Abstract
Aquaculture is a key food production sector responsible for meeting the nutritional needs of a rapidly growing global population. However, the emergence of disease outbreaks has become a major challenge for the aquaculture industry, resulting in significant economic losses. The use of costly and toxic antibiotics for treatment has a negative impact on the aquatic environment. Consequently, there has been a growing interest in probiotics as a non-antibiotic approach to manage disease outbreaks and improve fish performance. The use of the yeast Saccharomyces cerevisiae (SC) has shown remarkable benefits in aquaculture. In February 2025, a systematic search was conducted based on the Web of Science (WoS) database for the period 2015–2025 to identify relevant studies investigating the beneficial effects of SC in aquaculture. After searching on WoS, 466 documents were found and analyzed using R-bibliometric package for comprehensive analysis to identify research gap, trends, and distribution of global literature that focuses on SC in aquaculture. The most relevant and recent articles were reviewed, summarized and discussed. The yeast SC have shown a wide range of benefits, including improved growth performance, feed efficiency, enhanced diversity of the gut microbiome and immune response. The implementation of SC is becoming a recent trend and its efficacy in aquatic environments has been thoroughly investigated. This review aims to provide a valuable insight into SC as one of the most important aquaculture probiotics. It also emphasizes the need for further research to fully understand its benefits and the way it works.
1. Introduction
Aquaculture is one of the fast-growing animal food industries, supplying approximately 94 million tons of seafood and providing 15% of the world’s animal protein [1]. It plays a significant role in the global food supply [2,3]. Since 2001, the global aquaculture industry has shown a consistent annual growth rate of 5.8%, reflecting an increased demand for animal protein in rapidly growing economies. The aquaculture sector is largely driven by Asia, which accounts for 90% of global production. In 2016, China alone contributed more than 61% [4].
The occurrence of pathogens such as Vibrio anguillarum, V. harveyi, Aeromonas hydrophila, A. salmonicida, Flavobacterium psychrophilum, Yersinia ruckeri, Pseudomonas fluorescens, and Citrobacter freundii has been noted and could negatively impact the production of fish and other aquaculture species [5]. These pathogens and poor environmental factors are harmful to productivity and result in serious financial loss for aquaculture farmers. Fish practices, such as overfeeding, overcrowding, and water contamination, also contribute to the presence and spread of pathogens in aquatic environments [6,7,8].
Aquaculture frequently uses antibiotics for two primary reasons: to prevent and treat diseases, and to enhance fish performance [9,10]. However, the extensive use of antibiotics in fish farming has created conditions that favor the growth of drug-resistant bacterial strains, which can spread rapidly [11]. These resistant bacteria can negatively affect aquaculture production, fish consumers, and the surrounding environment [12,13,14]. Alternatively, probiotics have been utilized as an eco-friendly approach to enhance aquaculture sustainability [15,16,17].
The term “probiotic” is derived from the Greek words “pro” and “bios”, which together mean “for life” [18]. However, the initial definition of a probiotic was put forth by Parker [19], who defined a probiotic microorganism as one that contributes to intestinal microbial balance. According to the World Health Organization (WHO) and the Food and Agriculture Organization (FAO), probiotics are “live microorganisms which, when administered in adequate amounts, confer a health benefit to the host” [20]. A large number of studies have shown that probiotics are effective for a variety of fish and aquatic animals, such as African catfish (Clarias gariepinus), European sea bass (Dicentrarchus labrax), Nile tilapia (Oreochromis niloticus), Rainbow trout (Oncorhynchus mykiss), rohu (Labeo rohita), Indian Major Carp (Labeo rohita), snook (Centropomus undecimalis), common carp (Cyprinus carpio), and red seabream (Pagrus major) [21,22,23,24,25]. Among the probiotics, Saccharomyces cerevisiae is widely recognized as an important player in various domains of animal health, nutrition, and biotechnology.
The protein-rich probiotic Saccharomyces cerevisiae [26], due to its cellular components (e.g., β-glucan, glucooligosaccharides, mannooligosaccharides, and enzymes), has emerged as the most commonly used yeast in the aquaculture industry with multifaceted benefits for improving the health and productivity of various aquaculture species [27,28]. The probiotic potential of S. cerevisiae is supported by its resistance to various environmental stresses. Studies show that S. cerevisiae can survive in a variety of temperatures, making it a good choice for aquaculture, where temperatures can fluctuate [29]. Additionally, its tolerance of acidity and production of antimicrobial compounds provide an extra layer of protection against pathogens in aquatic environments. The secretion of antimicrobial peptides has been demonstrated in competitive interactions with non-Saccharomyces yeast strains, ensuring S. cerevisiae’s dominance during fermentation processes [30]. The various studies on the use of S. cerevisiae yeast in aquaculture have not been fully incorporated [31].
Therefore, the current study aims to discover the potential impact of S. cerevisiae in aquaculture based on the latest published scientific articles (2024–2025). It also aims to provide a bibliometric analysis of trends in the use of this yeast supplement in aquaculture. This analysis was designed to identify trends, limitations, and research gaps in the existing literature concerning the interaction between S. cerevisiae and other probiotics and prebiotics in aquaculture under various environmental conditions.
2. Results and Discussion
2.1. Situation of the Scientific Research on Saccharomyces cerevisiae (SC) in Aquaculture Based on WoS Database
2.1.1. Growth of SC-Related Documents 2015–2025
The number of published documents about the yeast probiotic SC in aquaculture covering the period between 2015 and 2025 was 466 in total. The publication trend demonstrates an increasing interest in the yeast SC, particularly after 2016. A notable increase occurred in 2020 and 2021, each recording 60 (12.88%) publications, suggesting a surge in research activity possibly driven by growing recognition of the potential use of SC in enhancing fish health and performance. In 2021, Dawood et al. [32] reported that S. cerevisiae in fish feeds aligns well with current trends in the aquaculture sector, emphasizing the shift away from antibiotics and towards more natural health-promoting alternatives. Although slight declines were observed in 2019 (32 publications, 6.87%) and 2023 (41 publications, 8.8%), interest increased quickly in 2024 with 58 publications (12.45%). The sharp drop to 20 (4.29%) publications in 2025 is likely due to incomplete data for the current year. Despite year-to-year variability, the fitted linear trend line (R2 = 0.0795) (Figure 1) indicates a modest but consistent growth in the number of publications over the past decade. This trend reflects a sustained and expanding research focus on SC as a functional probiotic and prebiotic in aquaculture, underscoring its growing relevance in fish nutrition, immunity, and disease resistance.
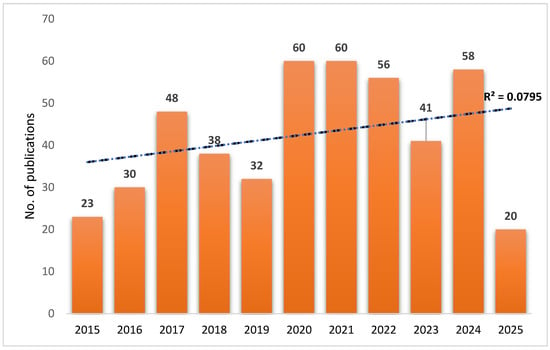
Figure 1.
Growth of scientific publications of SC-related documents in aquaculture, based on the WoS database. The correlation coefficient (R2) of the exponential curve between 2000 and 2025 was 0.0795.
2.1.2. Leading Countries on SC Research in Aquaculture
In total, 52 countries have published at least one SC-related document on the WoS. In fact, 466 documents have been published by these 52 countries. Table 1 presents the distribution of SC-related publications in aquaculture by country, highlighting both the volume and nature of international collaboration. China alone leads with 84 publications, accounting for 18.02% of the total output, followed by Egypt (10.30%) and Brazil (7.08%). These top-contributing countries indicate strong regional research activities, especially in Asia and South America. China also showed the highest number of single-country publications (SCP = 63), indicating a substantial volume of domestic research, while maintaining international collaboration through 21 multi-country publications (MCP), corresponding to 25% of its total. In fact, China is responsible for about 35% of the world’s fish and seafood production, making it the world’s largest producer of aquatic species [33]. China’s aquaculture industry is characterized by its vast size and diversity. In 2020, it produced over 49 million tons of fish. This includes not only finfish, but also mollusks and crustaceans, allowing for a wide range of products that meet the demands of both domestic and international markets [33].

Table 1.
The top 20 producing countries of SC-related documents in aquaculture (2015–2025) based on WoS database. SC: Saccharomyces cerevisiae.
In contrast, countries like Sweden (57.14%), Bangladesh (45.45%), Malaysia, and Norway (each 63.63%) exhibited relatively high MCP%, reflecting a strong inclination toward international collaboration despite their lower overall output. Japan stands out with a 100% MCP, suggesting that all its contributions are co-authored internationally. Similarly, countries like Australia (66.66%) and Pakistan (55.55%) showed higher proportions of collaborative research, underscoring the importance of global partnerships in advancing this niche field. Overall, the data indicate that while certain countries dominate in terms of quantity, others play significant collaborative roles in the global research network on Saccharomyces cerevisiae in aquaculture.
The international collaboration network among countries contributing to the SC research in aquaculture is presented in Figure 2. This map shows the global network of countries collaborating on SC-related aquaculture research. The intensity of the blue color represents the volume of publications, with darker colors indicating a higher research output. China, Egypt, Brazil, and the United States are notable contributors with relatively high publication counts. Red lines connecting countries indicate international co-authorships, reflecting research collaboration. The United States, China, and European nations are strongly interconnected, acting as major focal points in the global research network. Despite having fewer total publications, countries like Japan, Malaysia, and Norway demonstrate strong international engagement, as evidenced by their high proportion of multi-country publications (MCPs). This finding aligns with our previous bibliometric analysis, which showed that China, Egypt, and the USA are the most prominent countries in aquaculture probiotic research [15].
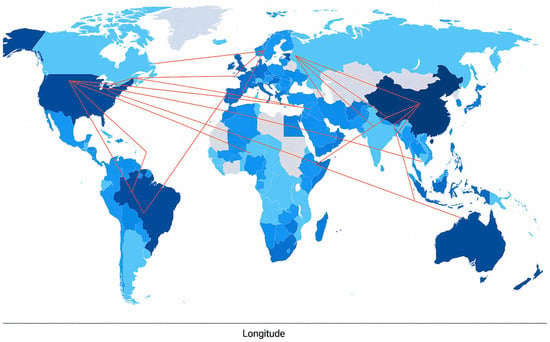
Figure 2.
Patterns of international collaboration on publications related to Saccharomyces cerevisiae in aquaculture (2015–2025). Countries with darker colors indicate higher productivity. The linking lines represent collaboration between countries.
The map also reveals a geographically diverse and interconnected research landscape in which emerging economies, such as Egypt, India, and Brazil, collaborate with established research leaders. This trend emphasized the increasing global significance of probiotics in aquaculture and the collaborative nature of the current scientific investigation into SC as a functional feed supplement and health enhancer in aquatic animals.
2.1.3. The Core Sources, and Most Cited Publications on SC Research
The use of Saccharomyces cerevisiae (SC), a beneficial yeast, has gained increasing focus in aquaculture due to its probiotic and synbiotic properties. Figure 3 shows the distribution of documents related to the application of SC in aquaculture across various scientific journals. Aquaculture was the most prominent source, with 60 publications, followed by Aquaculture Research (41 publications) and Fish & Shellfish Immunology (34 publications). These findings showed the important role these journals play in sharing aquaculture and fish health research. Additional journals, including Aquaculture Nutrition, Aquaculture Reports, and Aquaculture International, also made significant contributions, reflecting a rising interest in nutritional and health-related aquaculture topics. Specialized journals such as Fish Physiology and Biochemistry and Frontiers in Microbiology suggest that this research topic intersects with disciplines such as microbiology and fish physiology. The presence of multidisciplinary journals, such as PLoS ONE and Scientific Reports, has pointed to broader scientific interest and applicability. The dominance of aquaculture-specific journals emphasizes the maturity and specialization of the field, while contributions from related fields indicate the interdisciplinary nature of current research trends. The wide distribution of SC-related documents in various journals reflects the growing importance of this research topic. This aligns with the findings of Tucciarone et al. [34], who reported a rise in publications on sustainable aquaculture based on a recent trend analysis.
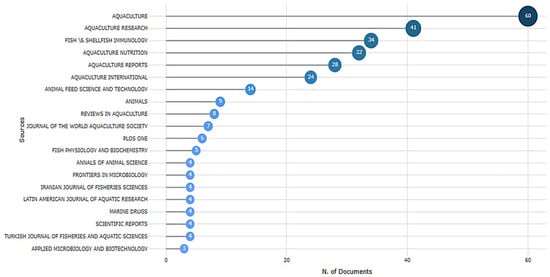
Figure 3.
Top journals publishing research on SC-related documents (2015–2025) based on the WoS database. SC: Saccharomyces cerevisiae.
Figure 4 introduces the core journals that published research on SC-related documents. The plot shows a decline in the number of articles beyond the first few journals, indicating a concentration of research output within a few sources, according to Bradford’s principle (a small number of sources account for most publications on a topic) [35]. Specifically, Aquaculture, Aquaculture Research, Fish & Shellfish Immunology, and Aquaculture Nutrition were identified as core sources within the shaded region of the graph. The sharp drop in productivity among the remaining journals supports Bradford’s principle. This finding aligns with the results shown in Figure 3, confirming the importance of these core journals in aquaculture and fish health research. Understanding the distribution of publications helps researchers prioritize target journals for their work and identify influential platforms that shape the academic landscape in this area.
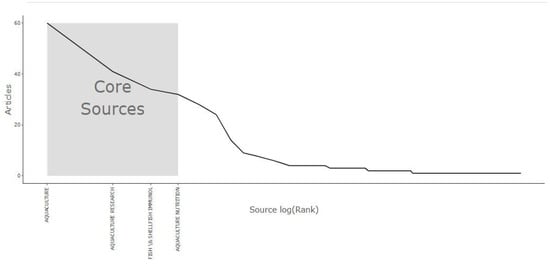
Figure 4.
Bradford’s principle application: identifying core journals in SC-related documents in aquaculture. Based on the WoS database (2015–2025). SC: Saccharomyces cerevisiae.
Table 2 presents the 20 most-cited articles in the research domain, emphasizing influential studies that have significantly impacted the field. The two most-cited papers are by Dawood et al. (2016, Aquaculture) [36] and Oberbeckmann et al. (2016, PLOS ONE) [37]. Each paper has received 372 total citations, averaging 37.2 citations per year, which indicates their foundational role in the field. Hai (2015, Journal of Applied Microbiology) [38] and Carbone (2016, Fish & Shellfish Immunology) [39] achieved similar citation counts, reflecting sustained academic interest in their research on microbiota modulation and host immune responses.

Table 2.
The top-cited documents on SC-related documents in aquaculture (2015–2025). SC: Saccharomyces cerevisiae.
Several papers published by Dawood appeared in the top 20 list, indicating his significant contributions to research on probiotics and functional feeds in aquaculture. Additionally, recent publications, such as Rohani M.F. (2022, Fish & Shellfish Immunology) [46], demonstrated strong annual citation rates (39.25 citations per year), indicating emerging research that is rapidly gaining recognition. The dominance of journals such as Fish & Shellfish Immunology, Aquaculture, and the Journal of Applied Microbiology among these highly cited works aligns with earlier identification of these sources as core journals (Figure 3 and Figure 4). This further supports their central role in disseminating impactful research. This citation analysis helps to identify key authors and studies that have laid the groundwork for future investigations into functional diets, host–microbe interactions, and immune modulation in aquaculture species.
2.2. The Potential Use of the Yeast SC in Aquaculture Based on Keyword Analysis
2.2.1. Dendrogram of Keyword Clusters
The dendrogram, derived from a factorial analysis of author keywords (Figure 5), shows how key terms in the literature cluster together based on their co-occurrence. This hierarchical clustering approach reveals the thematic structure of the research domain. Keywords that are closely linked form clusters that represent distinct research subfields, and the branching height indicates the degree of dissimilarity between groups of keywords. The analysis identified multiple thematic clusters. For example, terms such as probiotics, gut microbiota, and immune response frequently appear together, indicating a significant research focus on the health advantages of the yeast SC in aquaculture species.
The addition of yeast-based probiotics resulted in notable improvements in growth metrics and strengthened immune responses against pathogens, including Aeromonas spp. [27], Vibrio spp. [56], and Citrobacter spp. [57]. Other clusters may reflect related themes, such as growth performance, digestive enzyme activity, histomorphology, and disease resistance. These clusters indicate that the literature is organized around several interconnected yet distinct research trends.
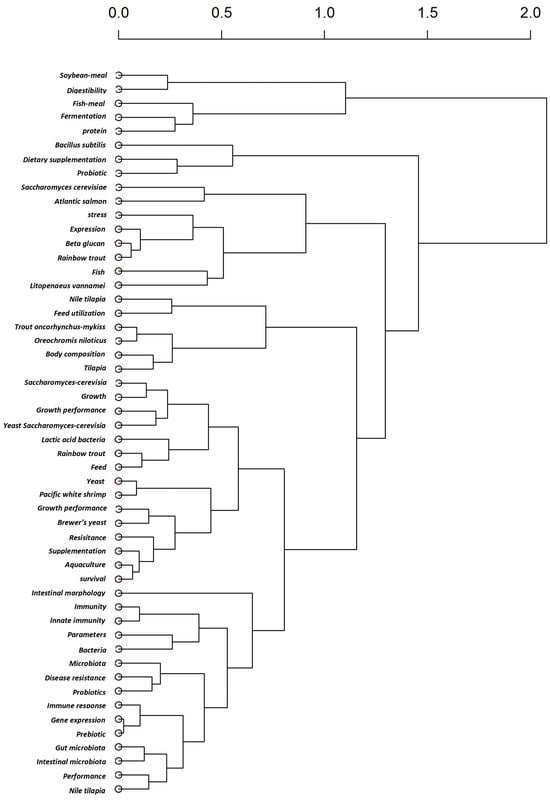
Figure 5.
Dendrogram of keyword clusters based on factorial analysis of the yeast SC-related document in aquaculture (2015–2025) based on the WoS database.
2.2.2. Thematic Map Analysis
The density of the two largest thematic clusters, “disease resistance, probiotics, and fish” and “Saccharomyces cerevisiae, growth performance, and rainbow trout”, revealed two distinct interconnected areas of research within aquaculture studies (Figure 6). The first cluster emphasizes the role of probiotics in enhancing disease resistance across various fish species, reflecting a broad and foundational theme and indicating a strong interest in health management and sustainable aquaculture practices. Its high density suggests a substantial body of research and collaboration on probiotic use and immune response in general. The second cluster, centered on S. cerevisiae and its impact on the growth performance of rainbow trout, points to a more specific line of inquiry. Its density highlights concentrated research efforts on this particular yeast probiotic. Numerous studies have investigated the effects of yeast on rainbow trout (O. mykiss). These studies have found that the dietary inclusion of S. cerevisiae improves intestinal microbiota composition and growth performance [28,58]. The coexistence of these dense themes underscores a layered research landscape in which general health-promoting strategies and species-specific probiotic applications are both critical to advancing aquaculture science.
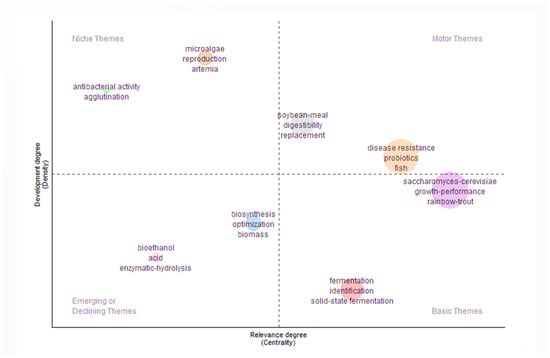
Figure 6.
Thematic map of aquaculture probiotic research (1999–2020) based on Web of Science data. Bubble size represents keyword frequency, and position is based on centrality and density.
2.2.3. Insights from the Three-Field Plot
The three-field plot highlights the growing global interest in the application of the probiotic S. cerevisiae within aquaculture, based on keyword co-occurrence across countries, research themes, and journals (Figure 7). Notably, Egypt, China, and India emerge as leading contributors to this area, reflecting a strong regional research focus. The central linkage of S. cerevisiae with key terms such as “growth”, “growth performance”, “probiotics”, and “immunity” underscores its potential as a multifunctional additive in fish diets. Moreover, its association with economically important species like Nile tilapia and rainbow trout, and frequent publication in prominent journals such as Aquaculture, Aquaculture Nutrition, and Fish & Shellfish Immunology, further emphasizes its significance in advancing both the nutritional and immunological dimensions of aquaculture. Collectively, the three-field plot illustrates how S. cerevisiae is becoming an integral component of sustainable and health-oriented aquafeed strategies.
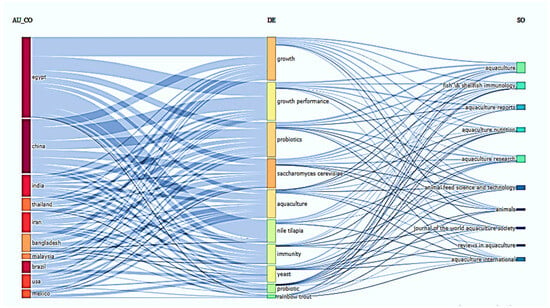
Figure 7.
Three-field plot illustrating the relationships between contributing countries (AU_CO), author keywords (DE), and journals (SO) in research on the use of Saccharomyces cerevisiae and probiotics in aquaculture.
2.2.4. Insights from the Trend Topics
Trend topic analysis reveals an increasing research interest regarding S. cerevisiae and its role in aquaculture, especially since 2019 (Figure 8). The appearance of this term alongside high-frequency keywords such as “growth”, “disease resistance”, “probiotic”, and “growth performance” indicates a clear thematic convergence on using this yeast to enhance the health and productivity of aquatic species. Associated topics such as “antioxidant status”, “immunostimulation”, and “beta-glucan” suggest an expanding scope of investigation into the functional and immunomodulatory properties of S. cerevisiae. The alignment of these terms with economically significant species, such as tilapia and rainbow trout, reflects their practical relevance in industry-driven research. This temporal trend indicates the emergence of S. cerevisiae as a key component in sustainable aquaculture strategies, particularly in the context of antibiotic alternatives and functional feed innovations. Its use contributes to healthier aquaculture systems and aligns with current global objectives aimed at reducing environmental impact of antibiotics and promoting food safety and aquaculture sustainability [31].
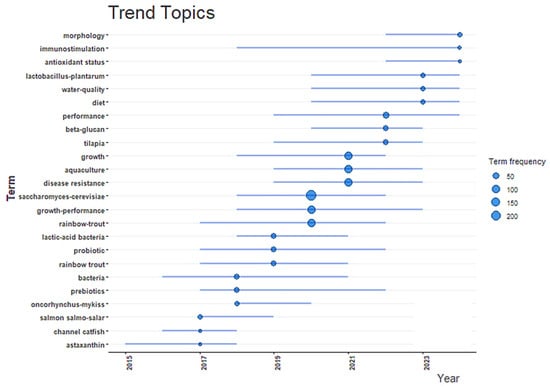
Figure 8.
Trend topic analysis showing the temporal evolution and frequency of key terms related to Saccharomyces cerevisiae in aquaculture (2015–2025).
2.3. Use of Saccharomyces cerevisiae (SC) in Aquaculture Based on the Recent Published Documents (2024–2025)
2.3.1. Enhancement of Growth, Feed Efficiency, and Digestibility
The main parameters used to evaluate the growth of animals after treatment with probiotic-based feed include final body weight (FBW), feed conversion ratio (FCR), survival (%), and specific growth rate (SGR). The FCR value is calculated using the formula: FCR = Feed intake/(Wf − Wi), where Wf is the final body weight (g), and Wi is the initial wet body weight (g). SGR is determined by the formula: SGR = (lnWf − lnWi)/t × 100, where t is the time (days). The enhanced growth indices result in faster harvesting times and improved production efficiency, both of which are vital for meeting the increasing global demand for seafood [15,59].
Numerous studies discussed the beneficial impact of probiotic yeast Saccharomyces cerevisiae (SC) on growth performance of different aquatic animals. For instance, rainbow trout (O. mykiss) fed on supplemented diets (1 × 108 CFU/g of SC) and 250–500 mg/kg for 4 weeks could enhance (p < 0.05) fish body weight and feed efficiency [60]. A report from China stated that the growth performance (weight gain, FCR, and body weight) of channel catfish (Ictalurus punctatus) can be significantly (p < 0.05) improved when animals received diet supplemented with the yeast S. cerevisiae for 12 weeks [27]. In addition, the FCR value and growth rate of Striped Catfish (Pangasianodon hypophthalmus) can be significantly improved when fish receive a diet supplemented with freeze-dried microencapsulated SC for 120 days [61]. Similarly, a report from Indonesia indicated that the supplementation by S. cerevisiae at 10 g/kg could significantly (p < 0.05) increase protein efficiency, and the growth rate of Saline Red Tilapia (Oreochromis spp.) when fish (1.07 ± 0.07 g) received a diet supplemented with yeast probiotic for 28 days [62]. Recently, extensive research studies have been performed on a wide range of aquaculture species, especially fish and shrimp, to evaluate the impact of probiotic yeast S. cerevisiae on growth parameters and feed efficiency (Table 3).
Many studies have confirmed a strong correlation between probiotics and fish growth parameters. However, the specific mechanisms by which probiotics improve growth parameters are not fully understood. As reported by Eliopoulos et al. [63] and Yang et al. [64], probiotics are important for increasing feed palatability via fermentation. This improvement can be linked to their ability to modify the sensory properties of feed. For example, certain probiotic strains ferment feed and produce metabolites that enhance its aroma, which plays an essential role in sensory perception, making feed more appealing to animals. Yang et al. [64] showed that probiotic fermentation can effectively enhance the palatability of traditional Chinese herbs by improving their flavor and taste. Furthermore, a recent study showed that the flavor and palatability of plants can be altered by fermentation and subsequently stimulate animals’ appetites through the formation of aroma and flavor compounds [65].
2.3.2. Impact on Disease Resistance, Immune Response, and Gut Integrity
In addition to enhancing growth parameters, the integration of probiotic S. cerevisiae in aquaculture has gained considerable attention for its ability to enhance disease resistance, modulate the immune system, improve gut integrity, and enhance microbial diversity within the gut of diverse fish and shrimp species (Table 3). Therefore, it fosters overall health in aquatic animals and contributes to the sustainability of aquaculture.
In the context of disease resistance and immune response, Mohammed et al. [66] examined the effect of S. cerevisiae fermented product (Diamond V Original XPC) on hybrid catfish (Ictalurus furcatus × I. punctatus). They noticed that marginally increased resistance to columnaris disease, enhanced level of immune effectors in the serum, such as lysozyme, complement, and immunoglobulin, were found when fish received a yeast-supplemented diet for 6 weeks. In addition, crayfish (Procambarus clarkia) exhibited significantly (p < 0.05) upregulated levels for prophenoloxidase and lysozyme when fish received a diet supplemented with S. cerevisiae YFI-SC2 at 107 CFU/g for 28 days. Additionally, the resistance against Citrobacter freundii can be enhanced by S. cerevisiae at 107 CFU/g feed supplementation. Furthermore, supplementation with S. cerevisiae could reduce the expression of intestinal inflammatory factors, and remarkably improve resistance to Vibrio harveyi infection in orange-spotted groupers (Epinephelus coioides) [67], resistance to A. hydrophila of ornamental fish (Poecilia latipinna) [68], resistance to a mixture of A. hydrophila NJ-1 and A. veronii HM091 of channel catfish (Ictalurus punctatus) [27], resistance to A. hydrophila AH2 in Indian major carp (Labeo rohita) [69], resistance to Streptococcus agalactiae in tambaqui (Colossoma macropomum) [70], and resistance of Nile tilapia (O. niloticus) to the sudden exposure to high water temperature (40 °C) [71].
Regarding gut health and microbiota, numerous research works have shown the potential use of S. cerevisiae for improving intestinal microbiota and gut health (Table 3). For example, El-Mokhlesany et al. [72] noticed that when mullet (Liza ramada) received diet contaminated with mycotoxins (AFB1) at 1 mg/kg and were supplemented with S. cerevisiae (5 × 106 cells/g), a remarkable improvement was recorded with regard to their intestinal structure and gut health in comparison to those fed solely an AFB1-contaminated diet. In addition, the length, width, and villus area of Nile tilapia (O. niloticus) can be significantly increased when fish receive a diet supplemented with S. cerevisiae at 4 g kg−1. Likewise, intestinal mucosal fold, width of lamina propria, width of enterocytes, and number of goblet cells can be increased [73]. Similarly, immunity and gut microbiota of Nile tilapia (O. niloticus) reared in low-input ponds can be altered under S. cerevisiae feed supplementation after 180 days [74]. Furthermore, the administration of the microencapsulated probiotic yeast S. cerevisiae has been correlated with an enhancement of length and width of the intestinal folds, and intestinal microbiota [75]. Taking all this into account, the improvement of the histomorphological indices of intestinal villi is directly correlated to the absorption and digestion efficiency [76]. However, the specific mechanisms by which probiotics and prebiotics affect intestinal absorption are not clear. The cells at the end of the villi are constantly sloughed off, and the intestinal epithelium’s renewal rate is high enough to replace them [77]. Moreover, the beneficial effect of S. cerevisiae on intestinal histomorphology could be attributed to the monosaccharides that compose its cell wall.
2.3.3. The Synbiotic Effect
Integrating the yeast probiotic S. cerevisiae into a synbiotic strategy gives aquaculture practitioners a powerful means of improving health and productivity of various fish species under various aquaculture systems (Table 3). For example, Siddik et al. [78] reported that the growth indices, immune response (TNF-alpha and IL-10), intestinal goblet cells, microvillous length, and gut health of barramundi (Lates calcarifer) juveniles remarkably improved when fish received co-supplementation of S. cerevisiae and Lactobacillus casei. Additionally, Vidakovic et al. [79] noticed that the combination of yeasts (Wickerhamomyces anomalus and Saccharomyces cerevisiae) can replace 40% of fishmeal for rainbow trout (Oncorhynchus mykiss) without affecting growth performance, intestinal health, or nutrient digestibility.
Furthermore, a combined probiotic containing Bacillus subtilis (1.5 × 109 CFU/g), Aspergillus oryzae (2 × 109 CFU/g), and S. cerevisiae (109 CFU/g) has beneficial effects on Nile tilapia (O. niloticus) juveniles. These probiotics provide resistance against Aeromonas hydrophila and Streptococcus iniae after three weeks of challenge, growth rates, and feed conversion improvements [48].

Table 3.
The significant role of the yeast Saccharomyces cerevisiae in aquaculture based on the most recent published research papers (2024–2025) on WoS.
Table 3.
The significant role of the yeast Saccharomyces cerevisiae in aquaculture based on the most recent published research papers (2024–2025) on WoS.
| Country | Host | Experimental Conditions | Key Findings | Date | Citation |
|---|---|---|---|---|---|
| Pakistan | Pacific whit shrimp (Litopenaeus vannamei) |
|
| 2025 | [80] |
| Canada | Atlantic Salmon (Salmo salar L.) |
|
| 2025 | [81] |
| Portugal | Green macroalga (Ulva rigida) |
|
| 2025 | [82] |
| UK | Atlantic Salmon (Salmo salar L.) |
|
| 2025 | [83] |
| Thailand | Nile tilapia (Oreochromis niloticus) |
|
| 2025 | [84] |
| Australia | Barramundi (Lates calcarifer) |
|
| 2025 | [85] |
| Iran | Rainbow trout (Oncorhynchus mykiss) |
|
| 2025 | [86] |
| Canada | Zebrafish (Danio rerio) |
|
| 2024 | [87] |
| Italy | Gilthead Seabream (Sparus aurata) |
|
| 2024 | [88] |
| Egypt | Nile tilapia (Oreochromis niloticus) |
|
| 2024 | [89] |
| Egypt | Nile tilapia (Oreochromis niloticus) |
|
| 2024 | [90] |
| Egypt | common carp (Cyprinus carpio) |
|
| 2024 | [91] |
| Peru | freshwater prawn (Cryphiops caementarius) |
|
| 2024 | [92] |
| Iran | Zebrafish (Danio rerio) |
|
| 2024 | [93] |
| Iran | Nile tilapia (Oreochromis niloticus) |
|
| 2024 | [94] |
| China | Salmo trutta |
|
| 2024 | [95] |
| Brazil | Shrimp (Penaeus vannamei) |
|
| 2024 | [96] |
| Malaysia | freshwater prawn (Macrobrachium rosenbergii) |
|
| 2024 | [97] |
| Egypt | Nile tilapia (Oreochromis niloticus) |
|
| 2024 | [98] |
| Egypt | Mugil capito |
|
| 2024 | [99] |
| Egypt | Nile tilapia (Oreochromis niloticus) |
|
| 2024 | [100] |
| USA | white sturgeon (Acipenser transmontanus) |
|
| 2024 | [101] |
“↑”: increase or upregulation at p < 0.05, “↓”: decrease or downregulation at p < 0.05, “IBW”: initial body weight, “CD”: control diet. “NE”: No effect.
2.4. Use of Machine Learning and AI
Integrating Artificial Intelligence (AI) and machine learning (ML) into the study of gut microbiota, with a particular focus on S. cerevisiae in aquaculture, holds significant potential for innovation. AI and ML techniques can improve our understanding of microbial interactions and functional dynamics within aquatic ecosystems. A key use of ML in aquaculture is predicting microbial community compositions and their functional interactions. In 2022, Nakanishi et al. [102] created a machine learning prediction model that used data from absorbance spectroscopy to estimate the density of cells in microbial mixtures, including S. cerevisiae. This predictive model is essential for optimizing conditions in aquaculture environments, where maintaining microbial balance is crucial for host health and optimal productivity.
Machine learning also provides innovative solutions for monitoring the health and efficiency of microbial activity in aquaculture. Machine learning-based profiling techniques can analyze gut microbiota and identify key microbial species, evaluating their functional roles. This assists in developing interventions that utilize Saccharomyces cerevisiae or similar microbes to improve the health of aquatic species [103]. Moreover, Palomba et al. [104] presented that the relevance of real-time monitoring in the context of bioprocesses was also underscored, with an emphasis placed on the importance of integrated systems using machine learning (ML) for the effective management of microbial populations.
On the whole, developing predictive models that use AI and ML is a reliable way to improve our understanding of gut microbiota behavior, especially with regard to probiotic applications such as S. cerevisiae. Combining functional genomics, microbiome analysis, and machine learning paves the way for significant improvements in aquaculture management practices, which aim to enhance the health and productivity of aquatic species.
3. Materials and Methods
3.1. Research Questions
- What are the main research areas, research quantity, global distribution of publications, and leading sources of studies focusing on the impact of the yeast Saccharomyces cerevisiae in aquaculture? This investigation was based on a selection of keywords, including “Saccharomyces cerevisiae” and “probiotics” (Figure 9).
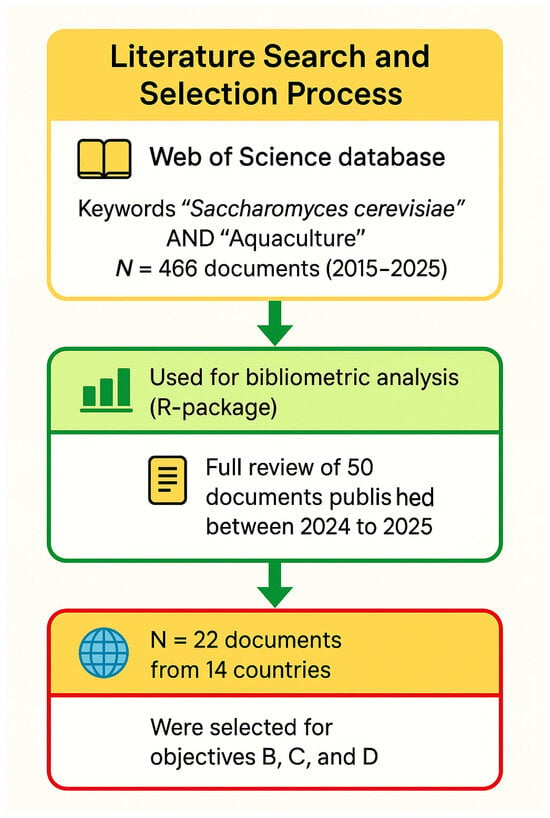 Figure 9. Workflow of document extraction, screening and processing using metadata obtained from the Web of Science database (WoS), 2015–2025.
Figure 9. Workflow of document extraction, screening and processing using metadata obtained from the Web of Science database (WoS), 2015–2025. - What are the effects of the yeast S. cerevisiae on the feed utilization and growth parameters of aquaculture species?
- What impact does S. cerevisiae have on disease prevention, survival (%), gut microbiome, and water quality in aquaculture species?
- What is the interactive effect between S. cerevisiae and other probiotics and prebiotics in aquaculture systems?
3.2. Searching Strategy
A comprehensive investigation was performed by examining the global literature in the Web of Science (WoS) database. WoS is considered the most comprehensive and well-known database for reviews and bibliometric analyses [15], which is why we selected it. The search was performed using a couple of keywords, including “Saccharomyces cerevisiae” and “Aquaculture” during the past decade (2015 to 2025). All documents written in English were considered. In addition, all research articles, review papers, or book chapters were also considered and counted; however, other types of documents were excluded. The search yielded a considerable amount of documents, which were 466 in total.
3.3. Data Processing and Bibliometric Analysis
For objective A, we exported the bibliometric parameters including authors, document titles, countries where the study was performed, publication dates, and author keywords, as BibTeX format from the WoS database. We performed in-depth analyses, including co-occurrence keyword assessment, citation analysis, trend, and factorial analysis, using RStudio v. 2025.05.0 + 496 (Boston, MA, USA) in connection with the bibliometric R package. The flexibility and statistical capabilities of R Studio v. 2025.05.0 + 496 (Boston, MA, USA) is essential for effectively visualizing complex and large amounts of data [105]. In brief, the metadata obtained from WoS database were converted to BibTeX format and uploaded to the R-bibliometric package for comprehensive analysis. The analysis yielded multiple knowledge maps illustrating the nature of emerging research of the yeast S. cerevisiae in aquaculture. The results and summary statistics were then exported to Microsoft® Excel (Redmond, WA, USA) for organization and processing.
3.4. Narrative Review (2024–2025)
Regarding objectives B–D, data including country where study was conducted, animal host, growth indices, feed utilization efficiency, disease incidence, gut health and integrity, intestinal microbiome of animal host, immune parameters, level of ammonia and toxic nitrogen in water, and activity of digestive enzymes were extracted directly from the results of narratively reviewed articles or by using Web Plot Digitizer (Version 5.0) if the data were presented as figures. The results that were extracted were then presented in tables, and they were summarized and discussed.
4. Conclusions
The current review highlights the growing interest in the yeast Saccharomyces cerevisiae as a probiotic and prebiotic in aquaculture based on bibliometric analysis and applied scientific perspectives. The growing number of research publications from 2015 to 2025 reflects the global interest in sustainable, antibiotic-free methods for promoting the health and nutrition of aquatic animals. The bibliometric analysis of 466 documents using RStudio v. 2025.05.0 + 496 reveals strong international collaboration, particularly among China, Egypt, and Brazil. It also identifies core journals such as Aquaculture (60 publications), Aquaculture Research (41 publications), and Fish & Shellfish Immunology (34 publications), in addition to the influential publications that shape the current literature on yeast-based prebiotics and probiotics, such as papers by Dawood et al. (2016, Aquaculture) [36] and Oberbeckmann et al. (2016, PLOS ONE) [37]. The author’s keyword analysis identified multiple thematic clusters. For example, terms such as gut microbiota and immune response frequently appear together, indicating a significant research focus on the health advantages of the S. cerevisiae in aquatic species. The trend topic analysis showed an increasing interest regarding S. cerevisiae and its role in aquaculture, especially from 2019 to 2025. The appearance of high-frequency terms such as “growth”, “disease resistance”, and “growth performance” indicates a considerable interest in using this yeast to enhance the health and productivity of fish and other aquatic animals.
A recent narrative review confirms that S. cerevisiae supplementation contributes to enhanced growth performance, improved feed efficiency, stronger immune responses, better gut integrity, and higher disease resistance in various aquaculture species based on different case studies from different countries. Furthermore, its synergistic effects in synbiotic formulations highlight its potential as a multifunctional supplement in functional animal feed. Despite these advances, gaps remain in our understanding of the precise mechanisms through which S. cerevisiae exhibits probiotic effects, especially under different environmental conditions and when combined with other microbes. Emerging areas like AI and machine learning-based microbiome profiling are not well-represented in the current literature. These findings offer valuable insight on research trends and gaps and provide a foundation for future studies on the multifaceted role of SC in sustainable aquaculture.
Future research should clarify these mechanisms, optimize dosage and delivery methods, and evaluate long-term effects in commercial aquaculture systems. Continued integration of bibliometric tools and experimental evidence is essential to identify research gaps, guide research priorities, and maximize the benefits of S. cerevisiae in sustainable aquaculture. Future research directions should also focus on uses of multi-omics technologies, strain-specific functional analysis, and the integration of machine learning-driven approaches to better understand host–microbe interactions and optimize S cerevisiae-based formulations.
Author Contributions
Conceptualization, E.A.H.M.; methodology, E.A.H.M.; software, E.A.H.M.; formal analysis, E.A.H.M.; investigation, E.A.H.M.; resources, K.P., B.K. and E.A.H.M.; data curation, E.A.H.M. writing—original draft preparation, E.A.H.M.; writing—review and editing, K.P., B.K. and E.A.H.M.; visualization, E.A.H.M.; supervision, K.P.; project administration, K.P. and E.A.H.M.; funding acquisition, B.K. All authors have read and agreed to the published version of the manuscript.
Funding
Project No. TKP2021-NKTA-32 was implemented with support from the National Research, Development, and Innovation Fund of Hungary, financed under the TKP2021-NKTA funding scheme, and supported by the University of Debrecen Program for Scientific Publication.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data supporting this study’s findings are available from the corresponding author upon request.
Acknowledgments
The current study is supported by the Stipendium Hungaricum Scholarship (SHS) program administered by the Tempus Public Foundation (TPF), Hungary. In addition, we would like to express our deep thanks to the University of Debrecen, Hungary, for facilitating unrestricted open access publishing in MDPI.
Conflicts of Interest
The Author, Elshafia Ali Hamid Mohammed, was employed by the Agricultural Research Corporation. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- FAO. The State of World Fisheries and Aquaculture 2024: Blue Transformation in Action; FAO: Rome, Italy, 2024. [Google Scholar]
- Shen, X.; Jin, G.; Zhao, Y.; Shao, X. Prevalence and Distribution Analysis of Antibiotic Resistance Genes in a Large-Scale Aquaculture Environment. Sci. Total Environ. 2020, 711, 134626. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2020; FAO: Rome, Italy, 2020; ISBN 978-92-5-132692-3. [Google Scholar]
- Boyd, C.E.; McNevin, A.A.; Davis, R.P. The Contribution of Fisheries and Aquaculture to the Global Protein Supply. Food Sec. 2022, 14, 805–827. [Google Scholar] [CrossRef] [PubMed]
- Lewbart, G.A. Bacteria and Ornamental Fish. Semin. Avian Exot. Pet. Med. 2001, 10, 48–56. [Google Scholar] [CrossRef]
- Abarike, E.D.; Jian, J.; Tang, J.; Cai, J.; Yu, H.; Lihua, C.; Jun, L. Influence of Traditional Chinese Medicine and Bacillus Species (TCMBS) on Growth, Immune Response and Disease Resistance in Nile Tilapia, Oreochromis niloticus. Aquac. Res. 2018, 49, 2366–2375. [Google Scholar] [CrossRef]
- Hasan, K.N.; Banerjee, G. Recent Studies on Probiotics as Beneficial Mediator in Aquaculture: A Review. JoBAZ 2020, 81, 53. [Google Scholar] [CrossRef]
- Khan, M.S.K.; Salin, K.R.; Yakupitiyage, A.; Tsusaka, T.W.; Nguyen, L.T.; Siddique, M.A.M. L-Tryptophan Mitigates Cannibalism and Improves Growth of Asian Seabass, Lates calcarifer Reared in a RAS System. Aquac. J. 2023, 3, 168–180. [Google Scholar] [CrossRef]
- Cabello, F.C.; Godfrey, H.P.; Tomova, A.; Ivanova, L.; Dölz, H.; Millanao, A.; Buschmann, A.H. Antimicrobial Use in Aquaculture Re-examined: Its Relevance to Antimicrobial Resistance and to Animal and Human Health. Environ. Microbiol. 2013, 15, 1917–1942. [Google Scholar] [CrossRef]
- Ali, H.; Rico, A.; Murshed-e-Jahan, K.; Belton, B. An Assessment of Chemical and Biological Product Use in Aquaculture in Bangladesh. Aquaculture 2016, 454, 199–209. [Google Scholar] [CrossRef]
- Gao, P.; Mao, D.; Luo, Y.; Wang, L.; Xu, B.; Xu, L. Occurrence of Sulfonamide and Tetracycline-Resistant Bacteria and Resistance Genes in Aquaculture Environment. Water Res. 2012, 46, 2355–2364. [Google Scholar] [CrossRef]
- Watts, J.; Schreier, H.; Lanska, L.; Hale, M. The Rising Tide of Antimicrobial Resistance in Aquaculture: Sources, Sinks and Solutions. Mar. Drugs 2017, 15, 158. [Google Scholar] [CrossRef]
- Elsabagh, M.; Mohamed, R.; Moustafa, E.M.; Hamza, A.; Farrag, F.; Decamp, O.; Dawood, M.A.O.; Eltholth, M. Assessing the Impact of Bacillus Strains Mixture Probiotic on Water Quality, Growth Performance, Blood Profile and Intestinal Morphology of Nile Tilapia, Oreochromis niloticus. Aquacult Nutr. 2018, 24, 1613–1622. [Google Scholar] [CrossRef]
- Won, S.; Hamidoghli, A.; Choi, W.; Bae, J.; Jang, W.J.; Lee, S.; Bai, S.C. Evaluation of Potential Probiotics Bacillus subtilis WB60, Pediococcus pentosaceus, and Lactococcus lactis on Growth Performance, Immune Response, Gut Histology and Immune-Related Genes in Whiteleg Shrimp, Litopenaeus vannamei. Microorganisms 2020, 8, 281. [Google Scholar] [CrossRef]
- Mohammed, E.A.H.; Ahmed, A.E.M.; Kovács, B.; Pál, K. The Significance of Probiotics in Aquaculture: A Review of Research Trend and Latest Scientific Findings. Antibiotics 2025, 14, 242. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, E.A.H.; Ahmed, A.E.M.; Teye-Gaga, C.; Pál, K. The Potential Use of Pediococcus spp. Probiotic in Aquaculture: A Review. Acta Agrar. Debreceniensis 2025, 1, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, E.A.H.; Kovács, B.; Kuunya, R.; Mustafa, E.O.A.; Abbo, A.S.H.; Pál, K. Antibiotic Resistance in Aquaculture: Challenges, Trends Analysis, and Alternative Approaches. Antibiotics 2025, 14, 598. [Google Scholar] [CrossRef] [PubMed]
- Gismondo, M.R.; Drago, L.; Lombardi, A. Review of Probiotics Available to Modify Gastrointestinal Flora. Int. J. Antimicrob. Agents 1999, 12, 287–292. [Google Scholar] [CrossRef]
- Parker, R. Probiotics, the Other Half of the Antibiotic Story. Anim. Nutr. Health 1974, 29, 4–6. [Google Scholar]
- FAO; WHO. Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Liver Lactic Acid Bacteria; Food and Agriculture Organization and World Health Organization Joint Report; FAO: Rome, Italy; WHO: Geneva, Switzerland, 2001. [Google Scholar]
- Yanbo, W.; Zirong, X. Effect of Probiotics for Common Carp (Cyprinus carpio) Based on Growth Performance and Digestive Enzyme Activities. Anim. Feed. Sci. Technol. 2006, 127, 283–292. [Google Scholar] [CrossRef]
- Tovar-Ramırez, D.; Zambonino Infante, J.; Cahu, C.; Gatesoupe, F.J.; Vázquez-Juárez, R. Influence of Dietary Live Yeast on European Sea Bass (Dicentrarchus labrax) Larval Development. Aquaculture 2004, 234, 415–427. [Google Scholar] [CrossRef]
- Kari, Z.A.; Kabir, M.A.; Dawood, M.A.O.; Razab, M.K.A.A.; Ariff, N.S.N.A.; Sarkar, T.; Pati, S.; Edinur, H.A.; Mat, K.; Ismail, T.A.; et al. Effect of Fish Meal Substitution with Fermented Soy Pulp on Growth Performance, Digestive Enzyme, Amino Acid Profile, and Immune-Related Gene Expression of African Catfish (Clarias gariepinus). Aquaculture 2022, 546, 737418. [Google Scholar] [CrossRef]
- Kumar, R.; Mukherjee, S.C.; Prasad, K.P.; Pal, A.K. Evaluation of Bacillus subtilis as a Probiotic to Indian Major Carp Labeo rohita (Ham.). Aquac. Res. 2006, 37, 1215–1221. [Google Scholar] [CrossRef]
- Mohammed, E.A.H.; Fehér, M.; Bársony, P.; Pál, K. Alteration in Gut Microbiome of Common Carp (Cyprinus carpio L., 1758) Mediated by Probiotics and Yeast Prebiotic. Biol. Life Sci. Forum 2025, 45, 1. [Google Scholar] [CrossRef]
- Agboola, J.O.; Øverland, M.; Skrede, A.; Hansen, J.Ø. Yeast as Major Protein-rich Ingredient in Aquafeeds: A Review of the Implications for Aquaculture Production. Rev. Aquac. 2021, 13, 949–970. [Google Scholar] [CrossRef]
- Xia, R.; Hao, Q.; Xie, Y.; Zhang, Q.; Ran, C.; Yang, Y.; Zhou, W.; Chu, F.; Zhang, X.; Wang, Y.; et al. Effects of Dietary Saccharomyces cerevisiae on Growth, Intestinal and Liver Health, Intestinal Microbiota and Disease Resistance of Channel Catfish (Ictalurus punctatus). Aquac. Rep. 2022, 24, 101157. [Google Scholar] [CrossRef]
- Adel, M.; Lazado, C.C.; Safari, R.; Yeganeh, S.; Zorriehzahra, M.J. Aqualase®, a Yeast-Based in-Feed Probiotic, Modulates Intestinal Microbiota, Immunity and Growth of Rainbow Trout Oncorhynchus mykiss. Aquac. Res. 2017, 48, 1815–1826. [Google Scholar] [CrossRef]
- Smukowski Heil, C.S.; Large, C.R.L.; Patterson, K.; Hickey, A.S.-M.; Yeh, C.-L.C.; Dunham, M.J. Temperature Preference Can Bias Parental Genome Retention during Hybrid Evolution. PLoS Genet. 2019, 15, e1008383. [Google Scholar] [CrossRef]
- Benito, Á.; Calderón, F.; Palomero, F.; Benito, S. Quality and Composition of Airen Wines Fermented by Sequential Inoculation of Lachancea Thermotolerans and Saccharomyces cerevisiae. Food Technol. Biotechnol. 2016, 54, 135–144. [Google Scholar] [CrossRef]
- Del Valle, J.C.; Bonadero, M.C.; Fernández-Gimenez, A.V. Saccharomyces cerevisiae as Probiotic, Prebiotic, Synbiotic, Postbiotics and Parabiotics in Aquaculture: An Overview. Aquaculture 2023, 569, 739342. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Abd El-Kader, M.F.; Farid, M.A.; Abd-Elghany, M.F.; Alkafafy, M.; Van Doan, H. Saccharomyces cerevisiae Enhanced the Growth, Immune and Antioxidative Responses of European Seabass (Dicentrarchus labrax). Ann. Anim. Sci. 2021, 21, 1423–1433. [Google Scholar] [CrossRef]
- Chen, W.; Gao, S. Current Status of Industrialized Aquaculture in China: A Review. Environ. Sci. Pollut. Res. 2023, 30, 32278–32287. [Google Scholar] [CrossRef]
- Tucciarone, I.; Secci, G.; Contiero, B.; Parisi, G. Sustainable Aquaculture over the Last 30 Years: An Analysis of the Scientific Literature by the Text Mining Approach. Rev. Aquac. 2024, 16, 2064–2076. [Google Scholar] [CrossRef]
- Brookes, B.C. “Sources of Information on Specific Subjects” by S.C. Bradford. J. Inf. Sci. 1985, 10, 173–175. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Koshio, S. Recent Advances in the Role of Probiotics and Prebiotics in Carp Aquaculture: A Review. Aquaculture 2016, 454, 243–251. [Google Scholar] [CrossRef]
- Oberbeckmann, S.; Osborn, A.M.; Duhaime, M.B. Microbes on a Bottle: Substrate, Season and Geography Influence Community Composition of Microbes Colonizing Marine Plastic Debris. PLoS ONE 2016, 11, e0159289. [Google Scholar] [CrossRef]
- Hai, N.V. The Use of Probiotics in Aquaculture. J. Appl. Microbiol. 2015, 119, 917–935. [Google Scholar] [CrossRef] [PubMed]
- Carbone, D.; Faggio, C. Importance of Prebiotics in Aquaculture as Immunostimulants. Effects on Immune System of Sparus aurata and Dicentrarchus labrax. Fish Shellfish Immunol. 2016, 54, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Kabeya, N.; Fonseca, M.M.; Ferrier, D.E.K.; Navarro, J.C.; Bay, L.K.; Francis, D.S.; Tocher, D.R.; Castro, L.F.C.; Monroig, Ó. Genes for de Novo Biosynthesis of Omega-3 Polyunsaturated Fatty Acids Are Widespread in Animals. Sci. Adv. 2018, 4, eaar6849. [Google Scholar] [CrossRef] [PubMed]
- Hai, N.V. Research Findings from the Use of Probiotics in Tilapia Aquaculture: A Review. Fish Shellfish Immunol. 2015, 45, 592–597. [Google Scholar] [CrossRef]
- Sharif, M.; Zafar, M.H.; Aqib, A.I.; Saeed, M.; Farag, M.R.; Alagawany, M. Single Cell Protein: Sources, Mechanism of Production, Nutritional Value and Its Uses in Aquaculture Nutrition. Aquaculture 2021, 531, 735885. [Google Scholar] [CrossRef]
- Jannathulla, R.; Rajaram, V.; Kalanjiam, R.; Ambasankar, K.; Muralidhar, M.; Dayal, J.S. Fishmeal Availability in the Scenarios of Climate Change: Inevitability of Fishmeal Replacement in Aquafeeds and Approaches for the Utilization of Plant Protein Sources. Aquac. Res. 2019, 50, 3493–3506. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Koshio, S.; Ishikawa, M.; Yokoyama, S.; El Basuini, M.F.; Hossain, M.S.; Nhu, T.H.; Dossou, S.; Moss, A.S. Effects of Dietary Supplementation of Lactobacillus rhamnosus or/and Lactococcus lactis on the Growth, Gut Microbiota and Immune Responses of Red Sea Bream, Pagrus major. Fish Shellfish Immunol. 2016, 49, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Standen, B.T.; Peggs, D.L.; Rawling, M.D.; Foey, A.; Davies, S.J.; Santos, G.A.; Merrifield, D.L. Dietary Administration of a Commercial Mixed-Species Probiotic Improves Growth Performance and Modulates the Intestinal Immunity of Tilapia, Oreochromis niloticus. Fish Shellfish Immunol. 2016, 49, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Rohani, M.F.; Islam, S.M.; Hossain, M.K.; Ferdous, Z.; Siddik, M.A.; Nuruzzaman, M.; Padeniya, U.; Brown, C.; Shahjahan, M. Probiotics, Prebiotics and Synbiotics Improved the Functionality of Aquafeed: Upgrading Growth, Reproduction, Immunity and Disease Resistance in Fish. Fish Shellfish Immunol. 2022, 120, 569–589. [Google Scholar] [CrossRef] [PubMed]
- Øverland, M.; Skrede, A. Yeast Derived from Lignocellulosic Biomass as a Sustainable Feed Resource for Use in Aquaculture. J. Sci. Food Agric. 2017, 97, 733–742. [Google Scholar] [CrossRef]
- Iwashita, M.K.P.; Nakandakare, I.B.; Terhune, J.S.; Wood, T.; Ranzani-Paiva, M.J.T. Dietary Supplementation with Bacillus subtilis, Saccharomyces cerevisiae and Aspergillus oryzae Enhance Immunity and Disease Resistance against Aeromonas hydrophila and Streptococcus iniae Infection in Juvenile Tilapia Oreochromis niloticus. Fish Shellfish Immunol. 2015, 43, 60–66. [Google Scholar] [CrossRef]
- Huyben, D.; Sun, L.; Moccia, R.; Kiessling, A.; Dicksved, J.; Lundh, T. Dietary Live Yeast and Increased Water Temperature Influence the Gut Microbiota of Rainbow Trout. J. Appl. Microbiol. 2018, 124, 1377–1392. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Koshio, S.; Ishikawa, M.; Yokoyama, S.; El Basuini, M.F.; Hossain, M.S.; Nhu, T.H.; Moss, A.S.; Dossou, S.; Wei, H. Dietary Supplementation of β-Glucan Improves Growth Performance, the Innate Immune Response and Stress Resistance of Red Sea Bream, Pagrus major. Aquacult Nutr. 2017, 23, 148–159. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Samir, F.; Abd El-Naby, A.S.; Monier, M.N. Antioxidative and Immunostimulatory Effect of Dietary Cinnamon Nanoparticles on the Performance of Nile Tilapia, Oreochromis niloticus (L.) and Its Susceptibility to Hypoxia Stress and Aeromonas hydrophila Infection. Fish Shellfish Immunol. 2018, 74, 19–25. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Koshio, S.; Ishikawa, M.; El-Sabagh, M.; Esteban, M.A.; Zaineldin, A.I. Probiotics as an Environment-Friendly Approach to Enhance Red Sea Bream, Pagrus major Growth, Immune Response and Oxidative Status. Fish Shellfish Immunol. 2016, 57, 170–178. [Google Scholar] [CrossRef]
- Zhou, P.; Ye, L.; Xie, W.; Lv, X.; Yu, H. Highly Efficient Biosynthesis of Astaxanthin in Saccharomyces cerevisiae by Integration and Tuning of Algal crtZ and Bkt. Appl. Microbiol. Biotechnol. 2015, 99, 8419–8428. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Hu, S.-Y.; Chiu, C.-S.; Liu, C.-H. Multiple-Strain Probiotics Appear to Be More Effective in Improving the Growth Performance and Health Status of White Shrimp, Litopenaeus vannamei, than Single Probiotic Strains. Fish Shellfish Immunol. 2019, 84, 1050–1058. [Google Scholar] [CrossRef]
- Lin, H.-L.; Shiu, Y.-L.; Chiu, C.-S.; Huang, S.-L.; Liu, C.-H. Screening Probiotic Candidates for a Mixture of Probiotics to Enhance the Growth Performance, Immunity, and Disease Resistance of Asian Seabass, Lates calcarifer (Bloch), Against Aeromonas hydrophila. Fish Shellfish Immunol. 2017, 60, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Pooljun, C.; Daorueang, S.; Weerachatyanukul, W.; Direkbusarakom, S.; Jariyapong, P. Enhancement of Shrimp Health and Immunity with Diets Supplemented with Combined Probiotics: Application to Vibrio Parahaemolyticus Infections. Dis. Aquat. Org. 2020, 140, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, Y.; Xue, M.; Yang, T.; Luo, X.; Fan, Y.; Meng, Y.; Liu, W.; Lin, G.; Li, B.; et al. Effects of Dietary Saccharomyces cerevisiae YFI-SC2 on the Growth Performance, Intestinal Morphology, Immune Parameters, Intestinal Microbiota, and Disease Resistance of Crayfish (Procambarus clarkia). Animals 2021, 11, 1963. [Google Scholar] [CrossRef]
- Heidarieh, M.; Mirvaghefi, A.R.; Akbari, M.; Sheikhzadeh, N.; Kamyabi-Moghaddam, Z.; Askari, H.; Shahbazfar, A.A. Evaluations of HilysesTM, Fermented Saccharomyces cerevisiae, on Rainbow Trout (Oncorhynchus mykiss) Growth Performance, Enzymatic Activities and Gastrointestinal Structure. Aquacult. Nutr. 2013, 19, 343–348. [Google Scholar] [CrossRef]
- Amenyogbe, E. Application of Probiotics for Sustainable and Environment-Friendly Aquaculture Management—A Review. Cogent Food Agric. 2023, 9, 2226425. [Google Scholar] [CrossRef]
- Yousefi, M.; Adineh, H.; Taheri Mirghaed, A.; Hoseini, S.M. Co-Supplementation of Diet with Saccharomyces cerevisiae and Thymol: Effects on Growth Performance, Antioxidant and Immunological Responses of Rainbow Trout, Oncorhynchus mykiss. Animals 2025, 15, 302. [Google Scholar] [CrossRef]
- Boonanuntanasarn, S.; Ditthab, K.; Jangprai, A.; Nakharuthai, C. Effects of Microencapsulated Saccharomyces cerevisiae on Growth, Hematological Indices, Blood Chemical, and Immune Parameters and Intestinal Morphology in Striped Catfish, Pangasianodon hypophthalmus. Probiotics Antimicro. Prot. 2019, 11, 427–437. [Google Scholar] [CrossRef]
- Samidjan, I.; Rachmawati, D.; Dody, S.; Riyadi, P.H. Effects of Various Doses of Saccharomyces cerevisiae on the Growth, Survival Rate, and Blood Profile of Saline Red Tilapia (Oreochromis spp.) in the Semi-Intensive Culture Conditions. Pertanika J. Sci. Technol. 2022, 31, 529–541. [Google Scholar] [CrossRef]
- Eliopoulos, C.; Markou, G.; Chorianopoulos, N.; Haroutounian, S.A.; Arapoglou, D. Transformation of Mixtures of Olive Mill Stone Waste and Oat Bran or Lathyrus Clymenum Pericarps into High Added Value Products Using Solid State Fermentation. Waste Manag. 2022, 149, 168–176. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Y.; Zheng, L.; Peng, M.; Mai, Y.; Wang, X. Comparative Analysis of the Effect of Dietary Supplementation with Fermented and Water-Extracted Leaf Extracts of Eucommia Ulmoides on Egg Production and Egg Nutrition. Foods 2024, 13, 1521. [Google Scholar] [CrossRef]
- Wei, G.; Chitrakar, B.; Regenstein, J.M.; Sang, Y.; Zhou, P. Microbiology, Flavor Formation, and Bioactivity of Fermented Soybean Curd (Furu): A Review. Food Res. Int. 2023, 163, 112183. [Google Scholar] [CrossRef]
- Mohammed, H.H.; Brown, T.L.; Beck, B.H.; Yildirim-Aksoy, M.; Eljack, R.M.; Peatman, E. The Effects of Dietary Inclusion of a Saccharomyces cerevisiae Fermentation Product in a Commercial Catfish Ration on Growth, Immune Readiness, and Columnaris Disease Susceptibility. J. Appl. Aquac. 2019, 31, 193–209. [Google Scholar] [CrossRef]
- Yang, X.; He, Y.; Chi, S.; Tan, B.; Lin, S.; Dong, X.; Yang, Q.; Liu, H.; Zhang, S. Supplementation with Saccharomyces cerevisiae Hydrolysate in a Complex Plant Protein, Low-Fishmeal Diet Improves Intestinal Morphology, Immune Function and Vibrio harveyi Disease Resistance in Epinephelus Coioides. Aquaculture 2020, 529, 735655. [Google Scholar] [CrossRef]
- Rezaei Aminlooi, V.; Ahmadifard, N.; Tukmechi, A.; Agh, N. Improvement of Reproductive Indices, Lysozyme Activity, and Disease Resistance in Live-bearing Ornamental Fish, Poecilia latipinna Using Artemia Supplementation with Treated Yeast Cell, Saccharomyces cerevisiae. Aquac. Res. 2019, 50, 72–79. [Google Scholar] [CrossRef]
- Bandyopadhyay, P.; Mishra, S.; Sarkar, B.; Swain, S.K.; Pal, A.; Tripathy, P.P.; Ojha, S.K. Dietary Saccharomyces cerevisiae Boosts Growth and Immunity of IMC Labeo rohita (Ham.) Juveniles. Indian J. Microbiol. 2015, 55, 81–87. [Google Scholar] [CrossRef]
- Da Paixão, A.E.M.; Dos Santos, J.C.; Pinto, M.S.; Pereira, D.S.P.; De Oliveira Ramos, C.E.C.; Cerqueira, R.B.; Navarro, R.D.; Da Silva, R.F. Effect of Commercial Probiotics (Bacillus subtilis and Saccharomyces cerevisiae) on Growth Performance, Body Composition, Hematology Parameters, and Disease Resistance against Streptococcus agalactiae in Tambaqui (Colossoma macropomum). Aquacult Int. 2017, 25, 2035–2045. [Google Scholar] [CrossRef]
- Abass, D.A.; Obirikorang, K.A.; Campion, B.B.; Edziyie, R.E.; Skov, P.V. Dietary Supplementation of Yeast (Saccharomyces cerevisiae) Improves Growth, Stress Tolerance, and Disease Resistance in Juvenile Nile Tilapia (Oreochromis niloticus). Aquacult Int. 2018, 26, 843–855. [Google Scholar] [CrossRef]
- El-Mokhlesany, S.A.I.; Ibrahim, M.A.; Amer, A.A.; Gewaily, M.S.; Zaineldin, A.I.; Soliman, A.; Baromh, M.Z.; Gouda, A.H.; Dawood, M.A.O. The Protective Effects of Saccharomyces cerevisiae on the Growth Performance, Intestinal Health, and Antioxidative Capacity of Mullet (Liza Ramada) Fed Diets Contaminated with Aflatoxin B1. Ann. Anim. Sci. 2023, 23, 859–868. [Google Scholar] [CrossRef]
- Islam, S.M.M.; Rohani, M.F.; Shahjahan, M. Probiotic Yeast Enhances Growth Performance of Nile Tilapia (Oreochromis niloticus) through Morphological Modifications of Intestine. Aquac. Rep. 2021, 21, 100800. [Google Scholar] [CrossRef]
- Opiyo, M.A.; Jumbe, J.; Ngugi, C.C.; Charo-Karisa, H. Dietary Administration of Probiotics Modulates Non-Specific Immunity and Gut Microbiota of Nile Tilapia (Oreochromis niloticus) Cultured in Low Input Ponds. Int. J. Vet. Sci. Med. 2019, 7, 1–9. [Google Scholar] [CrossRef]
- De Moraes, A.V.; Owatari, M.S.; Da Silva, E.; De Oliveira Pereira, M.; Piola, M.; Ramos, C.; Farias, D.R.; Schleder, D.D.; Jesus, G.F.A.; Jatobá, A. Effects of Microencapsulated Probiotics-Supplemented Diet on Growth, Non-Specific Immunity, Intestinal Health and Resistance of Juvenile Nile Tilapia Challenged with Aeromonas hydrophila. Anim. Feed. Sci. Technol. 2022, 287, 115286. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Magouz, F.I.; Essa, M.; Mansour, M. Impact of Yeast Fermented Poultry By-Product Meal on Growth, Digestive Enzyme Activities, Intestinal Morphometry and Immune Response Traits of Common Carp (Cyprinus carpio). Ann. Anim. Sci. 2020, 20, 939–959. [Google Scholar] [CrossRef]
- Aubin, J.; Gatesoupe, F.-J.; Labbe, L.; Lebrun, L. Trial of Probiotics to Prevent the Vertebral Column Compression Syndrome in Rainbow Trout (Oncorhynchus mykiss Walbaum). Aquac. Res. 2005, 36, 758–767. [Google Scholar] [CrossRef]
- Siddik, M.A.B.; Foysal, M.J.; Fotedar, R.; Francis, D.S.; Gupta, S.K. Probiotic Yeast Saccharomyces cerevisiae Coupled with Lactobacillus casei Modulates Physiological Performance and Promotes Gut Microbiota in Juvenile Barramundi, Lates calcarifer. Aquaculture 2022, 546, 737346. [Google Scholar] [CrossRef]
- Vidakovic, A.; Huyben, D.; Sundh, H.; Nyman, A.; Vielma, J.; Passoth, V.; Kiessling, A.; Lundh, T. Growth Performance, Nutrient Digestibility and Intestinal Morphology of Rainbow Trout (Oncorhynchus mykiss) Fed Graded Levels of the Yeasts Saccharomyces cerevisiae and Wickerhamomyces anomalus. Aquacult. Nutr. 2020, 26, 275–286. [Google Scholar] [CrossRef]
- Amin, I.; Can, E.; Khan, T.; Fatima, J.; Khalid, M. Synergistic Effects of Combined Probiotic Supplementation on Growth Performance, Survival, and Health Status in Labeo rohita. Aquacult. Int. 2025, 33, 340. [Google Scholar] [CrossRef]
- Stangroom, J.; Marana, M.; Booman, M.; Andrew, S.; Poley, J.; Wilderjans, E.; Ghillebert, R.; Sabbadin Zanuzzo, F. Aspergillus niger β-Glucan, MycoFence®, Efficacy Against Ulcerative Disease in Atlantic Salmon Compared to Commercial Yeast β-Glucan. Aquaculture 2025, 603, 742350. [Google Scholar] [CrossRef]
- Brandão, M.; Marques, D.J.; Sousa, S.; Mateus, M.; Pinheiro, H.M.; Da Fonseca, M.M.R.; Pires, C.; Nunes, M.L.; Marques, A.; Cesário, M.T. Lactic Acid Bacteria and Yeast Fermentation to Improve the Nutritional Value of Ulva rigida. Mar. Drugs 2025, 23, 106. [Google Scholar] [CrossRef]
- Momoh, T.A.; Odu-Onikosi, S.G.; Amulejoye, F.D.; Wilson, J.; Eynon, B.; Kühlwein, H.; Kuri, V.; Merrifield, D.L. Brewers’ Yeast (Saccharomyces cerevisiae) Purified Functional Feed Additives Mitigate Soybean Meal-Induced Enteritis in Atlantic Salmon (Salmo salar) Parr. Aquac. Nutr. 2025, 2025, 8555658. [Google Scholar] [CrossRef]
- Phinyo, M.; Khlaithim, P.; Boonsrangsom, T.; Pongpadung, P.; Janpoom, S.; Klinbunga, S.; Sujipuli, K. Improved Growth and Immunity in Nile Tilapia Oreochromis niloticus Fed a Fermented Rice Bran Supplement. Anim. Feed. Sci. Technol. 2025, 319, 116160. [Google Scholar] [CrossRef]
- Siddik, M.A.B.; Francis, D.S.; Islam, S.M.M.; Salini, M.J.; Fotedar, R. Fermentation and Fortification of Sargassum linearifolium with Multi-Strain Probiotics Improves Mucosal Barrier Status, Inflammatory Response and Resistance to Vibrio harveyi Infection in Barramundi Lates calcarifer. Aquaculture 2025, 595, 741502. [Google Scholar] [CrossRef]
- Hoseini, S.M.; Pagheh, E.; Aghaei Moghaddam, A.; Gharavi, B.; Ghelichpour, M. Dietary Nonenriched and Iron-Enriched Yeasts Improve Hematological and Antioxidant Parameters in Rainbow Trout, Oncorhynchus mykiss, Fed on Diets Containing Cottonseed Meal. Aquac. Nutr. 2025, 2025, 9955172. [Google Scholar] [CrossRef]
- Gao, N.; Zhang, J.; Shandilya, U.K.; Lumsden, J.S.; Barzrgar, A.B.; Huyben, D.; Karrow, N.A. Hepatic Gene Expression Changes of Zebrafish Fed Yeast Prebiotic, Yeast Probiotic, Black Soldier Fly Meal, and Butyrate. Fishes 2024, 9, 495. [Google Scholar] [CrossRef]
- Alfonso, S.; Toomey, L.; Fiocchi, E.; Manfrin, A.; Boscarato, M.; Vasilaki, P.; Zupa, W.; Bertazzo, V.; Bégout, M.-L.; Spedicato, M.T.; et al. Growth Performance, Immune Characteristics, and Health and Welfare of Gilthead Seabream (Sparus aurata) Fed a Tailor-Made Environmentally Sustainable Diet Formulated Using Novel Ingredients. Aquac. Res. 2024, 2024, 8234882. [Google Scholar] [CrossRef]
- Awad, A.; Mohammady, E.Y.; Soaudy, M.R.; Rabetimarghezar, N.; El-Haroun, E.R.; Hassaan, M.S. Growth and Physiological Response of Nile Tilapia (Oreochromis niloticus) Fed a Fermented Mixture of Plant Protein Sources. Anim. Feed. Sci. Technol. 2024, 315, 116034. [Google Scholar] [CrossRef]
- Mohammady, E.Y.; Aboseif, A.M.; Al-Afify, A.D.G.; Abdelhameed, M.S.; Shawer, E.E.; Abdo, S.M.; Ramadan, E.A.; Hegab, M.H.; El-Dein, A.N.; Hassaan, M.S. Growth and Physiological Responses of Nile Tilapia, Oreochromis niloticus Fed Dietary Fermented Sugar Beet Bagasse and Reared in Biofloc System. Anim. Feed. Sci. Technol. 2024, 318, 116124. [Google Scholar] [CrossRef]
- El-Mashtoly, M.; Magouz, F.I.; Darwish, S.; Amer, A.A.; Zaineldin, A.I.; Gewaily, M.S.; Dawood, M.A.O. Dietary Chlorella vulgaris and Saccharomyces cerevisiae Enhanced the Growth Performance, Blood Biomarkers, and Antioxidative Capacity of Common Carp (Cyprinus carpio). Sci. Afr. 2024, 26, e02407. [Google Scholar] [CrossRef]
- Reyes-Avalos, W.; Azañero-Díaz, C.; Melgarejo-Velasquez, G.; Yzásiga-Barrera, C.; Alegre-Calvo, B.; Lezama-Salazar, R. Effect of Diets Supplemented with Yeast, Chitin, and Chitosan on the Growth, Immune, and Antioxidant Responses of the Freshwater Prawn Cryphiops (Cryphiops) caementarius. Aquac. Nutr. 2024, 2024, 1727130. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Sudaagar, M.; Zakariaee, H.; Paknejad, H.; Baruah, K.; Norouzitalab, P. Evaluation of the Synbiotic Effects of Saccharomyces cerevisiae and Mushroom Extract on the Growth Performance, Digestive Enzyme Activity, and Immune Status of Zebrafish Danio rerio. BMC Microbiol. 2024, 24, 331. [Google Scholar] [CrossRef]
- Adineh, H.; Yousefi, M.; Al Sulivany, B.S.A.; Ahmadifar, E.; Farhangi, M.; Hoseini, S.M. Effects of Dietary Yeast, Saccharomyces cerevisiae, and Costmary, Tanacetum balsamita, Essential Oil on Growth Performance, Digestive Enzymes, Biochemical Parameters, and Disease Resistance in Nile Tilapia, Oreochromis niloticus. Aquac. Nutr. 2024, 2024, 1388002. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Z.; He, H.; He, W.; Zhang, Z.; Sun, S.; Wang, W. Multi-Omics Analysis Reveals the Regulatory Mechanism of Different Probiotics on Growth Performance and Intestinal Health of Salmo trutta (S. trutta). Microorganisms 2024, 12, 1410. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, O.A.L.F.; Wasielesky, W.; Sena, R.P.O.; Ramiro, B.D.O.; Bezerra, A.; Krummenauer, D. Microbial Community Composition, Nitrification Process, and Growth of Penaeus vannamei in a Synbiotic Nursery System Inoculated with Different Probiotic Microorganisms. Aquaculture 2024, 592, 741254. [Google Scholar] [CrossRef]
- Taguemount, R.; Pratoomyot, J.; Shinn, A.P.; Waiho, K.; Rasid, R. Dietary Supplements of β-1,3/1,6-Glucan Derived from Baker’s Yeast Results in Enhanced Seed Production and Robustness in Larvae of the Freshwater Prawn Macrobrachium rosenbergii (De Man, 1879). Aquac. Int. 2024, 32, 8095–8113. [Google Scholar] [CrossRef]
- Abd El-Naby, A.S.; El Asely, A.M.; Hussein, M.N.; Khattaby, A.E.-R.A.; Sabry, E.A.; Abdelsalam, M.; Samir, F. Effects of Dietary Fermented Saccharomyces cerevisiae Extract (Hilyses) Supplementation on Growth, Hematology, Immunity, Antioxidants, and Intestinal Health in Nile Tilapia. Sci. Rep. 2024, 14, 12583. [Google Scholar] [CrossRef]
- Shehata, A.I.; Soliman, A.A.; Ahmed, H.A.; Gewaily, M.S.; Amer, A.A.; Shukry, M.; Abdel-Latif, H.M.R. Evaluation of Different Probiotics on Growth, Body Composition, Antioxidant Capacity, and Histoarchitecture of Mugil capito. Sci. Rep. 2024, 14, 7379. [Google Scholar] [CrossRef]
- Abdel-Latif, H.M.R.; Soliman, A.A.; Gewaily, M.S.; Amer, A.A.; Shukry, M.; Khalil, R.H.; Shehata, A.I. Dietary Effects of Saccharomyces cerevisiae and Allium sativum on Growth, Antioxidant Status, Hepatic and Intestinal Histoarchitecture, Expression of Growth- and Immune-Related Genes, and Resistance of Oreochromis niloticus to Aeromonas sobria. Fish Shellfish Immunol. 2024, 148, 109493. [Google Scholar] [CrossRef]
- McDonald, S.; Yazdi, Z.; Camus, A.; Soto, E. Evaluation of Three Inactive Vaccines Against Veronaea botryosa Infection in White Sturgeon (Acipenser transmontanus). Fish Shellfish Immunol. 2024, 145, 109368. [Google Scholar] [CrossRef]
- Nakanishi, A.; Fukunishi, H.; Matsumoto, R.; Eguchi, F. Development of a Prediction Method of Cell Density in Autotrophic/Heterotrophic Microorganism Mixtures by Machine Learning Using Absorbance Spectrum Data. BioTech 2022, 11, 46. [Google Scholar] [CrossRef]
- Yukgehnaish, K.; Kumar, P.; Sivachandran, P.; Marimuthu, K.; Arshad, A.; Paray, B.A.; Arockiaraj, J. Gut Microbiota Metagenomics in Aquaculture: Factors Influencing Gut Microbiome and Its Physiological Role in Fish. Rev. Aquac. 2020, 12, 1903–1927. [Google Scholar] [CrossRef]
- Palomba, E.; Tirelli, V.; De Alteriis, E.; Parascandola, P.; Landi, C.; Mazzoleni, S.; Sanchez, M. A Cytofluorimetric Analysis of a Saccharomyces cerevisiae Population Cultured in a Fed-Batch Bioreactor. PLoS ONE 2021, 16, e0248382. [Google Scholar] [CrossRef]
- Aria, M.; Cuccurullo, C. Bibliometrix: An R-Tool for Comprehensive Science Mapping Analysis. J. Informetr. 2017, 11, 959–975. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).