1,5-Diarylidene-4-Piperidones as Promising Antifungal Candidates Against Cryptococcus neoformans
Abstract
1. Introduction
2. Results
2.1. Synthesis of Piperidones
2.2. Antifungal Activities and Toxicity of Synthesized Piperidones
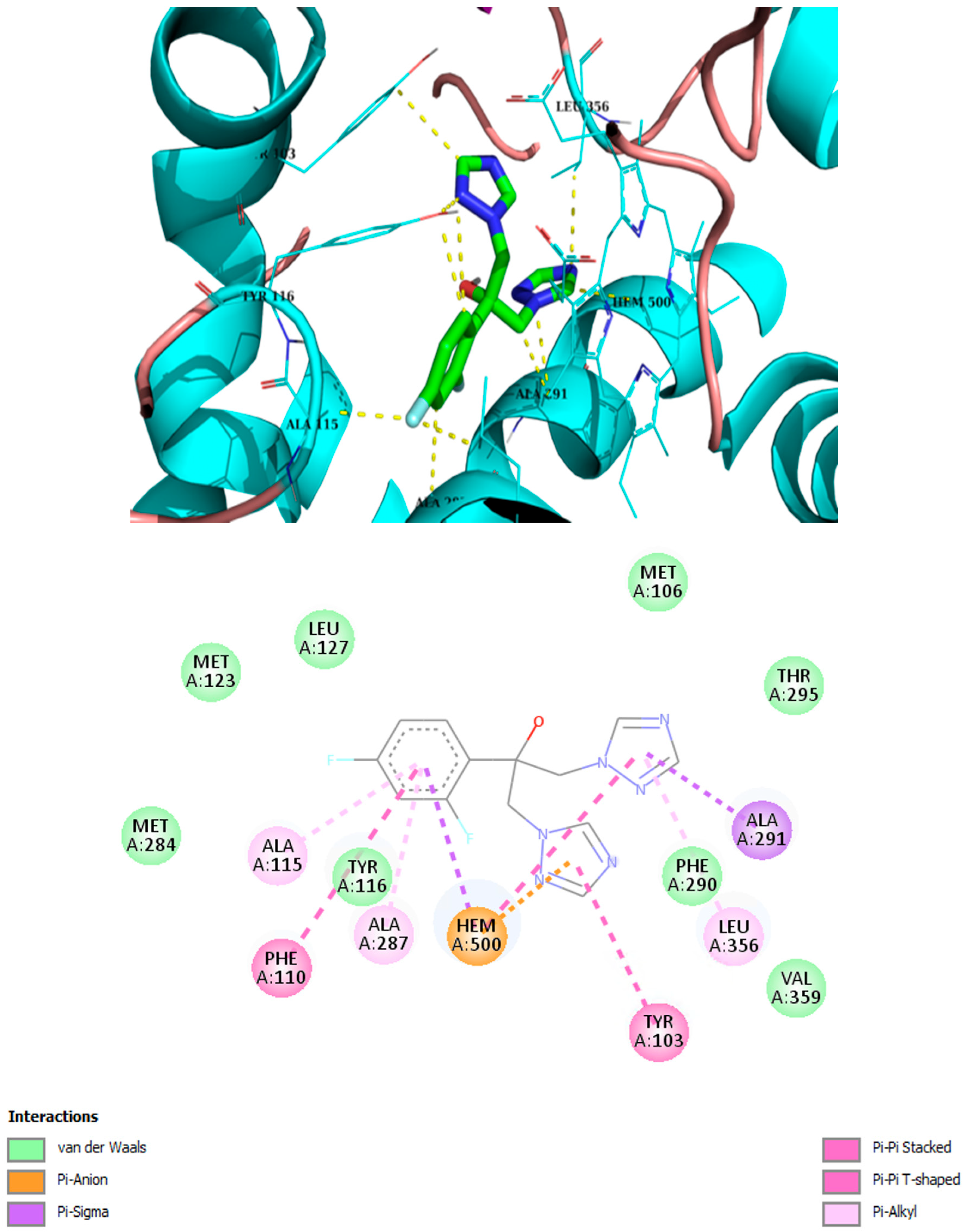
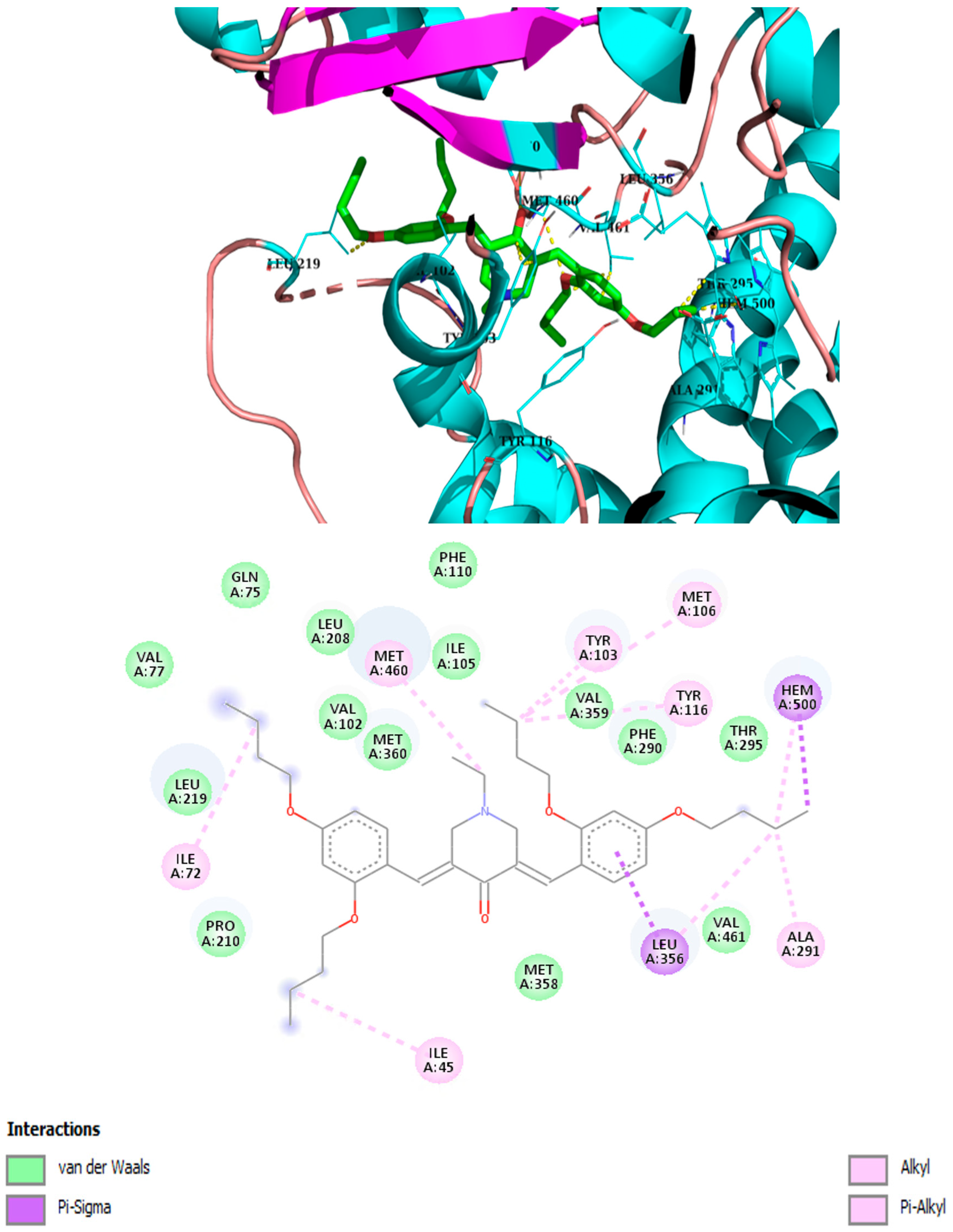
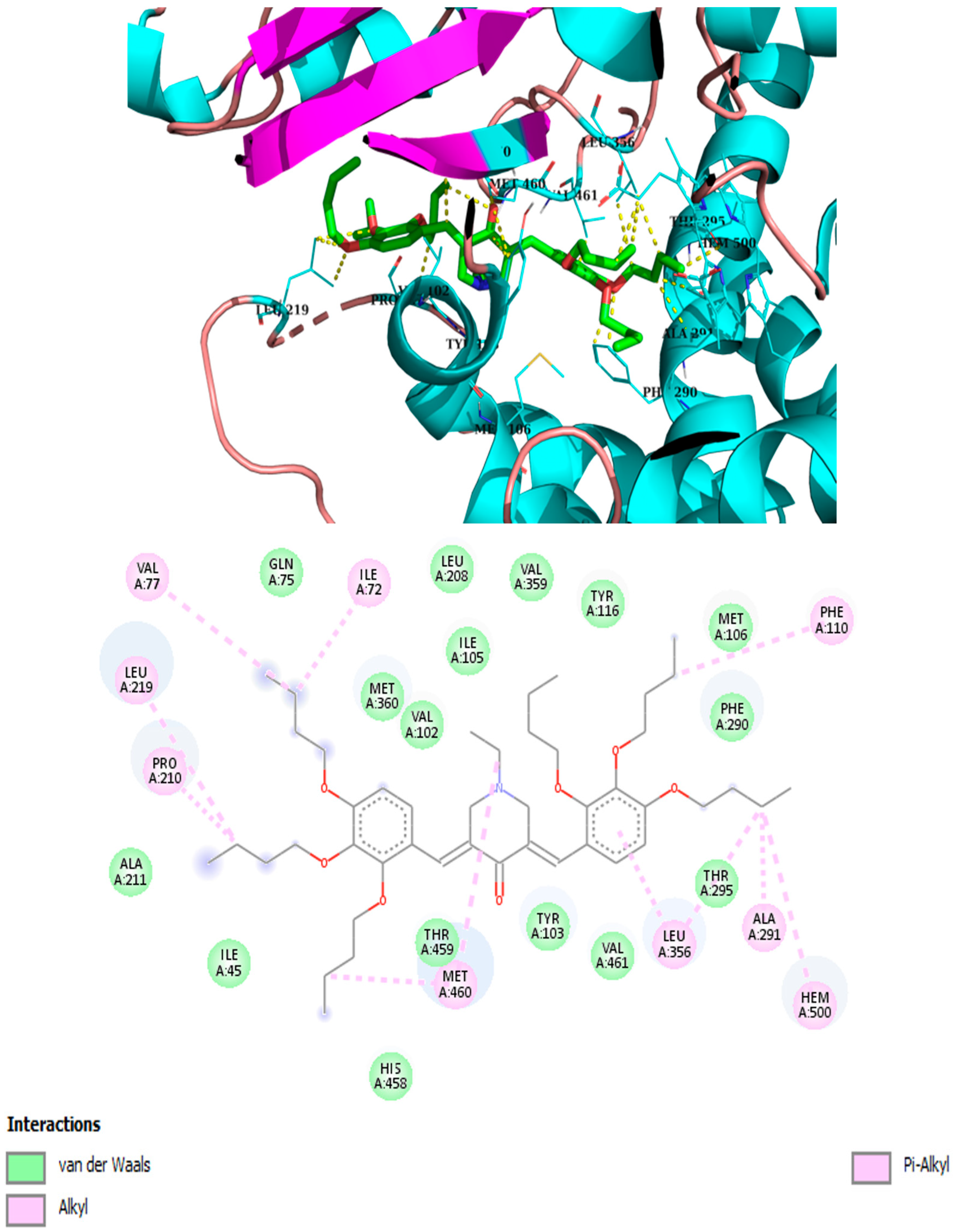
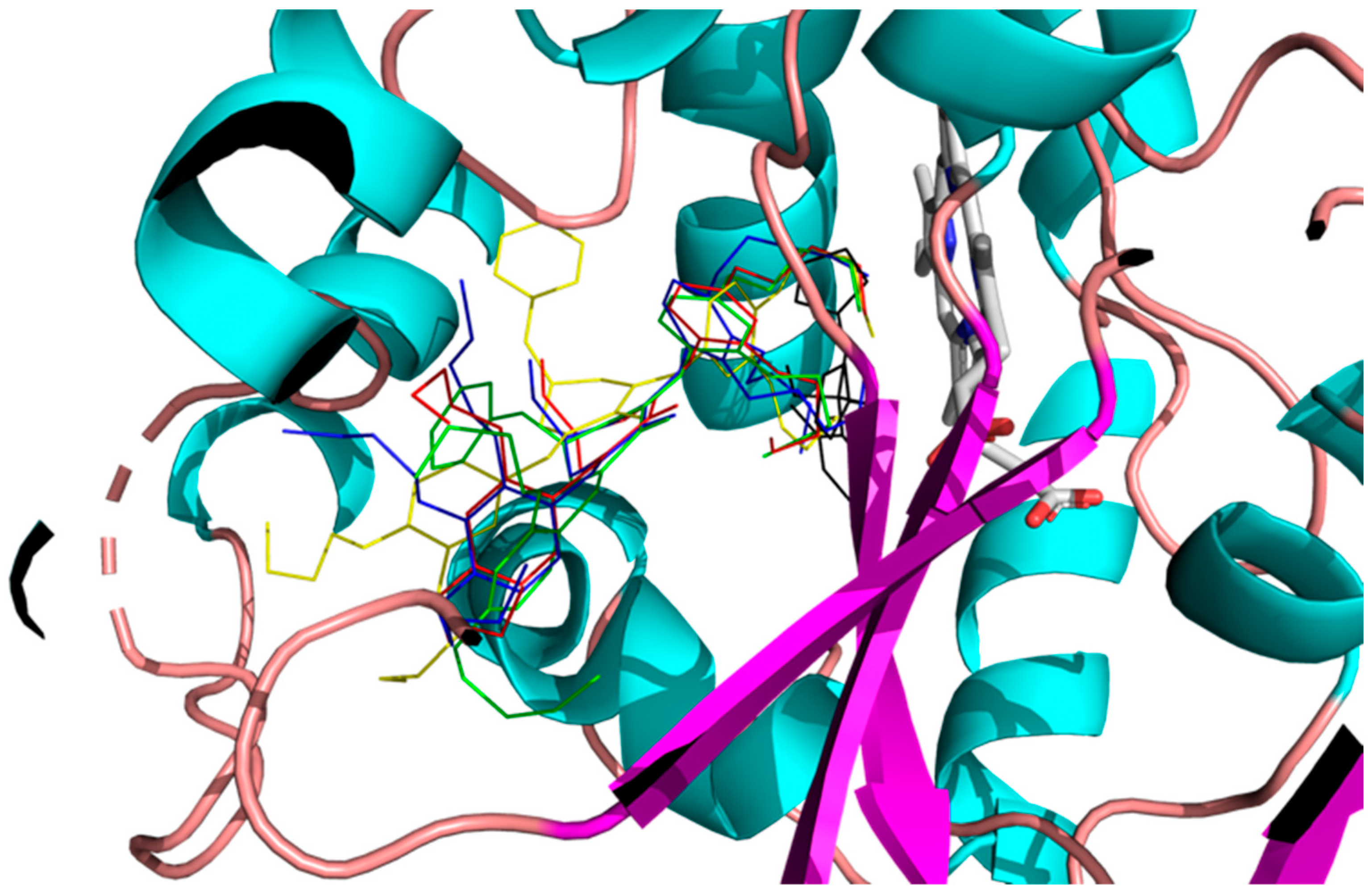
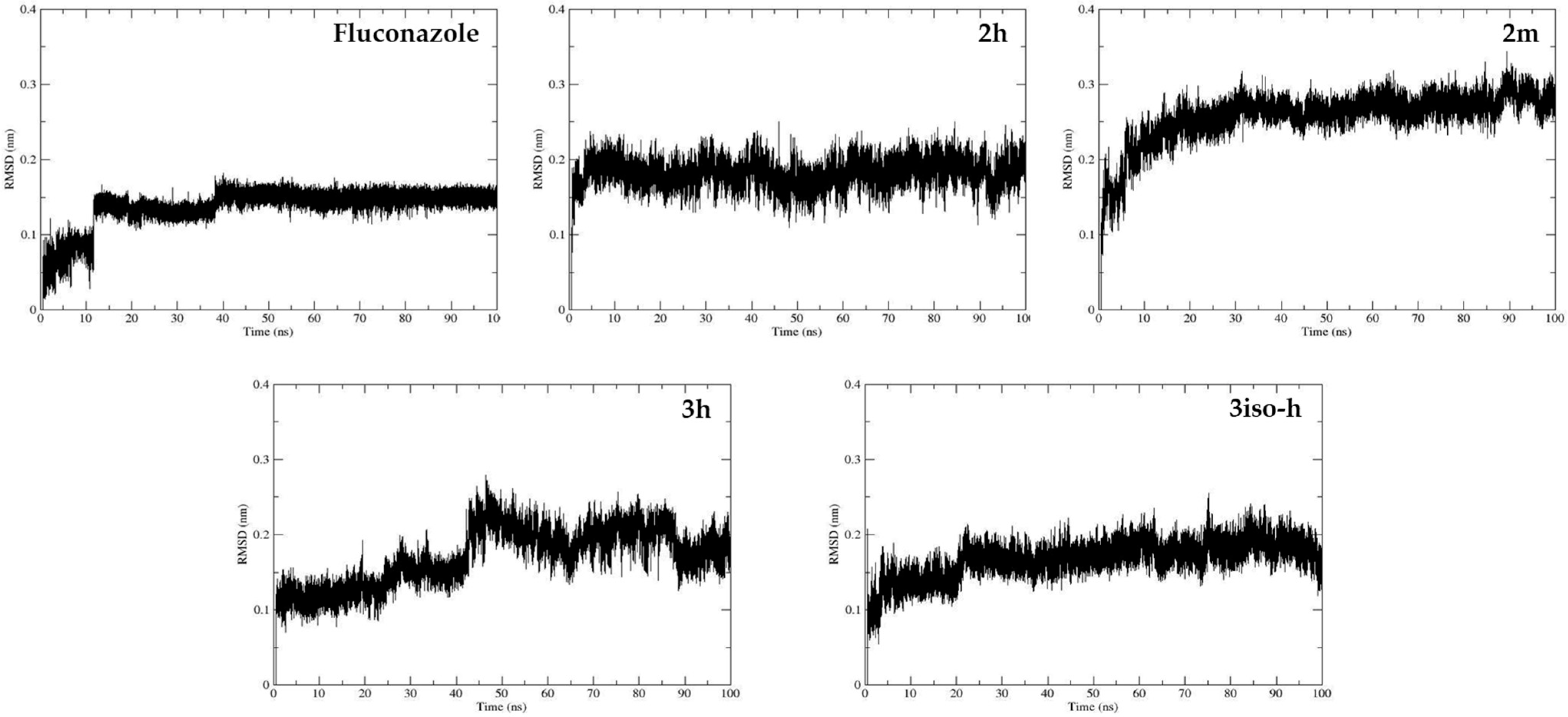
2.3. Molecular Modeling
3. Discussion
4. Materials and Methods
4.1. Chemistry
4.2. Antimicrobial Activity
4.3. Cytotoxicity Studies
4.4. Molecular Docking
4.5. Molecular Dynamics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perfect, J.R. Efficiently Killing a Sugar-Coated Yeast. N. Engl. J. Med. 2013, 368, 1354–1356. [Google Scholar] [CrossRef]
- Francis, V.I.; Liddle, C.; Camacho, E.; Kulkarni, M.; Junior, S.R.S.; Harvey, J.A.; Ballou, E.R.; Thomson, D.D.; Brown, G.D.; Hardwick, J.M.; et al. Cryptococcus neoformans Rapidly Invades the Murine Brain by Sequential Breaching of Airway and Endothelial Tissues Barriers, Followed by Engulfment by Microglia. mBio 2024, 15, e03078-23. [Google Scholar] [CrossRef]
- Poley, M.; Koubek, R.; Walsh, L.; McGillen, B. Cryptococcal Meningitis in an Apparent Immunocompetent Patient. J. Investig. Med. High Impact Case Rep. 2019, 7, 2324709619834578. [Google Scholar] [CrossRef]
- Goldman, D.L.; Khine, H.; Abadi, J.; Lindenberg, D.J.; Pirofski, L.; Niang, R.; Casadevall, A. Serologic Evidence for Cryptococcus neoformans Infection in Early Childhood. Pediatrics 2001, 107, e66. [Google Scholar] [CrossRef]
- Coelho, C.; Farrer, R.A. Pathogen and Host Genetics Underpinning Cryptococcal Disease. In Advances in Genetics; Elsevier: Amsterdam, The Netherlands, 2020; Volume 105, pp. 1–66. ISBN 978-0-12-821685-9. [Google Scholar]
- Alanio, A. Dormancy in Cryptococcus Neoformans: 60 Years of Accumulating Evidence. J. Clin. Investig. 2020, 130, 3353–3360. [Google Scholar] [CrossRef] [PubMed]
- Calegari-Alves, Y.P.; Costa, R.P.; Innocente-Alves, C.; Soares, G.D.N.; Lima, E.S.; Saciloto-de-Oliveira, L.R.; Alves, L.R.; Vainstein, M.H.; Beys-da-Silva, W.O.; Santi, L. A Review of Bioactive Plant Compounds against WHO Priority Fungal Pathogens. Microb. Pathog. 2025, 207, 107930. [Google Scholar] [CrossRef]
- Briner, S.L.; Doering, T.L. Cryptococcus. Curr. Biol. 2025, 35, R518–R522. [Google Scholar] [CrossRef] [PubMed]
- Casadevall, A.; Shoham, S. Cryptococcosis: Update on Therapeutics and New Targets. Curr. Opin. Infect. Dis. 2025. [Google Scholar] [CrossRef]
- Dao, A.; Kim, H.Y.; Garnham, K.; Kidd, S.; Sati, H.; Perfect, J.; Sorrell, T.C.; Harrison, T.; Rickerts, V.; Gigante, V.; et al. Cryptococcosis—A Systematic Review to Inform the World Health Organization Fungal Priority Pathogens List. Med. Mycol. 2024, 62, myae043. [Google Scholar] [CrossRef] [PubMed]
- Day, J.N.; Chau, T.T.H.; Wolbers, M.; Mai, P.P.; Dung, N.T.; Mai, N.H.; Phu, N.H.; Nghia, H.D.; Phong, N.D.; Thai, C.Q.; et al. Combination Antifungal Therapy for Cryptococcal Meningitis. N. Engl. J. Med. 2013, 368, 1291–1302. [Google Scholar] [CrossRef]
- Hartland, K.; Pu, J.; Palmer, M.; Dandapani, S.; Moquist, P.N.; Munoz, B.; DiDone, L.; Schreiber, S.L.; Krysan, D.J. High-Throughput Screen in Cryptococcus neoformans Identifies a Novel Molecular Scaffold That Inhibits Cell Wall Integrity Pathway Signaling. ACS Infect. Dis. 2016, 2, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Beattie, S.R.; Schnicker, N.J.; Murante, T.; Kettimuthu, K.; Williams, N.S.; Gakhar, L.; Krysan, D.J. Benzothiourea Derivatives Target the Secretory Pathway of the Human Fungal Pathogen Cryptococcus neoformans. ACS Infect. Dis. 2020, 6, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Bongomin, F.; Oladele, R.O.; Gago, S.; Moore, C.B.; Richardson, M.D. A Systematic Review of Fluconazole Resistance in Clinical Isolates of Cryptococcus Species. Mycoses 2018, 61, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Moreira, I.D.M.B.; Cortez, A.C.A.; De Souza, É.S.; Pinheiro, S.B.; De Souza Oliveira, J.G.; Sadahiro, A.; Cruz, K.S.; Matsuura, A.B.J.; Melhem, M.D.S.C.; Frickmann, H.; et al. Investigation of Fluconazole Heteroresistance in Clinical and Environmental Isolates of Cryptococcus neoformans Complex and Cryptococcus gattii Complex in the State of Amazonas, Brazil. Med. Mycol. 2022, 60, myac005. [Google Scholar] [CrossRef]
- Pati, H.N.; Das, U.; Das, S.; Bandy, B.; De Clercq, E.; Balzarini, J.; Kawase, M.; Sakagami, H.; Quail, J.W.; Stables, J.P.; et al. The Cytotoxic Properties and Preferential Toxicity to Tumour Cells Displayed by Some 2,4-Bis(Benzylidene)-8-Methyl-8-Azabicyclo[3.2.1] Octan-3-Ones and 3,5-Bis(Benzylidene)-1-Methyl-4-Piperidones. Eur. J. Med. Chem. 2009, 44, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K.; Aggarwal, B.B. Curcumin, a Component of Golden Spice: From Bedside to Bench and Back. Biotechnol. Adv. 2014, 32, 1053–1064. [Google Scholar] [CrossRef]
- Bazzaro, M.; Linder, S. Dienone Compounds: Targets and Pharmacological Responses. J. Med. Chem. 2020, 63, 15075–15093. [Google Scholar] [CrossRef]
- Noureddin, S.A.; El-Shishtawy, R.M.; Al-Footy, K.O. Curcumin Analogues and Their Hybrid Molecules as Multifunctional Drugs. Eur. J. Med. Chem. 2019, 182, 111631. [Google Scholar] [CrossRef] [PubMed]
- Dao, T.T.; Sehgal, P.; Tung, T.T.; Møller, J.V.; Nielsen, J.; Palmgren, M.; Christensen, S.B.; Fuglsang, A.T. Demethoxycurcumin Is a Potent Inhibitor of P-Type ATPases from Diverse Kingdoms of Life. PLoS ONE 2016, 11, e0163260. [Google Scholar] [CrossRef]
- Lawson, S.; Arumugam, N.; Almansour, A.I.; Suresh Kumar, R.; Thangamani, S. Dispiropyrrolidine Tethered Piperidone Heterocyclic Hybrids with Broad-Spectrum Antifungal Activity against Candida Albicans and Cryptococcus Neoformans. Bioorganic Chem. 2020, 100, 103865. [Google Scholar] [CrossRef]
- Nagargoje, A.A.; Akolkar, S.V.; Subhedar, D.D.; Shaikh, M.H.; Sangshetti, J.N.; Khedkar, V.M.; Shingate, B.B. Propargylated Monocarbonyl Curcumin Analogues: Synthesis, Bioevaluation and Molecular Docking Study. Med. Chem. Res. 2020, 29, 1902–1913. [Google Scholar] [CrossRef]
- Pigot, C.; Noirbent, G.; Bui, T.-T.; Péralta, S.; Gigmes, D.; Nechab, M.; Dumur, F. Push-Pull Chromophores Based on the Naphthalene Scaffold: Potential Candidates for Optoelectronic Applications. Materials 2019, 12, 1342. [Google Scholar] [CrossRef]
- Xu, Y.; Noirbent, G.; Brunel, D.; Ding, Z.; Gigmes, D.; Graff, B.; Xiao, P.; Dumur, F.; Lalevée, J. Allyloxy Ketones as Efficient Photoinitiators with High Migration Stability in Free Radical Polymerization and 3D Printing. Dyes Pigment. 2021, 185, 108900. [Google Scholar] [CrossRef]
- Nagargoje, A.A.; Akolkar, S.V.; Siddiqui, M.M.; Subhedar, D.D.; Sangshetti, J.N.; Khedkar, V.M.; Shingate, B.B. Quinoline Based Monocarbonyl Curcumin Analogs as Potential Antifungal and Antioxidant Agents: Synthesis, Bioevaluation and Molecular Docking Study. Chem. Biodivers. 2020, 17, e1900624. [Google Scholar] [CrossRef]
- Fioravanti, R.; Biava, M.; Porretta, G.; Landolfi, C.; Simonetti, N.; Villa, A.; Conte, E.; Porta-Puglia, A. Research on Antibacterial and Antifungal Agents. XI. Synthesis and Antimicrobial Activity of N-Heteroaryl Benzylamines and Their Schiff Bases. Eur. J. Med. Chem. 1995, 30, 123–132. [Google Scholar] [CrossRef]
- Hammoudi Halat, D.; Younes, S.; Mourad, N.; Rahal, M. Allylamines, Benzylamines, and Fungal Cell Permeability: A Review of Mechanistic Effects and Usefulness against Fungal Pathogens. Membranes 2022, 12, 1171. [Google Scholar] [CrossRef]
- Perfect, J.R.; Dismukes, W.E.; Dromer, F.; Goldman, D.L.; Graybill, J.R.; Hamill, R.J.; Harrison, T.S.; Larsen, R.A.; Lortholary, O.; Nguyen, M.-H.; et al. Clinical Practice Guidelines for the Management of Cryptococcal Disease: 2010 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2010, 50, 291–322. [Google Scholar] [CrossRef] [PubMed]
- Loyse, A.; Burry, J.; Cohn, J.; Ford, N.; Chiller, T.; Ribeiro, I.; Koulla-Shiro, S.; Mghamba, J.; Ramadhani, A.; Nyirenda, R.; et al. Leave No One behind: Response to New Evidence and Guidelines for the Management of Cryptococcal Meningitis in Low-Income and Middle-Income Countries. Lancet Infect. Dis. 2019, 19, e143–e147. [Google Scholar] [CrossRef]
- Denning, D.W. Global Incidence and Mortality of Severe Fungal Disease. Lancet Infect. Dis. 2024, 24, e428–e438. [Google Scholar] [CrossRef] [PubMed]
- Long, B.; Lacy, A.J.; Koyfman, A.; Liang, S.Y. Candida auris: A Focused Review for Emergency Clinicians. Am. J. Emerg. Med. 2024, 84, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Kinjo, Y.; Koshikawa, T.; Miyazaki, Y. Basic Research on Candida Species: Disease Mechanism, Virulence, and Relationship with Environmental Factors. Med. Mycol. J. 2024, 65, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Dawoud, A.M.; Saied, S.A.; Torayah, M.M.; Ramadan, A.E.; Elaskary, S.A. Antifungal Susceptibility and Virulence Determinants Profile of Candida Species Isolated from Patients with Candidemia. Sci. Rep. 2024, 14, 11597. [Google Scholar] [CrossRef]
- Beardsley, J.; Kim, H.Y.; Dao, A.; Kidd, S.; Alastruey-Izquierdo, A.; Sorrell, T.C.; Tacconelli, E.; Chakrabarti, A.; Harrison, T.S.; Bongomin, F.; et al. Candida glabrata (Nakaseomyces glabrata): A Systematic Review of Clinical and Microbiological Data from 2011 to 2021 to Inform the World Health Organization Fungal Priority Pathogens List. Med. Mycol. 2024, 62, myae041. [Google Scholar] [CrossRef]
- Keighley, C.; Kim, H.Y.; Kidd, S.; Chen, S.C.-A.; Alastruey, A.; Dao, A.; Bongomin, F.; Chiller, T.; Wahyuningsih, R.; Forastiero, A.; et al. Candida tropicalis—A Systematic Review to Inform the World Health Organization of a Fungal Priority Pathogens List. Med. Mycol. 2024, 62, myae040. [Google Scholar] [CrossRef]
- Dhingra, S.; Cramer, R.A. Regulation of Sterol Biosynthesis in the Human Fungal Pathogen Aspergillus fumigatus: Opportunities for Therapeutic Development. Front. Microbiol. 2017, 8, 92. [Google Scholar] [CrossRef]
- Liu, S.; Giacoletto, N.; Schmitt, M.; Nechab, M.; Graff, B.; Morlet-Savary, F.; Xiao, P.; Dumur, F.; Lalevée, J. Effect of Decarboxylation on the Photoinitiation Behavior of Nitrocarbazole-Based Oxime Esters. Macromolecules 2022, 55, 2475–2485. [Google Scholar] [CrossRef]
- Deligeorgakis, C.; Magro, C.; Skendi, A.; Gebrehiwot, H.H.; Valdramidis, V.; Papageorgiou, M. Fungal and Toxin Contaminants in Cereal Grains and Flours: Systematic Review and Meta-Analysis. Foods 2023, 12, 4328. [Google Scholar] [CrossRef]
- Maebashi, K.; Niimi, M.; Kudoh, M.; Fischer, F.J.; Makimura, K.; Niimi, K.; Piper, R.J.; Uchida, K.; Arisawa, M.; Cannon, R.D.; et al. Mechanisms of Fluconazole Resistance in Candida Albicans Isolates from Japanese AIDS Patients. J. Antimicrob. Chemother. 2001, 47, 527–536. [Google Scholar] [CrossRef]
- Janowski, M.; Demchuk, O.M.; Wujec, M. Fluconazole Analogs and Derivatives: An Overview of Synthesis, Chemical Transformations, and Biological Activity. Molecules 2024, 29, 2855. [Google Scholar] [CrossRef] [PubMed]
- Wiederhold, N. Antifungal Resistance: Current Trends and Future Strategies to Combat. Infect. Drug Resist. 2017, 10, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Olleik, H.; Nicoletti, C.; Lafond, M.; Courvoisier-Dezord, E.; Xue, P.; Hijazi, A.; Baydoun, E.; Perrier, J.; Maresca, M. Comparative Structure–Activity Analysis of the Antimicrobial Activity, Cytotoxicity, and Mechanism of Action of the Fungal Cyclohexadepsipeptides Enniatins and Beauvericin. Toxins 2019, 11, 514. [Google Scholar] [CrossRef]
- Notteghem, J.L. Distribution of the Mating Type Alleles in Magnaporthe grisea Populations Pathogenic on Rice. Phytopathology 1992, 82, 421. [Google Scholar] [CrossRef]
- Benkhaled, B.T.; Hadiouch, S.; Olleik, H.; Perrier, J.; Ysacco, C.; Guillaneuf, Y.; Gigmes, D.; Maresca, M.; Lefay, C. Elaboration of Antimicrobial Polymeric Materials by Dispersion of Well-Defined Amphiphilic Methacrylic SG1-Based Copolymers. Polym. Chem. 2018, 9, 3127–3141. [Google Scholar] [CrossRef]
- Olleik, H.; Yacoub, T.; Hoffer, L.; Gnansounou, S.M.; Benhaiem-Henry, K.; Nicoletti, C.; Mekhalfi, M.; Pique, V.; Perrier, J.; Hijazi, A.; et al. Synthesis and Evaluation of the Antibacterial Activities of 13-Substituted Berberine Derivatives. Antibiotics 2020, 9, 381. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Pall, S.; Smith, J.C.; Hess, B.; Lindahk, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. Software X 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general amber force field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Madura, J.D. Investigating novel thiazolyl-indazole derivatives as scaffolds for SARS-CoV-2 MPro inhibitors. J. Am. Chem. Soc. 1983, 105, 1407–1473. [Google Scholar] [CrossRef]
- Schmitt, F.; Subramaniam, D.; Anant, S.; Padhye, S.; Begemann, G.; Schobert, R.; Biersack, B. Halogenated Bis(Methoxybenzylidene)-4-piperidone Curcuminoids with Improved Anticancer Activity. ChemMedChem 2018, 13, 1115–1123. [Google Scholar] [CrossRef]
- Andreani, A.; Cavalli, A.; Granaiola, M.; Guardigli, M.; Leoni, A.; Locatelli, A.; Morigi, R.; Rambaldi, M.; Recanatini, M.; Roda, A. Synthesis and Screening for Antiacetylcholinesterase Activity of (1-Benzyl-4-Oxopiperidin-3-Ylidene)Methylindoles and -Pyrroles Related to Donepezil. J. Med. Chem. 2001, 44, 4011–4014. [Google Scholar] [CrossRef]
- Bayomi, S.M.; El-Kashef, H.A.; El-Ashmawy, M.B.; Nasr, M.N.A.; El-Sherbeny, M.A.; Abdel-Aziz, N.I.; El-Sayed, M.A.-A.; Suddek, G.M.; El-Messery, S.M.; Ghaly, M.A. Synthesis and Biological Evaluation of New Curcumin Analogues as Antioxidant and Antitumor Agents: Molecular Modeling Study. Eur. J. Med. Chem. 2015, 101, 584–594. [Google Scholar] [CrossRef]
- Girgis, A.S.; Panda, S.S.; Farag, I.S.A.; El-Shabiny, A.M.; Moustafa, A.M.; Ismail, N.S.M.; Pillai, G.G.; Panda, C.S.; Hall, C.D.; Katritzky, A.R. Synthesis, and QSAR Analysis of Anti-Oncological Active Spiro-Alkaloids. Org. Biomol. Chem. 2015, 13, 1741–1753. [Google Scholar] [CrossRef]
- Parlar, S. Synthesis and Cholinesterase Inhibitory Activity Studies of Some Piperidinone Derivatives. Org. Commun. 2019, 12, 202–209. [Google Scholar] [CrossRef]
- Hanachi, R.; Said, R.B.; Rahali, S.; Tangour, B.; Abdelwahab, S.I.; Farasani, A.; Taha, M.M.E.; Bidwai, A.; Koko, W.S.; Khan, T.A.; et al. P-Trifluoromethyl- and p-Pentafluorothio-Substituted Curcuminoids of the 2,6-Di[(E)-Benzylidene)]Cycloalkanone Type: Syntheses and Activities against Leishmania Major and Toxoplasma Gondii Parasites. Bioorg. Chem. 2021, 114, 105099. [Google Scholar] [CrossRef]
- Insuasty, B.; Becerra, D.; Quiroga, J.; Abonia, R.; Nogueras, M.; Cobo, J. Microwave-Assisted Synthesis of Pyrimido[4,5-b][1,6]Naphthyridin-4(3H)-Ones with Potential Antitumor Activity. Eur. J. Med. Chem. 2013, 60, 1–9. [Google Scholar] [CrossRef]
- Schmitt, F.; Gold, M.; Begemann, G.; Andronache, I.; Biersack, B.; Schobert, R. Fluoro and Pentafluorothio Analogs of the Antitumoral Curcuminoid EF24 with Superior Antiangiogenic and Vascular-Disruptive Effects. Bioorg. Med. Chem. 2017, 25, 4894–4903. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Ding, N.; Zhao, S.; Li, D.; Van Doren, J.; Qian, Y.; Wei, X.; Zheng, X. Synthesis and Evaluation of Curcumin-Related Compounds Containing Inden-2-One for Their Effects on Human Cancer Cells. Biol. Pharm. Bull. 2014, 37, 1977–1981. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, A.K.; Mathew, J.; Achalkumar, A.S.; Mathews, M. Synthesis and Liquid Crystalline Properties of Low Molecular Weight Bis-Chalcone Compounds. Curr. Org. Synth. 2022, 19, 463–475. [Google Scholar] [CrossRef] [PubMed]


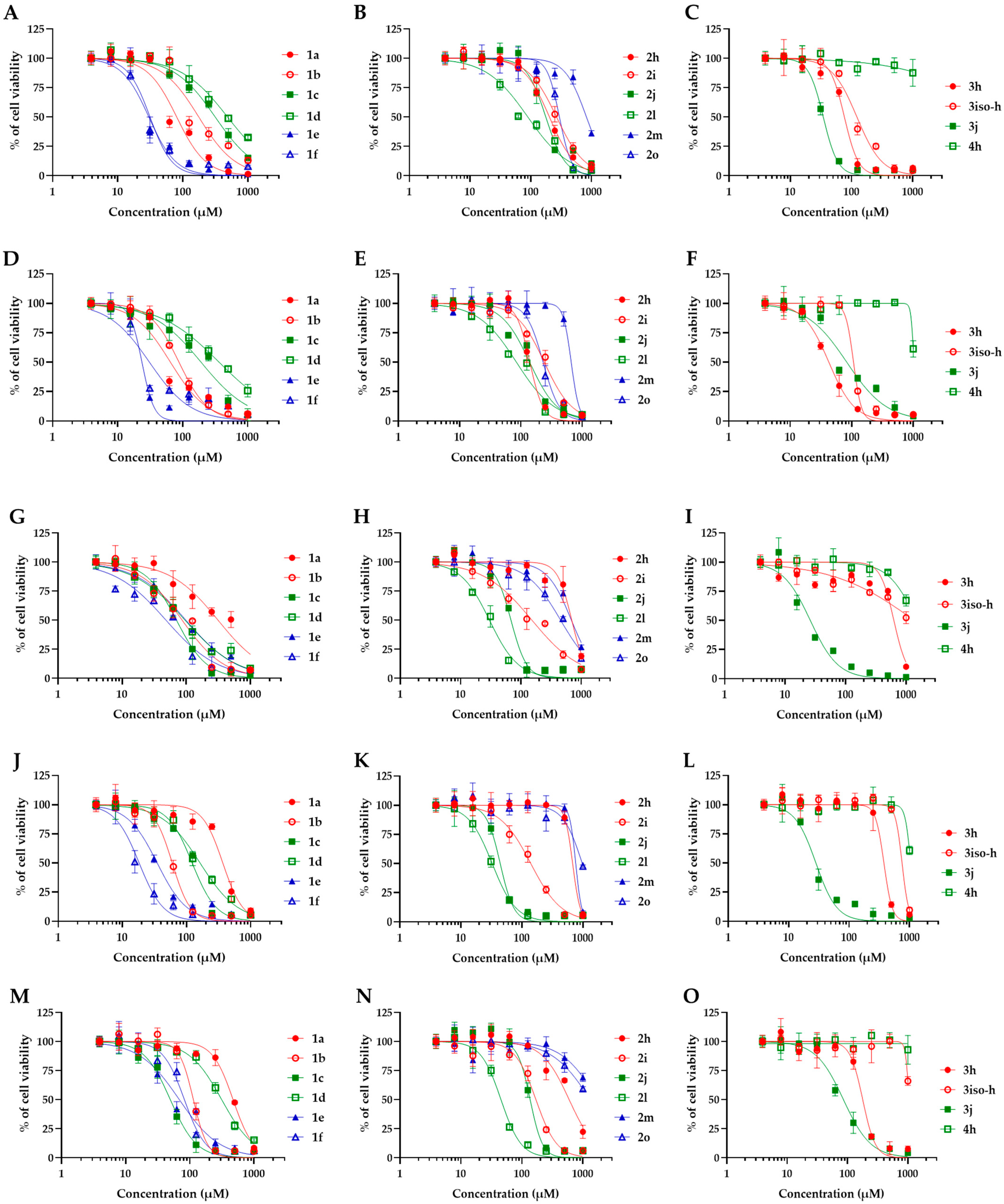




| Compound | MIC (μM) | CC50 (μM) | SI | ||||
|---|---|---|---|---|---|---|---|
| A498 | BEAS-2B | Caco-2 | HaCaT | HepG2 | Min–Max | ||
| 1a | 125 | 80.4 | 64.9 | 313.7 | 368.6 | 471.9 | 0.5–3.7 |
| 1b | 250 | 175.6 | 87.9 | 86.2 | 60.0 | 113.5 | 0.2–0.7 |
| 1c | 250 | 313.9 | 191.8 | 70.3 | 122.7 | 49.5 | 0.1–1.2 |
| 1d | 250 | 486.2 | 363.5 | 95.4 | 162.9 | 313.6 | 0.3–1.9 |
| 1e | 250 | 31.3 | 23.7 | 98.6 | 34.9 | 68.5 | 0.09–0.4 |
| 1f | 250 | 30.0 | 30.9 | 47.0 | 18.3 | 82.1 | 0.07–0.3 |
| 2a | >250 | NT | NT | NT | NT | NT | NT |
| 2g | >250 | NT | NT | NT | NT | NT | NT |
| 2h | 31.2 | 203.7 | 140.2 | 696.6 | 680.3 | 595.6 | 4.5–22.3 |
| 2i | 250 | 266.6 | 241.2 | 152.0 | 140.0 | 169.6 | 0.5–1.0 |
| 2j | 250 | 177.3 | 139.0 | 68.3 | 44.2 | 136.4 | 0.1–0.7 |
| 2k | >250 | NT | NT | NT | NT | NT | ND |
| 2l | 250 | 89.5 | 84.5 | 30.0 | 32.6 | 45.3 | 0.1–0.3 |
| 2m | 62.5 | 829.4 | 663.7 | 686.9 | 764.3 | >1000 | 10.6–>16 |
| 2n | >250 | NT | NT | NT | NT | NT | NT |
| 2o | 125 | 297.4 | 230.5 | 454.7 | 979.3 | >1000 | 1.8->8 |
| 3a | >250 | NT | NT | NT | NT | NT | NT |
| 3h | 7.8 | 76.4 | 42.4 | 628.6 | 377.3 | 178.3 | 5.4–80.5 |
| 3iso-h | 62.5 | 122.4 | 106.9 | >1000 | 753.8 | >1000 | 1.7–>16 |
| 3j | 62.5 | 34.5 | 82.9 | 26.1 | 28.2 | 85.6 | 0.4–1.3 |
| 4h | 250 | >1000 | >1000 | >1000 | >1000 | >1000 | >4 |
| 5h | >250 | NT | NT | NT | NT | NT | NT |
| 6h | >250 | NT | NT | NT | NT | NT | NT |
| 7h | >250 | NT | NT | NT | NT | NT | NT |
| 8h | >250 | NT | NT | NT | NT | NT | NT |
| 9h | >250 | NT | NT | NT | NT | NT | NT |
| 10h | >250 | NT | NT | NT | NT | NT | NT |
| Fluconazole | 25 | 993.6 | 942.5 | 977.0 | 841.2 | >1000 | 33.6–>40 |
| Compound | C. albicans | C. auris | C. glabrata | C. tropicalis | C. neoformans |
|---|---|---|---|---|---|
| 2h | 250 | 31.2 | 31.2 | 250 | 31.2 |
| 2m | 125 | 31.2 | 15.6 | 250 | 62.5 |
| 3h | 62.5 | 7.8 | 7.8 | 125 | 7.8 |
| 3iso-h | 250 | 31.2 | 62.5 | 125 | 62.5 |
| Fluconazole | 6.25 | 500 | 250 | 1000 | 25 |
| Compound | A. flavus | A. fumigatus | C. graminicola | F. graminearum | M. oryzae | M. bolleyi | P. verrucosum | T. rubrum |
|---|---|---|---|---|---|---|---|---|
| 2h | >250 | 125 | >250 | >250 | 15.6 | 62.5 | 125 | 62.5 |
| 2m | >250 | 250 | >250 | >250 | 62.5 | >250 | >250 | 31.2 |
| 3h | 31.2 | 31.2 | 250 | 125 | 7.8 | 31.2 | 62.5 | 7.8 |
| 3iso-h | >250 | 125 | 125 | >250 | 15.6 | 62.5 | 125 | 125 |
| Fluconazole | >1000 | >1000 | 50 | >1000 | 12.5 | >1000 | >1000 | >1000 |
| Compound | Binding Energy kcal/mol | Hydrophobic Interactions with Amino Acids | Aromatic Interactions |
|---|---|---|---|
| Fluconazole | −8.1 | Phe110, Tyr116, Leu127, Ala287, Ala291, Leu356 | T-Shaped (Tyr103), Hem500 |
| 2h | −7.8 | Tyr103, Met106, Tyr116, Leu219, Phe290, Ala291, Leu356, Met360, Met460 | Hem500 T-Shaped (Tyr103), |
| 2m | −7.2 | Ile72, Tyr103, Phe110, Pro210, Leu219, Phe290, Thr295, Leu356, Met360, Met460, Val461 | Hem500 |
| 3h | −8.2 | Val102, Tyr103, Ile105, Pro210, Phe290, Ala291, Leu356, Met360, Met460 | Hem500 T-Shaped (Tyr103), |
| 3iso-h | −8.8 | Ile105, Met106, Leu208, Pro210, Leu219, Phe290, Ala291, Leu356, Thr459, Val461 | Hem500 T-Shaped (His294, Phe290), |
| Compound | Fluconazole | 2h | 2m | 3h | 3iso-h |
|---|---|---|---|---|---|
| RMSD mean value (nm) | 0.14 ± 0.03 | 0.18 ± 0.02 | 0.26 ± 0.03 | 0.17 ± 0.04 | 0.14 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Courvoisier-Dezord, E.; Ragusa, H.; Grandé, A.; Denudt, L.; Charmasson, Y.; Dumur, F.; Siri, D.; Maresca, M.; Nechab, M. 1,5-Diarylidene-4-Piperidones as Promising Antifungal Candidates Against Cryptococcus neoformans. Antibiotics 2025, 14, 883. https://doi.org/10.3390/antibiotics14090883
Courvoisier-Dezord E, Ragusa H, Grandé A, Denudt L, Charmasson Y, Dumur F, Siri D, Maresca M, Nechab M. 1,5-Diarylidene-4-Piperidones as Promising Antifungal Candidates Against Cryptococcus neoformans. Antibiotics. 2025; 14(9):883. https://doi.org/10.3390/antibiotics14090883
Chicago/Turabian StyleCourvoisier-Dezord, Elise, Hugo Ragusa, Axelle Grandé, Louise Denudt, Yolande Charmasson, Frédéric Dumur, Didier Siri, Marc Maresca, and Malek Nechab. 2025. "1,5-Diarylidene-4-Piperidones as Promising Antifungal Candidates Against Cryptococcus neoformans" Antibiotics 14, no. 9: 883. https://doi.org/10.3390/antibiotics14090883
APA StyleCourvoisier-Dezord, E., Ragusa, H., Grandé, A., Denudt, L., Charmasson, Y., Dumur, F., Siri, D., Maresca, M., & Nechab, M. (2025). 1,5-Diarylidene-4-Piperidones as Promising Antifungal Candidates Against Cryptococcus neoformans. Antibiotics, 14(9), 883. https://doi.org/10.3390/antibiotics14090883








