Prevalence, Antimicrobial Resistance, and Viable Alternative Approaches. Vet. Anim. Sci. 2023, 21, 100306. [Google Scholar] [CrossRef]
- Liang, Z.; Shen, J.; Liu, J.; Sun, X.; Yang, Y.; Lv, Y.; Zheng, J.; Mou, X.; Li, H.; Ding, X.; et al. Prevalence and Characterization of Serratia Marcescens Isolated from Clinical Bovine Mastitis Cases in Ningxia Hui Autonomous Region of China. Infect. Drug Resist. 2023, 16, 2727–2735. [Google Scholar] [CrossRef]
- Zivkovic Zaric, R.; Zaric, M.; Sekulic, M.; Zornic, N.; Nesic, J.; Rosic, V.; Vulovic, T.; Spasic, M.; Vuleta, M.; Jovanovic, J.; et al. Antimicrobial Treatment of Serratia Marcescens Invasive Infections: Systematic Review. Antibiotics 2023, 12, 367. [Google Scholar] [CrossRef]
- Ahmed, A.M.; Shimamoto, T. Molecular Characterization of Antimicrobial Resistance in Gram-Negative Bacteria Isolated from Bovine Mastitis in Egypt. Microbiol. Immunol. 2011, 55, 318–327. [Google Scholar] [CrossRef]
- Maseda, H.; Hashida, Y.; Shirai, A.; Omasa, T.; Nakae, T. Mutation in the sdeS Gene Promotes Expression of the sdeAB Efflux Pump Genes and Multidrug Resistance in Serratia Marcescens. Antimicrob. Agents Chemother. 2011, 55, 2922–2926. [Google Scholar] [CrossRef]
- Khayyat, A.N.; Abbas, H.A.; Khayat, M.T.; Shaldam, M.A.; Askoura, M.; Asfour, H.Z.; Khafagy, E.-S.; Abu Lila, A.S.; Allam, A.N.; Hegazy, W.A.H. Secnidazole Is a Promising Imidazole Mitigator of Serratia Marcescens Virulence. Microorganisms 2021, 9, 2333. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, W.A.H.; Khayat, M.T.; Ibrahim, T.S.; Youns, M.; Mosbah, R.; Soliman, W.E. Repurposing of Antidiabetics as Serratia Marcescens Virulence Inhibitors. Braz. J. Microbiol. 2021, 52, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Van Houdt, R.; Givskov, M.; Michiels, C.W. Quorum Sensing in Serratia. FEMS Microbiol. Rev. 2007, 31, 407–424. [Google Scholar] [CrossRef]
- Hnini, R.; Silva, E.; Pinho, L.; Najimi, M.; Thompson, G. Phenotypic Characteriza-Tion and Resistance Genes Detection of Staphylococcus Aureus Isolated from Bovine Mastitis in the Northwest of Portugal. Acta Vet. Eurasia 2023, 49, 127–136. [Google Scholar] [CrossRef]
- Marinho, C.M.; Santos, T.; Gonçalves, A.; Poeta, P.; Igrejas, G. A Decade-Long Commitment to Antimicrobial Resistance Surveillance in Portugal. Front. Microbiol. 2016, 7, 01650. [Google Scholar] [CrossRef]
- SciELO Brazil-Beta-Lactam Antimicrobials Activity and the Diversity of blaZ Gene in Staphylococcus Aureus Isolates from Bovine Mastitis in the Northwest of Portugal Beta-Lactam Antimicrobials Activity and the Diversity of blaZ Gene in Staphylococcus aureus Isolates from Bovine Mastitis in the Northwest of Portugal. Available online: https://www.scielo.br/j/rbz/a/kM7mH9RRF93SdBvVVydspNc/ (accessed on 25 July 2025).
- Quinteira, S.; Dantas, R.; Pinho, L.; Campos, C.; Freitas, A.R.; Brito, N.V.; Miranda, C. Dairy Cattle and the Iconic Autochthonous Cattle in Northern Portugal Are Reservoirs of Multidrug-Resistant Escherichia coli. Antibiotics 2024, 13, 1208. [Google Scholar] [CrossRef]
- Bento, J.T.; Gomes-Gonçalves, S.; Cruz, R.; Esteves, F.; Baptista, A.L.; Aires Pereira, M.; Caseiro, P.; Carreira, P.; Figueira, L.; Mesquita, J.R.; et al. The Prevalence of Antimicrobial Resistance Genes in the Environments of Small Ruminant Farms from Central Portugal. Antibiotics 2025, 14, 576. [Google Scholar] [CrossRef]
- Mash: Fast Genome and Metagenome Distance Estimation Using MinHash|Genome Biology|Full Text. Available online: https://genomebiology.biomedcentral.com/articles/10.1186/s13059-016-0997-x (accessed on 25 July 2025).
- Chen, S.; Blom, J.; Walker, E.D. Genomic, Physiologic, and Symbiotic Characterization of Serratia Marcescens Strains Isolated from the Mosquito Anopheles Stephensi. Front. Microbiol. 2017, 8, 1483. [Google Scholar] [CrossRef]
- Ruegg, P.L.; Guterbock, W.M.; Holmberg, C.A.; Gay, J.M.; Weaver, L.D.; Walton, R.W. Microbiologic Investigation of an Epizootic of Mastitis Caused by Serratia Marcescens in a Dairy Herd. J. Am. Vet. Med. Assoc. 1992, 200, 184–189. [Google Scholar] [CrossRef]
- Kamarudin, M.I.; Fox, L.K.; Gaskins, C.T.; Gay, J.M. Environmental Reservoirs for Serratia Marcescens Intramammary Infections in Dairy Cows. J. Am. Vet. Med. Assoc. 1996, 208, 555–558. [Google Scholar] [CrossRef]
- International Conference on Mastitis Control from Science to Practice. Mastitis Control: From Science to Practice, Proceedings of International Conference on Mastitis Control: From Science to Practice, The Hague, The Netherlands, 30 September–2 October 2008; Academic Publishers: Wageningen, The Netherlands, 2008. [Google Scholar]
- Stanek, P.; Żółkiewski, P.; Januś, E. A Review on Mastitis in Dairy Cows Research: Current Status and Future Perspectives. Agriculture 2024, 14, 1292. [Google Scholar] [CrossRef]
- Nagano, D.S.; Taniguchi, I.; Ono, T.; Nakamura, K.; Gotoh, Y.; Hayashi, T. Systematic Analysis of Plasmids of the Serratia Marcescens Complex Using 142 Closed Genomes. Microb. Genom. 2023, 9, 001135. [Google Scholar] [CrossRef]
- Saralegui, C.; Ponce-Alonso, M.; Pérez-Viso, B.; Moles Alegre, L.; Escribano, E.; Lázaro-Perona, F.; Lanza, V.F.; de Pipaón, M.S.; Rodríguez, J.M.; Baquero, F.; et al. Genomics of Serratia Marcescens Isolates Causing Outbreaks in the Same Pediatric Unit 47 Years Apart: Position in an Updated Phylogeny of the Species. Front. Microbiol. 2020, 11, 451. [Google Scholar] [CrossRef] [PubMed]
- Brandt, M.; McCullor, K.; Harris, D.; Ratzlaff, Z.; Thompson, E.; Pfeifer, C.M. Direct Inoculation Method for Identification and Antimicrobial Susceptibility Testing Using Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry and Both the Vitek 2 and MicroScan Walkaway 96 Plus Systems. Proc. Bayl. Univ. Med. Cent. 2023, 36, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Carattoli, A.; Zankari, E.; García-Fernández, A.; Voldby Larsen, M.; Lund, O.; Villa, L.; Møller Aarestrup, F.; Hasman, H. In Silico Detection and Typing of Plasmids Using PlasmidFinder and Plasmid Multilocus Sequence Typing. Antimicrob. Agents Chemother. 2014, 58, 3895–3903. [Google Scholar] [CrossRef]
- Lopes, T.S.; Fussieger, C.; Rizzo, F.A.; Silveira, S.; Lunge, V.R.; Streck, A.F. Species Identification and Antimicrobial Susceptibility Profile of Bacteria Associated with Cow Mastitis in Southern Brazil. Pesqui. Veterinária Bras. 2022, 42, e06958. [Google Scholar] [CrossRef]
- Abdullah, A.; Aljoburi, A. Isolation and Identification of Serratia Marcescens from Bovine Mastitis Infections in Iraq and Their Susceptibility to Antibiotics. J. Entomol. Zool. Stud. 2017, 5, 489–492. [Google Scholar] [CrossRef]
- Awoderu, B.; Omololu-Aso, J. Susceptibility Profile among Isolates of S. Marcescens Obtained from Clinical and Non-Clinical Sources, Ile-Ife, Osun State, Nigeria. Int. Arch. Public Health Community Med. 2021, 5, 071. [Google Scholar] [CrossRef]
- Hayashi, W.; Yoshida, S.; Izumi, K.; Koide, S.; Soga, E.; Takizawa, S.; Arakawa, Y.; Nagano, Y.; Nagano, N. Genomic Characterisation and Epidemiology of Nosocomial Serratia Marcescens Isolates Resistant to Ceftazidime and Their Plasmids Mediating Rare blaTEM-61. J. Glob. Antimicrob. Resist. 2021, 25, 124–131. [Google Scholar] [CrossRef]
- Traub, W.H. Antibiotic Susceptibility of Serratia Marcescens and Serratia Liquefaciens. Chemotherapy 2000, 46, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Chopra, D.A.; Oberoi, D.L.; Singh, D.K.; Sidhu, D.S. Serratia Marcescens Diabetic Foot: A Case Report and Review of Literature. Int. J. Health Sci. Res. 2019, 9, 316–319. [Google Scholar]
- Ballaben, A.; de Almeida, O.G.; Ferreira, J.; Garcia, D.; Doi, Y.; Ernst, R.; Kress, M.; Darini, A. Phenotypic and in Silico Characterization of Carbapenem-Resistant Serratia Marcescens Clinical Strains. J. Glob. Antimicrob. Resist. 2025, 42, 105–112. [Google Scholar] [CrossRef]
- Caneschi, A.; Bardhi, A.; Barbarossa, A.; Zaghini, A. Plant Essential Oils as a Tool in the Control of Bovine Mastitis: An Update. Molecules 2023, 28, 3425. [Google Scholar] [CrossRef]
- Tomanić, D.; Božin, B.; Kladar, N.; Stanojević, J.; Čabarkapa, I.; Stilinović, N.; Apić, J.; Božić, D.D.; Kovačević, Z. Environmental Bovine Mastitis Pathogens: Prevalence, Antimicrobial Susceptibility, and Sensitivity to Thymus Vulgaris L., Thymus Serpyllum L., and Origanum Vulgare L. Essential Oils. Antibiotics 2022, 11, 1077. [Google Scholar] [CrossRef]
- Hyyawi, S.; Abdulrasool, M.; Nadhom, B.; Al-Rubaye, S. The Growth Susceptibility Test of Serratia Marcescens in The Presence of Crude Capsicum Annum. Plant Arch. 2021, 20, 8690–8694. [Google Scholar]
- Galgano, M.; Pellegrini, F.; Mrenoshki, D.; Addante, L.; Sposato, A.; Del Sambro, L.; Capozzi, L.; Catalano, E.; Solito, M.; D’Amico, F.; et al. Inhibition of Biofilm Production and Determination of In Vitro Time-Kill Thymus Vulgaris L. Essential Oil (TEO) for the Control of Mastitis in Small Ruminants. Pathogens 2025, 14, 412. [Google Scholar] [CrossRef]
- Gómez-Lus, R.; Rivera, M.J.; Bobey, D.; Martín, C.; Navarro, M. Chromosomal Origin of Acetyltransferase AAC(6′) Specifying Amikacin Resistance in Serratia Marcescens. Microbiol. Madr. Spain 1987, 3, 185–194. [Google Scholar]
- Shaw, K.J.; Rather, P.N.; Sabatelli, F.J.; Mann, P.; Munayyer, H.; Mierzwa, R.; Petrikkos, G.L.; Hare, R.S.; Miller, G.H.; Bennett, P.; et al. Characterization of the Chromosomal Aac(6′)-Ic Gene from Serratia Marcescens. Antimicrob. Agents Chemother. 1992, 36, 1447–1455. [Google Scholar] [CrossRef]
- Tada, T.; Miyoshi-Akiyama, T.; Shimada, K.; Dahal, R.K.; Mishra, S.K.; Ohara, H.; Kirikae, T.; Pokhrel, B.M. A Novel 6′-N-Aminoglycoside Acetyltransferase, AAC(6′)-Ial, from a Clinical Isolate of Serratia Marcescens. Microb. Drug Resist. 2016, 22, 103–108. [Google Scholar] [CrossRef]
- Hou, J.; Mao, D.; Zhang, Y.; Huang, R.; Li, L.; Wang, X.; Luo, Y. Long-Term Spatiotemporal Variation of Antimicrobial Resistance Genes within the Serratia Marcescens Population and Transmission of S. Marcescens Revealed by Public Whole-Genome Datasets. J. Hazard. Mater. 2022, 423, 127220. [Google Scholar] [CrossRef]
- Franzon, J.H.; Santos, D.S. A Role for Histone-like Protein H1 (H-NS) in the Regulation of Hemolysin Expression by Serratia Marcescens. Braz. J. Med. Biol. Res. Rev. Bras. Pesqui. Medicas E Biol. 2004, 37, 1763–1769. [Google Scholar] [CrossRef][Green Version]
- Wu, L.-T.; Tsou, M.-F.; Wu, H.-J.; Chen, H.-E.; Chuang, Y.-C.; Yu, W.-L. Survey of CTX-M-3 Extended-Spectrum Beta-Lactamase (ESBL) among Cefotaxime-Resistant Serratia Marcescens at a Medical Center in Middle Taiwan. Diagn. Microbiol. Infect. Dis. 2004, 49, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Piccirilli, A.; Cherubini, S.; Brisdelli, F.; Fazii, P.; Stanziale, A.; Di Valerio, S.; Chiavaroli, V.; Principe, L.; Perilli, M. Molecular Characterization by Whole-Genome Sequencing of Clinical and Environmental Serratia Marcescens Strains Isolated during an Outbreak in a Neonatal Intensive Care Unit (NICU). Diagnostics 2022, 12, 2180. [Google Scholar] [CrossRef] [PubMed]
- Klages, L.J.; Kaup, O.; Busche, T.; Kalinowski, J.; Rückert-Reed, C. Classification of a Novel Serratia Species, Isolated from a Wound Swab in North Rhine-Westphalia: Proposal of Serratia sarumanii sp. Nov. Syst. Appl. Microbiol. 2024, 47, 126527. [Google Scholar] [CrossRef] [PubMed]
- Cunha, B.A.; Schoch, P.E.; Hage, J.R. Nitrofurantoin: Preferred Empiric Therapy for Community-Acquired Lower Urinary Tract Infections. Mayo Clin. Proc. 2011, 86, 1243–1244. [Google Scholar] [CrossRef]
- Osei Sekyere, J. Genomic Insights Into Nitrofurantoin Resistance Mechanisms and Epidemiology in Clinical Enterobacteriaceae. Future Microbiol. 2018, 4, FSO293. [Google Scholar] [CrossRef]
- Thompson, S.A.; Maani, E.V.; Lindell, A.H.; King, C.J.; McArthur, J.V. Novel Tetracycline Resistance Determinant Isolated from an Environmental Strain of Serratia Marcescens. Appl. Environ. Microbiol. 2007, 73, 2199–2206. [Google Scholar] [CrossRef]
- Bolourchi, N.; Noori Goodarzi, N.; Giske, C.G.; Nematzadeh, S.; Haririzadeh Jouriani, F.; Solgi, H.; Badmasti, F. Comprehensive Pan-Genomic, Resistome and Virulome Analysis of Clinical OXA-48 Producing Carbapenem-Resistant Serratia Marcescens Strains. Gene 2022, 822, 146355. [Google Scholar] [CrossRef]
- Tavares-Carreon, F.; De Anda-Mora, K.; Rojas-Barrera, I.C.; Andrade, A. Serratia Marcescens Antibiotic Resistance Mechanisms of an Opportunistic Pathogen: A Literature Review. PeerJ 2023, 11, e14399. [Google Scholar] [CrossRef]
- Pérez-Viso, B.; Hernández-García, M.; Rodríguez, C.M.; Fernández-de-Bobadilla, M.D.; Serrano-Tomás, M.I.; Sánchez-Díaz, A.M.; Avendaño-Ortiz, J.; Coque, T.M.; Ruiz-Garbajosa, P.; Del Campo, R.; et al. A Long-Term Survey of Serratia spp. Bloodstream Infections Revealed an Increase of Antimicrobial Resistance Involving Adult Population. Microbiol. Spectr. 2024, 12, e0276223. [Google Scholar] [CrossRef]
- Katsikogianni, M.; Missirlis, Y.F. Concise Review of Mechanisms of Bacterial Adhesion to Biomaterials and of Techniques Used in Estimating Bacteria-Material Interactions. Eur. Cells Mater. 2004, 8, 37–57. [Google Scholar] [CrossRef]
- Zahller, J.; Stewart, P.S. Transmission Electron Microscopic Study of Antibiotic Action on Klebsiella Pneumoniae Biofilm. Antimicrob. Agents Chemother. 2002, 46, 2679–2683. [Google Scholar] [CrossRef] [PubMed]
- Haiko, J.; Westerlund-Wikström, B. The Role of the Bacterial Flagellum in Adhesion and Virulence. Biology 2013, 2, 1242–1267. [Google Scholar] [CrossRef] [PubMed]
- Anderl, J.N.; Franklin, M.J.; Stewart, P.S. Role of Antibiotic Penetration Limitation in Klebsiella Pneumoniae Biofilm Resistance to Ampicillin and Ciprofloxacin. Antimicrob. Agents Chemother. 2000, 44, 1818–1824. [Google Scholar] [CrossRef] [PubMed]
- Melchior, M.B.; Vaarkamp, H.; Fink-Gremmels, J. Biofilms: A Role in Recurrent Mastitis Infections? Vet. J. 2006, 171, 398–407. [Google Scholar] [CrossRef]
- Pedersen, R.R.; Krömker, V.; Bjarnsholt, T.; Dahl-Pedersen, K.; Buhl, R.; Jørgensen, E. Biofilm Research in Bovine Mastitis. Front. Vet. Sci. 2021, 8, 656810. [Google Scholar] [CrossRef]
- Tang, B.; Zhao, H.; Li, J.; Liu, N.; Huang, Y.; Wang, J.; Yue, M. Detection of Clinical Serratia Marcescens Isolates Carrying BlaKPC-2 in a Hospital in China. Heliyon 2024, 10, e29702. [Google Scholar] [CrossRef]
- Abreo, E.; Altier, N. Pangenome of Serratia Marcescens Strains from Nosocomial and Environmental Origins Reveals Different Populations and the Links between Them. Sci. Rep. 2019, 9, 46. [Google Scholar] [CrossRef]
- Sandner-Miranda, L.; Vinuesa, P.; Cravioto, A.; Morales-Espinosa, R. The Genomic Basis of Intrinsic and Acquired Antibiotic Resistance in the Genus Serratia. Front. Microbiol. 2018, 9, 00828. [Google Scholar] [CrossRef] [PubMed]
- McNally, A.; Oren, Y.; Kelly, D.; Pascoe, B.; Dunn, S.; Sreecharan, T.; Vehkala, M.; Välimäki, N.; Prentice, M.; Ashour, A.; et al. Combined Analysis of Variation in Core, Accessory and Regulatory Genome Regions Provides a Super-Resolution View into the Evolution of Bacterial Populations. PLoS Genet. 2016, 12, e1006280. [Google Scholar] [CrossRef] [PubMed]
- Isaksson, A.; Holmberg, O. Serratia-mastitis in cows as a herd problem. Nord. Vet. Med. 1984, 36, 354–360. [Google Scholar] [PubMed]
- Ono, T.; Taniguchi, I.; Nakamura, K.; Nagano, D.S.; Nishida, R.; Gotoh, Y.; Ogura, Y.; Sato, M.P.; Iguchi, A.; Murase, K.; et al. Global Population Structure of the Serratia Marcescens Complex and Identification of Hospital-Adapted Lineages in the Complex. Microb. Genom. 2022, 8, 000793. [Google Scholar] [CrossRef]
- Shikov, A.E.; Merkushova, A.V.; Savina, I.A.; Nizhnikov, A.A.; Antonets, K.S. The Man, the Plant, and the Insect: Shooting Host Specificity Determinants in Serratia Marcescens Pangenome. Front. Microbiol. 2023, 14, 1211999. [Google Scholar] [CrossRef]
- Hejazi, A.; Falkiner, F.R. Serratia Marcescens. J. Med. Microbiol. 1997, 46, 903–912. [Google Scholar] [CrossRef]
- Fusco, V.; Abriouel, H.; Benomar, N.; Kabisch, J.; Chieffi, D.; Cho, G.-S.; Franz, C.M.A.P. Chapter 10-Opportunistic Food-Borne Pathogens. In Food Safety and Preservation; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 269–306. ISBN 978-0-12-814956-0. [Google Scholar]
- Gonzalez, T.D.J.B.; van Gelderen, B.; Harders, F.; Vloet, R.; Voorbergen-Laarman, M.; de Ruiter, B.; Haenen, O.L.M. Molecular Characterization of Serratia Marcescens Strain Isolated from Yellow Mealworms, Tenebrio Molitor, in The Netherlands. Insects 2023, 14, 770. [Google Scholar] [CrossRef]
- Eucast: Clinical Breakpoints and Dosing of Antibiotics. Available online: https://www.eucast.org/clinical_breakpoints (accessed on 30 June 2025).
- M07|Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. Available online: https://clsi.org/shop/standards/m07/ (accessed on 30 June 2025).
- M100|Performance Standards for Antimicrobial Susceptibility Testing. Available online: https://clsi.org/shop/standards/m100/ (accessed on 30 June 2025).
- van Tongeren, S.P.; Degener, J.E.; Harmsen, H.J.M. Comparison of Three Rapid and Easy Bacterial DNA Extraction Methods for Use with Quantitative Real-Time PCR. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 1053–1061. [Google Scholar] [CrossRef]
- Smith, B.; Li, N.; Andersen, A.S.; Slotved, H.C.; Krogfelt, K.A. Optimising Bacterial DNA Extraction from Faecal Samples: Comparison of Three Methods. Open Microbiol. J. 2011, 5, 14–17. [Google Scholar] [CrossRef]
- Ligation Sequencing Amplicons-Native Barcoding Kit 96 V14 (SQK-NBD114.96). Available online: https://nanoporetech.com/document/ligation-sequencing-amplicons-native-barcoding-v14-sqk-nbd114-96 (accessed on 25 July 2025).
- De Coster, W.; Rademakers, R. NanoPack2: Population-Scale Evaluation of Long-Read Sequencing Data. Bioinformatics 2023, 39, btad311. [Google Scholar] [CrossRef]
- Wick, R. rrwick/Porechop 2024. Available online: https://github.com/rrwick/Porechop (accessed on 23 June 2025).
- Wick, R. rrwick/Filtlong 2025. Available online: https://github.com/rrwick/Filtlong (accessed on 23 June 2025).
- Li, H. Minimap and Miniasm: Fast Mapping and de Novo Assembly for Noisy Long Sequences. Bioinformatics 2016, 32, 2103–2110. [Google Scholar] [CrossRef] [PubMed]
- Kolmogorov, M.; Yuan, J.; Lin, Y.; Pevzner, P.A. Assembly of Long, Error-Prone Reads Using Repeat Graphs. Nat. Biotechnol. 2019, 37, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Nanoporetech/Medaka 2025. Available online: https://github.com/nanoporetech/medaka (accessed on 23 June 2025).
- Beyvers, S.; Jelonek, L.; Goesmann, A.; Schwengers, O. Bakta Web–Rapid and Standardized Genome Annotation on Scalable Infrastructures. Nucleic Acids Res. 2025, 53, gkaf335. [Google Scholar] [CrossRef] [PubMed]
- Parks, D.H.; Imelfort, M.; Skennerton, C.T.; Hugenholtz, P.; Tyson, G.W. CheckM: Assessing the Quality of Microbial Genomes Recovered from Isolates, Single Cells, and Metagenomes. Genome Res. 2015, 25, 1043–1055. [Google Scholar] [CrossRef]
- Ondov, B.D.; Starrett, G.J.; Sappington, A.; Kostic, A.; Koren, S.; Buck, C.B.; Phillippy, A.M. Mash Screen: High-Throughput Sequence Containment Estimation for Genome Discovery. Genome Biol. 2019, 20, 232. [Google Scholar] [CrossRef]
- O’Leary, N.A.; Wright, M.W.; Brister, J.R.; Ciufo, S.; Haddad, D.; McVeigh, R.; Rajput, B.; Robbertse, B.; Smith-White, B.; Ako-Adjei, D.; et al. Reference Sequence (RefSeq) Database at NCBI: Current Status, Taxonomic Expansion, and Functional Annotation. Nucleic Acids Res. 2016, 44, D733–D745. [Google Scholar] [CrossRef]
- Garcillán-Barcia, M.P.; Redondo-Salvo, S.; Vielva, L.; de la Cruz, F. MOBscan: Automated Annotation of MOB Relaxases. In Horizontal Gene Transfer. Methods in Molecular Biology; Humana: New York, NY, USA, 2020; Volume 2075, pp. 295–308. [Google Scholar] [CrossRef]
- Seemann, T. Tseemann/Abricate 2025. Available online: https://github.com/tseemann/abricate (accessed on 23 June 2025).
- Jia, B.; Raphenya, A.R.; Alcock, B.; Waglechner, N.; Guo, P.; Tsang, K.K.; Lago, B.A.; Dave, B.M.; Pereira, S.; Sharma, A.N.; et al. CARD 2017: Expansion and Model-Centric Curation of the Comprehensive Antibiotic Resistance Database. Nucleic Acids Res. 2017, 45, D566–D573. [Google Scholar] [CrossRef]
- Chen, L.; Yang, J.; Yu, J.; Yao, Z.; Sun, L.; Shen, Y.; Jin, Q. VFDB: A Reference Database for Bacterial Virulence Factors. Nucleic Acids Res. 2005, 33, D325–D328. [Google Scholar] [CrossRef]
- Tonkin-Hill, G.; MacAlasdair, N.; Ruis, C.; Weimann, A.; Horesh, G.; Lees, J.A.; Gladstone, R.A.; Lo, S.; Beaudoin, C.; Floto, R.A.; et al. Producing Polished Prokaryotic Pangenomes with the Panaroo Pipeline. Genome Biol. 2020, 21, 180. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v4: Recent Updates and New Developments. Nucleic Acids Res. 2019, 47, W256–W259. [Google Scholar] [CrossRef]
| Neg-Urine-Combo98 Panel | Isolate 1-DH1 | Isolate 2-DH1 | Isolate 3-DH2 | Isolate 4-DH3 |
|---|---|---|---|---|
| Bacteria Identification % of probability | S. marcescens 99.99 | S. marcescens 99.99 | S. odorifera 94.66 | S. marcescens 99.99 |
| Antimicrobials | ||||
| β-lactam | ||||
| Amoxicillin-clavulanate acid-E (Aug-E) | R * (>32.00) | R * (>32.00) | R * (>32.00) | R * (32.00) |
| Ampicillin (AM) | R * (>8.00) | R * (>8.00) | R * (>8.00) | R * (>8.00) |
| Cefepime (Cpe) | S * (≤1.00) | S * (≤1.00) | S * (≤1.00) | S * (≤1.00) |
| Cefotaxime (Cft) | S * (<1.00) | S * (<1.00) | S * (<1.00) | S * (<1.00) |
| Ceftazidime-clavulanate acid (Caz/CA) | S * (≤0.25) | S * (≤0.25) | S * (≤0.25) | S * (≤0.25) |
| Ertapenem (Etp) | S * (≤0.12) | S * (≤0.12) | S * (≤0.12) | S * (≤0.12) |
| Imipenem (Imp) | S * (≤2.00) | S * (≤2.00) | S * (≤2.00) | S * (≤2.00) |
| Meropenem (Mer) | S * (≤0.12) | S * (≤0.12) | S * (≤0.12) | S * (≤0.12) |
| Quinolone | ||||
| Nalidixic Acid (NA) | S * (≤0.16) | S * (≤0.16) | S * (≤0.16) | S * (≤0.16) |
| Ciprofloxacin (Cp) | S * (0.25) | S * (0.25) | S * (0.25) | S * (≤0.06) |
| Levofloxacin (Lvx) | S * (≤0.50) | S * (≤0.50) | S * (≤0.50) | S * (≤0.50) |
| Norfloxacine (Nxn) | S * (≤0.50) | S * (≤0.50) | S * (≤0.50) | S * (≤0.50) |
| Aminoglycoside | ||||
| Amikacin (AK) | S * (≤8.00) | S * (≤8.00) | S * (≤8.00) | S * (≤8.00) |
| Gentamicin (Gm) | S * (≤0.20) | S * (≤0.20) | S * (≤0.20) | S * (≤0.20) |
| Tobramycin (To) | R * (4.00) | R * (4.00) | R * (4.00) | R * (≤0.20) |
| Sulphonamide | ||||
| Trimethoprim-Sulfamethoxazole (T/S) | S * (≤2/38) | S * (≤2/38) | S * (≤2/38) | S * (≤2/38) |
| Monobactam | ||||
| Aztreonam (AZT) | S * (≤1.00) | S * (≤1.00) | S * (≤1.00) | S * (≤1.00) |
| Cephalosporin | ||||
| Cefoxitin (Cfx) | R * (≤8.00) | R * (≤8.00) | S * (≤8.00) | R * (≤8.00) |
| Ceftazidime (Caz) | S * (≤1.00) | S * (≤1.00) | S * (≤1.00) | S * (≤1.00) |
| Cefuroxime (Crm) | R * (>8.00) | R * (>8.00) | R * (>8.00) | R * (>8.00) |
| Polymyxin | ||||
| Colistin (Cl) | R * (>4.00) | R * (>4.00) | R * (>4.00) | R * (>4.00) |
| Phosphonic | ||||
| Fosfomycin (Fos) | S * (≤8.00) | S * (≤8.00) | S * (≤8.00) | S * (≤8.00) |
| Nitrofuran | ||||
| Nitrofurantoin (Fd) | R * (>64.00) | R * (>64.00) | R * (>64.00) | R * (>64.00) |
| Cephalosporin/β-lactam | ||||
| Cefotaxime-clavulanate acid (Cft/CA) | S * (≤0.25) | S * (≤0.25) | S * (≤0.25) | S * (≤0.25) |
| Penicillin/β-lactam | ||||
| Piperacillin-Tazobactam (P/T) | S * (≤8.00) | S * (≤8.00) | S * (≤8.00) | S * (≤8.00) |
| Total | 25 | 25 | 25 | 25 |
| % Susceptible | 72.00 | 72.00 | 76.00 | 72.00 |
| % Intermediate | 0.00 | 0.00 | 0.00 | 0.00 |
| % Resistant | 28.00 | 28.00 | 24.00 | 28.00 |
| Isolate | Length (bp) | Bacterial Species | % Identity |
|---|---|---|---|
| 1-DH1 | 5,083,761 | NZ_JGVB01000001.1 Serratia marcescens strain ATCC 14041 contig001 | 99.30 |
| 3-DH2 | 5,083,758 | NZ_JGVB01000001.1 Serratia marcescens strain ATCC 14041 contig001 | 99.30 |
| 4-DH3 | 5,082,903 | NZ_JGVB01000001.1 Serratia marcescens strain ATCC 14041 contig001 | 99.30 |
| Database | CARD | NCBI | ResFinder | * AMR in CARD, NCBI or ResFinder | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AMR Gene/Isolate | 1 | 3 | 4 | 1 | 3 | 4 | 1 | 3 | 4 | |
| AAC(6′)-Ic | x | x | x | Aminoglycoside | ||||||
| aac(6′)-Ic_1 | x | x | x | Amikacin; tobramycin | ||||||
| aac(6′)-Ial | x | x | x | Aminoglycoside | ||||||
| H-NS | x | x | x | Cephalosporin, fluoroquinolone, macrolide, penicillin, tetracycline, cephamycin | ||||||
| tet(41) | x | x | x | x | x | x | x | x | x | Acycline, doxycycline, tetracycline |
| SRT-2 | x | x | x | Cephalosporin, cefotaxime | ||||||
| oqxB | x | x | x | Diaminopyrimidine, fluoroquinolone, glycylcycline, nitrofuran, tetracycline | ||||||
| oqxB_1 | x | x | x | Nalidixic acid; ciprofloxacin | ||||||
| oqxB25 | x | x | x | Phenicol, quinolone | ||||||
| mexI | x | x | x | Acridine_dye, fluoroquinolone, tetracycline | ||||||
| CRP | x | x | x | fluoroquinolone, macrolide, penicillin | ||||||
| blaSST-1 | x | x | x | Cephalosporin | ||||||
| blaSST-1_1 | x | x | x | N/A | ||||||
| Database | VFDB | ** VF in VFDB | ||||||||
| VF gene/Isolate | 1 | 3 | 4 | |||||||
| fliG | x | x | x | Flagellar motor switch protein G | ||||||
| fliM | x | x | x | Flagellar motor switch protein FliM | ||||||
| fliP | x | x | x | Flagellar biosynthetic protein FliP | ||||||
| flgH | x | x | x | Flagellar L-ring protein precursor FlgH | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreira, G.; Pinho, L.; Mesquita, J.R.; Silva, E. Serratia marcescens Isolates from Bovine Mastitic Milk: Antimicrobial Resistance and Virulence Features. Antibiotics 2025, 14, 892. https://doi.org/10.3390/antibiotics14090892
Moreira G, Pinho L, Mesquita JR, Silva E. Serratia marcescens Isolates from Bovine Mastitic Milk: Antimicrobial Resistance and Virulence Features. Antibiotics. 2025; 14(9):892. https://doi.org/10.3390/antibiotics14090892
Chicago/Turabian StyleMoreira, Guilherme, Luís Pinho, João R. Mesquita, and Eliane Silva. 2025. "Serratia marcescens Isolates from Bovine Mastitic Milk: Antimicrobial Resistance and Virulence Features" Antibiotics 14, no. 9: 892. https://doi.org/10.3390/antibiotics14090892
APA StyleMoreira, G., Pinho, L., Mesquita, J. R., & Silva, E. (2025). Serratia marcescens Isolates from Bovine Mastitic Milk: Antimicrobial Resistance and Virulence Features. Antibiotics, 14(9), 892. https://doi.org/10.3390/antibiotics14090892





