The Impact of Antibiotic Prophylaxis on Antibiotic Resistance, Clinical Outcomes, and Costs in Adult Hemato-Oncological and Surgical Patients: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Literature Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Data Analysis
3. Results
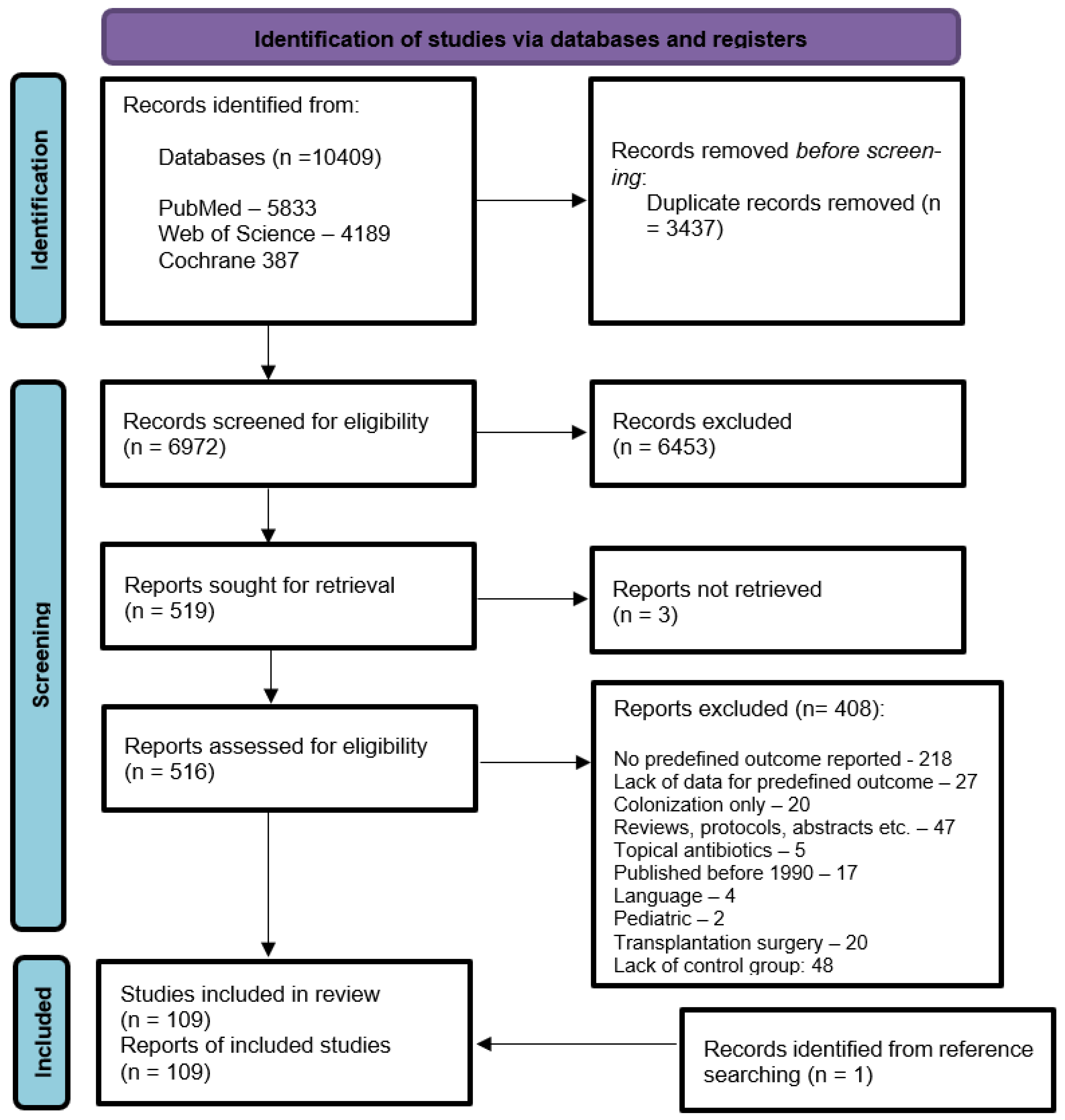
3.1. Study Selection and Study Characteristics
3.2. Impact of Antibiotic Prophylaxis Among Hemato-Oncological Patients
3.2.1. Risk of Infection
3.2.2. Antibiotic Resistance Rates Among Infections
3.2.3. Mortality
3.2.4. Length of Hospital Stay
3.2.5. Clinical Outcomes in Patients Colonized with Antibiotic-Resistant Bacteria Before Prophylaxis
3.2.6. Costs
3.3. Impact of Antibiotic Prophylaxis Among Surgical Patients
3.3.1. Risk of Infection
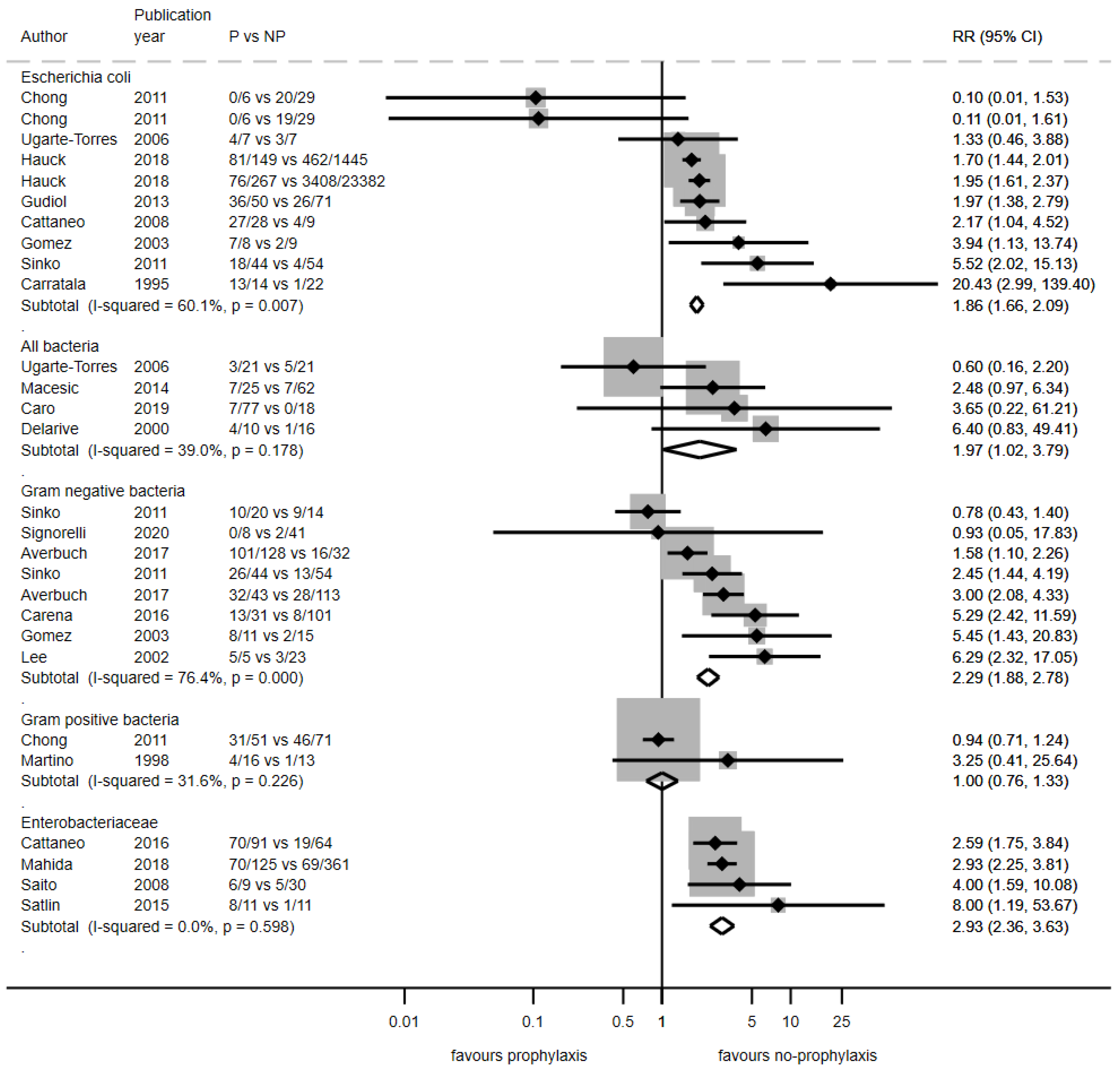
3.3.2. Antibiotic Resistance Rates Among Infections
3.3.3. Mortality
3.3.4. Extended Surgical Prophylaxis and Clinical Outcomes
3.3.5. Clinical Outcomes in Patients Colonized with Antibiotic-Resistant Bacteria Before Surgical Prophylaxis
3.3.6. Costs
3.4. Risk-of-Bias Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LOS | Length of Stay |
| AMR | Antimicrobial Resistance |
| GDP | Gross Domestic Product |
| ARB | Antibiotic-Resistant Bacteria |
| WMD | Weighted Mean Difference |
| FQRE | Fluoroquinolone-Resistant Enterobacterales |
| ESBL | Extended-Spectrum Beta-Lactamase |
| ESBL-PE | ESBL-Producing Enterobacterales |
| MRSA | Methicillin-Resistant Staphylococcus aureus |
| RR | Relative Risk |
| CI | Confidence Interval |
| SSI | Surgical-Site Infection |
| ASHP | American Society of Health-System Pharmacists |
| EAU | European Association of Urology |
| TRPB | Transrectal Prostate Biopsy |
| DGHO | Deutsche Gesellschaft für Hämatoonkologie/German Association of Hemato-Oncology |
| ASCO | American Society of Clinical Oncology |
| IDSA | Infectious Diseases Society of America |
References
- World Health Organization. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report: 2021; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef]
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Plachouras, D.; Karki, T.; Hansen, S.; Hopkins, S.; Lyytikainen, O.; Moro, M.L.; Reilly, J.; Zarb, P.; Zingg, W.; Kinross, P.; et al. Antimicrobial use in European acute care hospitals: Results from the second point prevalence survey (PPS) of healthcare-associated infections and antimicrobial use, 2016 to 2017. Eurosurveillance 2018, 23, 1800393. [Google Scholar] [CrossRef] [PubMed]
- Kolasiński, W. Surgical site infections—Review of current knowledge, methods of prevention. Pol. Przegl. Chir. 2018, 91, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.T. Wound infection after gastroduodenal operations: A 10-year review. Can. J. Surg. 1977, 20, 435–440. [Google Scholar] [PubMed]
- Eckmann, C.; Aghdassi, S.J.S.; Brinkmann, A.; Pletz, M.; Rademacher, J. Perioperative Antibiotic Prophylaxis: Indications and Modalities for the Prevention of Postoperative Wound Infection. Dtsch. Ärztebl. Int. 2024, 121, 233. [Google Scholar] [CrossRef]
- Stone, H.H.; Haney, B.B.; Kolb, L.D.; Geheber, C.E.; Hooper, C.A. Prophylactic and preventive antibiotic therapy: Timing, duration and economics. Ann. Surg. 1979, 189, 691–699. [Google Scholar] [CrossRef]
- Nayyar, R.; Kumar, S.; Collaborative Working Group on Use of Antibiotics in Endourology. Peri-operative antibiotic usage during endourological surgery: A multi-institutional, national-level, cross-sectional audit of prevalent practice pattern in India. Indian J. Urol. 2023, 39, 133–141. [Google Scholar] [CrossRef]
- Ierano, C.; Thursky, K.; Marshall, C.; Koning, S.; James, R.; Johnson, S.; Imam, N.; Worth, L.J.; Peel, T. Appropriateness of Surgical Antimicrobial Prophylaxis Practices in Australia. JAMA Netw. Open 2019, 2, e1915003. [Google Scholar] [CrossRef]
- Murri, R.; de Belvis, A.G.; Fantoni, M.; Tanzariello, M.; Parente, P.; Marventano, S.; Bucci, S.; Giovannenze, F.; Ricciardi, W.; Cauda, R.; et al. Impact of antibiotic stewardship on perioperative antimicrobial prophylaxis. Int. J. Qual. Health Care 2016, 28, 502–507. [Google Scholar] [CrossRef]
- Karamchandani, K.; Barden, K.; Prozesky, J. Adherence to surgical antimicrobial prophylaxis: “checking-the-box” is not enough. Int. J. Health Care Qual. Assur. 2019, 32, 470–473. [Google Scholar] [CrossRef] [PubMed]
- Mohamed Rizvi, Z.; Palasanthiran, P.; Wu, C.; Mostaghim, M.; McMullan, B. Adherence to surgical antibiotic prophylaxis guidelines in children: A cohort study. J. Paediatr. Child Health 2020, 56, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Prunty, M.; Rhodes, S.; Rivero, M.J.; Callegari, M.; Jesse, E.; Arenas-Gallo, C.; Brant, A.; Calaway, A.; Scherr, D.; Shoag, J.E. National Adherence to Guidelines for Antimicrobial Prophylaxis for Patients Undergoing Radical Cystectomy. J. Urol. 2023, 209, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Jung, N.; Tometten, L.; Draenert, R. Choosing Wisely internationally—Helpful recommendations for antimicrobial stewardship! Infection 2023, 51, 567–581. [Google Scholar] [CrossRef]
- Taplitz, R.A.; Kennedy, E.B.; Bow, E.J.; Crews, J.; Gleason, C.; Hawley, D.K.; Langston, A.A.; Nastoupil, L.J.; Rajotte, M.; Rolston, K.V.; et al. Antimicrobial Prophylaxis for Adult Patients With Cancer-Related Immunosuppression: ASCO and IDSA Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 3043–3054. [Google Scholar] [CrossRef]
- Gandra, S.; Trett, A.; Alvarez-Uria, G.; Solomkin, J.S.; Laxminarayan, R. Is the efficacy of antibiotic prophylaxis for surgical procedures decreasing? Systematic review and meta-analysis of randomized control trials. Infect. Control Hosp. Epidemiol. 2019, 40, 133–141. [Google Scholar] [CrossRef]
- Gafter-Gvili, A.; Fraser, A.; Paul, M.; Vidal, L.; Lawrie, T.A.; van de Wetering, M.D.; Kremer, L.C.; Leibovici, L. Antibiotic prophylaxis for bacterial infections in afebrile neutropenic patients following chemotherapy. Cochrane Database Syst. Rev. 2012, 1, Cd004386. [Google Scholar] [CrossRef]
- Kimura, S.; Akahoshi, Y.; Nakano, H.; Ugai, T.; Wada, H.; Yamasaki, R.; Ishihara, Y.; Kawamura, K.; Sakamoto, K.; Ashizawa, M.; et al. Antibiotic prophylaxis in hematopoietic stem cell transplantation. A meta-analysis of randomized controlled trials. J. Infect. 2014, 69, 13–25. [Google Scholar] [CrossRef]
- Mikulska, M.; Averbuch, D.; Tissot, F.; Cordonnier, C.; Akova, M.; Calandra, T.; Ceppi, M.; Bruzzi, P.; Viscoli, C. Fluoroquinolone prophylaxis in haematological cancer patients with neutropenia: ECIL critical appraisal of previous guidelines. J. Infect. 2018, 76, 20–37. [Google Scholar] [CrossRef]
- Righi, E.; Scudeller, L.; Mirandola, M.; Visentin, A.; Mutters, N.T.; Meroi, M.; Schwabe, A.; Erbogasto, A.; Vantini, G.; Cross, E.L.A.; et al. Colonisation with Extended-Spectrum Cephalosporin-Resistant Enterobacterales and Infection Risk in Surgical Patients: A Systematic Review and Meta-analysis. Infect. Dis. Ther. 2023, 12, 623–636. [Google Scholar] [CrossRef]
- Egan, G.; Robinson, P.D.; Martinez, J.P.D.; Alexander, S.; Ammann, R.A.; Dupuis, L.L.; Fisher, B.T.; Lehrnbecher, T.; Phillips, B.; Cabral, S.; et al. Efficacy of antibiotic prophylaxis in patients with cancer and hematopoietic stem cell transplantation recipients: A systematic review of randomized trials. Cancer Med. 2019, 8, 4536–4546. [Google Scholar] [CrossRef] [PubMed]
- Rink, M.; Beryl, P.; Tacconelli, E.; Bitzer, M.; Göpel, S. Secondary Burden of Antibiotic Resistance: Prophylactic Antibiotic Usage, Antibiotic Resistance and Clinical Outcomes—A Systematic Review and Meta-Analysis. PROSPERO: International Prospective Register of Systematic Reviews. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021267613 (accessed on 31 March 2025).
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef] [PubMed]
- StataCorp. Stata Statistical Software: Release 15; StataCorp LLC: College Station, TX, USA, 2017. [Google Scholar]
- JBI. JBI Critical Appraisal Tools. Available online: https://jbi.global/critical-appraisal-tools (accessed on 22 April 2025).
- Adibi, M.; Hornberger, B.; Bhat, D.; Raj, G.; Roehrborn, C.G.; Lotan, Y. Reduction in Hospital Admission Rates Due to Post-Prostate Biopsy Infections After Augmenting Standard Antibiotic Prophylaxis. J. Urol. 2013, 189, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Akhmedov, M.; Klyasova, G.; Kuzmina, L.; Fedorova, A.; Drokov, M.; Parovichnikova, E. Impact of fluoroquinolone administration and gut mucosal colonization on the risk of pre-engraftment bloodstream infections after allogeneic hematopoietic cell transplantation. Leuk. Lymphoma 2023, 64, 1102–1111. [Google Scholar] [CrossRef]
- Amelot, A.; Riche, M.; Latreille, S.; Degos, V.; Carpentier, A.; Mathon, B.; Korinek, A.M. Antimicrobial prophylaxis in noninstrumented spine surgery: A prospective study to determine efficacy and drawbacks. J. Neurosurg. Spine 2021, 35, 366–375. [Google Scholar] [CrossRef]
- Antsupova, V.; Norgaard, N.; Bisbjerg, R.; Jensen, J.N.; Boel, J.; Jarlov, J.O.; Arpi, M. Antibiotic prophylaxis for transrectal prostate biopsy-a new strategy. J. Antimicrob. Chemother. 2014, 69, 3372–3378. [Google Scholar] [CrossRef]
- Averbuch, D.; Tridello, G.; Hoek, J.; Mikulska, M.; Akan, H.; San Segundo, L.Y.; Pabst, T.; Ozcelik, T.; Klyasova, G.; Donnini, I.; et al. Antimicrobial Resistance in Gram-Negative Rods Causing Bacteremia in Hematopoietic Stem Cell Transplant Recipients: Intercontinental Prospective Study of the Infectious Diseases Working Party of the European Bone Marrow Transplantation Group. Clin. Infect. Dis. 2017, 65, 1819–1828. [Google Scholar] [CrossRef]
- Bains, S.S.; Dubin, J.A.; Hameed, D.; Chen, Z.; Moore, M.C.; Shrestha, A.; Nace, J.; Delanois, R.E. Addition of vancomycin to cefazolin is often unnecessary for preoperative antibiotic prophylaxis during total joint arthroplasties. Arthroplasty 2024, 6, 20. [Google Scholar] [CrossRef]
- Bartella, A.K.; Kamal, M.; Teichmann, J.; Kloss-Brandstatter, A.; Steiner, T.; Holzle, F.; Lethaus, B. Prospective comparison of perioperative antibiotic management protocols in oncological head and neck surgery. J. Cranio-Maxillofac. Surg. 2017, 45, 1078–1082. [Google Scholar] [CrossRef]
- Bartella, A.K.; Lemmen, S.; Burnic, A.; Kloss-Brandstatter, A.; Kamal, M.; Breisach, T.; Holzle, F.; Lethaus, B. Influence of a strictly perioperative antibiotic prophylaxis vs a prolonged postoperative prophylaxis on surgical site infections in maxillofacial surgery. Infection 2018, 46, 225–230. [Google Scholar] [CrossRef]
- Cammann, S.; Timrott, K.; Vonberg, R.P.; Vondran, F.W.R.; Schrem, H.; Suerbaum, S.; Klempnauer, J.; Bektas, H.; Kleine, M. Cholangitis in the postoperative course after biliodigestive anastomosis. Langenbecks Arch. Surg. 2016, 401, 715–724. [Google Scholar] [CrossRef]
- Cao, Y.; Pu, K.; Li, G.; Yan, X.; Ma, Y.; Xue, K.; Sun, Z.; Li, Q. The Role of Antibiotic Prophylaxis in Clean Neurosurgery. World Neurosurg. 2017, 100, 305–310. [Google Scholar] [CrossRef]
- Carena, A.A.; Jorge, L.; Bonvehi, P.; Temporiti, E.; Zarate, M.S.; Herrera, F. Levofloxacin prophylaxis in neutropenic patients. Medicina 2016, 76, 295–303. [Google Scholar]
- Caro, J.; Madero-Marroquin, R.; Zubizarreta, N.; Moshier, E.; Tremblay, D.; Coltoff, A.; Lancman, G.; Fuller, R.; Rana, M.; Mascarenhas, J.; et al. Impact of Fluoroquinolone Prophylaxis on Neutropenic Fever, Infections, and Antimicrobial Resistance in Newly Diagnosed AML Patients. Clin. Lymphoma Myeloma Leuk. 2022, 22, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Caro, J.; Moshier, E.; Tremblay, D.; Coltoff, A.; Lancman, G.; Rana, M.; Mascarenhas, J.; Jacobs, S. Impact of Primary Antibacterial Prophylaxis on Neutropenic Fever, Infections, and Antimicrobial Resistance in Newly Diagnosed AML Patients. Blood 2019, 134, 3849. [Google Scholar] [CrossRef]
- Carratalá, J.; Fernández-Sevilla, A.; Tubau, F.; Callis, M.; Gudiol, F. Emergence of quinolone-resistant Escherichia coli bacteremia in neutropenic patients with cancer who have received prophylactic norfloxacin. Clin. Infect. Dis. 1995, 20, 557–560; discussion 561–563. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, C.; Quaresmini, G.; Casari, S.; Capucci, M.A.; Micheletti, M.; Borlenghi, E.; Signorini, L.; Re, A.; Carosi, G.; Rossi, G. Recent changes in bacterial epidemiology and the emergence of fluoroquinolone-resistant Escherichia coli among patients with haematological malignancies: Results of a prospective study on 823 patients at a single institution. J. Antimicrob. Chemother. 2008, 61, 721–728. [Google Scholar] [CrossRef]
- Cattaneo, C.; Zappasodi, P.; Mancini, V.; Annaloro, C.; Pavesi, F.; Skert, C.; Ferrario, A.; Todisco, E.; Sacca, V.; Verga, L.; et al. Emerging resistant bacteria strains in bloodstream infections of acute leukaemia patients: Results of a prospective study by the Rete Ematologica Lombarda (Rel). Ann. Hematol. 2016, 95, 1955–1963. [Google Scholar] [CrossRef]
- Chong, Y.; Yakushiji, H.; Ito, Y.; Kamimura, T. Clinical impact of fluoroquinolone prophylaxis in neutropenic patients with hematological malignancies. Int. J. Infect. Dis. 2011, 15, E277–E281. [Google Scholar] [CrossRef]
- Clerici, D.; Galli, L.; Greco, R.; Lugli, A.P.; Erbella, F.; Ripa, M.; Din, C.T.; Nitti, R.; Giglio, F.; Mastaglio, S.; et al. Levofloxacin prophylaxis vs no prophylaxis in patients with neutropenia within an endemic country for carbapenem-resistant GNB. Blood Adv. 2023, 7, 1621–1634. [Google Scholar] [CrossRef]
- Cohen, M.E.; Salmasian, H.; Li, J.; Liu, J.; Zachariah, P.; Wright, J.D.; Freedberg, D.E. Surgical Antibiotic Prophylaxis and Risk for Postoperative Antibiotic-Resistant Infections. J. Am. Coll. Surg. 2017, 225, 631–638.e633. [Google Scholar] [CrossRef]
- Craig, M.; Cumpston, A.D.; Hobbs, G.R.; DeVetten, M.P.; Sarwari, A.R.; Ericson, S.G. The clinical impact of antibacterial prophylaxis and cycling antibiotics for febrile neutropenia in a hematological malignancy and transplantation unit. Bone Marrow Transpl. 2007, 39, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Cumpston, A.; Craig, M.; Hamadani, M.; Abraham, J.; Hobbs, G.R.; Sarwari, A.R. Extended follow-up of an antibiotic cycling program for the management of febrile neutropenia in a hematologic malignancy and hematopoietic cell transplantation unit. Transpl. Infect. Dis. 2013, 15, 142–149. [Google Scholar] [CrossRef] [PubMed]
- D’Antonio, D.; Piccolomini, R.; Iacone, A.; Fioritoni, G.; Parruti, G.; Betti, S.; Quaglietta, A.M.; Accorsi, P.; Dell’Isola, M.; Favalli, C. Comparison of ciprofloxacin, ofloxacin and pefloxacin for the prevention of the bacterial infection in neutropenic patients with haematological malignancies. J. Antimicrob. Chemother. 1994, 33, 837–844. [Google Scholar] [CrossRef] [PubMed]
- De Pastena, M.; Paiella, S.; Azzini, A.M.; Zaffagnini, A.; Scarlini, L.; Montagnini, G.; Maruccio, M.; Filippini, C.; Romeo, F.; Mazzariol, A.; et al. Antibiotic Prophylaxis with Piperacillin-Tazobactam Reduces Post-Operative Infectious Complication after Pancreatic Surgery: An Interventional, Non-Randomized Study. Surg. Infect. 2021, 22, 536–542. [Google Scholar] [CrossRef]
- Delarive, P.; Baumgartner, J.D.; Glauser, M.P.; Cometta, A. Evaluation of antibiotic prophylaxis in neutropenic patients with haematological malignancies. Schweiz. Med. Wochenschr. 2000, 130, 1837–1844. [Google Scholar]
- Dubinsky-Pertzov, B.; Temkin, E.; Harbarth, S.; Fankhauser-Rodriguez, C.; Carevic, B.; Radovanovic, I.; Ris, F.; Kariv, Y.; Buchs, N.C.; Schiffer, E.; et al. Carriage of Extended-spectrum Beta-lactamase-producing Enterobacteriaceae and the Risk of Surgical Site Infection After Colorectal Surgery: A Prospective Cohort Study. Clin. Infect. Dis. 2019, 68, 1699–1704. [Google Scholar] [CrossRef]
- Engels, E.A.; Ellis, C.A.; Supran, S.E.; Schmid, C.H.; Barza, M.; Schenkein, D.P.; Koc, Y.; Miller, K.B.; Wong, J.B. Early infection in bone marrow transplantation: Quantitative study of clinical factors that affect risk. Clin. Infect. Dis. 1999, 28, 256–266. [Google Scholar] [CrossRef]
- Fahmy, A.M.; Kotb, A.; Youssif, T.A.; Abdeldiam, H.; Algebaly, O.; Elabbady, A. Fosfomycin antimicrobial prophylaxis for transrectal ultrasound-guided biopsy of the prostate: A prospective randomised study. Arab. J. Urol. 2016, 14, 228–233. [Google Scholar] [CrossRef]
- Finkelstein, R.; Rabino, G.; Mashiah, T.; Bar-El, Y.; Adler, Z.; Kertzman, V.; Cohen, O.; Milo, S. Vancomycin versus cefazolin prophylaxis for cardiac surgery in the setting of a high prevalence of methicillin-resistant staphylococcal infections. J. Thorac. Cardiovasc. Surg. 2002, 123, 326–332. [Google Scholar] [CrossRef]
- Fong, Z.V.; McMillan, M.T.; Marchegiani, G.; Sahora, K.; Malleo, G.; De Pastena, M.; Loehrer, A.P.; Lee, G.C.; Ferrone, C.R.; Chang, D.C.; et al. Discordance Between Perioperative Antibiotic Prophylaxis and Wound Infection Cultures in Patients Undergoing Pancreaticoduodenectomy. JAMA Surg. 2016, 151, 432–439. [Google Scholar] [CrossRef]
- Ganti, B.R.; Marini, B.L.; Nagel, J.; Bixby, D.; Perissinotti, A.J. Impact of antibacterial prophylaxis during reinduction chemotherapy for relapse/refractory acute myeloid leukemia. Support. Care Cancer 2017, 25, 541–547. [Google Scholar] [CrossRef]
- Garnica, M.; Nouér, S.A.; Pellegrino, F.L.; Moreira, B.M.; Maiolino, A.; Nucci, M. Ciprofloxacin prophylaxis in high risk neutropenic patients: Effects on outcomes, antimicrobial therapy and resistance. BMC Infect. Dis. 2013, 13, 356. [Google Scholar] [CrossRef]
- Gentilotti, E.; De Nardo, P.; Nguhuni, B.; Piscini, A.; Damian, C.; Vairo, F.; Chaula, Z.; Mencarini, P.; Torokaa, P.; Zumla, A.; et al. Implementing a combined infection prevention and control with antimicrobial stewardship joint program to prevent caesarean section surgical site infections and antimicrobial resistance: A Tanzanian tertiary hospital experience. Antimicrob. Resist. Infect. Control. 2020, 9, 69. [Google Scholar] [CrossRef]
- Goldstein, E.J.; Citron, D.M.; Merriam, C.V.; Abramson, M.A. Infection after elective colorectal surgery: Bacteriological analysis of failures in a randomized trial of cefotetan vs. ertapenem prophylaxis. Surg. Infect. 2009, 10, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Gomez, L.; Garau, J.; Estrada, C.; Marquez, M.; Dalmau, D.; Xercavins, M.; Marti, J.M.; Estany, C. Ciprofloxacin prophylaxis in patients with acute leukemia and granulocytopenia in an area with a high prevalence of ciprofloxacin-resistant Escherichial coli. Cancer 2003, 97, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Guare, E.G.; Hale, C.M.; Sivik, J.; Lehman, E.; Inoue, Y.; Rakszawski, K.; Songdej, N.; Nickolich, M.; Zheng, H.; Naik, S.; et al. The addition of doxycycline to fluoroquinolones for bacterial prophylaxis in autologous stem cell transplantation for multiple myeloma. Transpl. Infect. Dis. 2024, 26, e14241. [Google Scholar] [CrossRef] [PubMed]
- Gudiol, C.; Bodro, M.; Simonetti, A.; Tubau, F.; Gonzalez-Barca, E.; Cisnal, M.; Domingo-Domenech, E.; Jimenez, L.; Carratala, J. Changing aetiology, clinical features, antimicrobial resistance, and outcomes of bloodstream infection in neutropenic cancer patients. Clin. Microbiol. Infect. 2013, 19, 474–479. [Google Scholar] [CrossRef]
- Guimaraes, T.; Borges, I.C.; Spadao, F.D.; Mariano, L.; Nascimento, M.D.; Higashino, H.; Rossi, F.; Rocha, V.; Costa, S.F. Impact of Discontinuing Levofloxacin Prophylaxis on Bloodstream Infections in Neutropenic Hematopoietic Stem Cell Transplantation Patients. Antibiotics 2022, 11, 1269. [Google Scholar] [CrossRef]
- Guiot, H.F.; van der Meer, J.W.; van den Broek, P.J.; Willemze, R.; van Furth, R. Prevention of viridans-group streptococcal septicemia in oncohematologic patients: A controlled comparative study on the effect of penicillin G and cotrimoxazole. Ann. Hematol. 1992, 64, 260–265. [Google Scholar] [CrossRef]
- Haga, N.; Ishida, H.; Ishiguro, T.; Kumamoto, K.; Ishibashi, K.; Tsuji, Y.; Miyazaki, T. A prospective randomized study to assess the optimal duration of intravenous antimicrobial prophylaxis in elective gastric cancer surgery. Int. Surg. 2012, 97, 169–176. [Google Scholar] [CrossRef]
- Hakki, M.; Humphries, R.M.; Hemarajata, P.; Tallman, G.B.; Shields, R.K.; Mettus, R.T.; Doi, Y.; Lewis, J.S. Fluoroquinolone Prophylaxis Selects for Meropenem-nonsusceptible Pseudomonas aeruginosa in Patients With Hematologic Malignancies and Hematopoietic Cell Transplant Recipients. Clin. Infect. Dis. 2019, 68, 2045–2052. [Google Scholar] [CrossRef] [PubMed]
- Harbarth, S.; Samore, M.H.; Lichtenberg, D.; Carmeli, Y. Prolonged antibiotic prophylaxis after cardiovascular surgery and its effect on surgical site infections and antimicrobial resistance. Circulation 2000, 101, 2916–2921. [Google Scholar] [CrossRef] [PubMed]
- Hauck, C.G.; Chong, P.P.; Miller, M.B.; Jamieson, K.; Fine, J.P.; Foster, M.C.; Shea, T.C.; van Duin, D. Increasing Rates of Fluoroquinolone Resistance in Escherichia coli Isolated From the Blood and Urine of Patients with Hematologic Malignancies and Stem Cell Transplant Recipients. Pathog. Immun. 2016, 1, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Higaki, E.; Abe, T.; Fujieda, H.; Hosoi, T.; Nagao, T.; Komori, K.; Ito, S.; Itoh, N.; Matsuo, K.; Shimizu, Y. Significance of Antimicrobial Prophylaxis for the Prevention of Early-Onset Pneumonia After Radical Esophageal Cancer Resection: A Retrospective Analysis of 356 Patients Undergoing Thoracoscopic Esophagectomy. Ann. Surg. Oncol. 2022, 29, 1374–1387. [Google Scholar] [CrossRef]
- Ho, H.S.S.; Ng, L.G.; Tan, Y.H.; Yeo, M.; Cheng, C.W.S. Intramuscular Gentamicin Improves the Efficacy of Ciprofloxacin as an Antibiotic Prophylaxis for Transrectal Prostate Biopsy. Ann. Acad. Med. Singap. 2009, 38, 212–216. [Google Scholar] [CrossRef]
- Hsueh, P.R.; Cheng, H.J.; Tang, J.L.; Yao, M.; Tien, H.F. Prophylactic use of moxifloxacin in patients receiving bone marrow transplants was not associated with increased ciprofloxacin resistance in Escherichia coli and enterococci. Clin. Infect. Dis. 2005, 40, 1862–1864. [Google Scholar] [CrossRef]
- Ishibashi, K.; Kuwabara, K.; Ishiguro, T.; Ohsawa, T.; Okada, N.; Miyazaki, T.; Yokoyama, M.; Ishida, H. Short-term intravenous antimicrobial prophylaxis in combination with preoperative oral antibiotics on surgical site infection and methicillin-resistant Staphylococcus aureus infection in elective colon cancer surgery: Results of a prospective randomized trial. Surg. Today 2009, 39, 1032–1039. [Google Scholar] [CrossRef]
- Ishida, H.; Yokoyama, M.; Nakada, H.; Inokuma, S.; Hashimoto, D. Impact of oral antimicrobial prophylaxis on surgical site infection and methicillin-resistant Staphylococcus aureus infection after elective colorectal surgery. Results of a prospective randomized trial. Surg. Today 2001, 31, 979–983. [Google Scholar] [CrossRef]
- Itani, K.M.; Wilson, S.E.; Awad, S.S.; Jensen, E.H.; Finn, T.S.; Abramson, M.A. Ertapenem versus cefotetan prophylaxis in elective colorectal surgery. N. Engl. J. Med. 2006, 355, 2640–2651. [Google Scholar] [CrossRef]
- Kalkanli, A.; Gezmis, C.T.; Ozkan, A.; Cilesiz, N.C.; Yanaral, F.; Aydin, M.; Tandogdu, Z. Comparison of Single and Prolonged Fluoroquinolone Prophylaxis and Risk Factors for Infectious Complications After Transrectal Prostate Biopsy. Balk. Med. J. 2018, 35, 373–377. [Google Scholar] [CrossRef]
- Kanayama, M.; Hashimoto, T.; Shigenobu, K.; Oha, F.; Togawa, D. Effective prevention of surgical site infection using a Centers for Disease Control and Prevention guideline-based antimicrobial prophylaxis in lumbar spine surgery. J. Neurosurg. Spine 2007, 6, 327–329. [Google Scholar] [CrossRef]
- Kern, W.V.; Klose, K.; Jellen-Ritter, A.S.; Oethinger, M.; Bohnert, J.; Kern, P.; Reuter, S.; von Baum, H.; Marre, R. Fluoroquinolone resistance of Escherichia coli at a cancer center: Epidemiologic evolution and effects of discontinuing prophylactic fluoroquinolone use in neutropenic patients with leukemia. Eur. J. Clin. Microbiol. Infect. Dis. 2005, 24, 111–118. [Google Scholar] [CrossRef]
- Kern, W.V.; Weber, S.; Dettenkofer, M.; Kaier, K.; Bertz, H.; Behnke, M.; Weisser, M.; Götting, T.; Widmer, A.F.; Theilacker, C. Impact of fluoroquinolone prophylaxis during neutropenia on bloodstream infection: Data from a surveillance program in 8755 patients receiving high-dose chemotherapy for haematologic malignancies between 2009 and 2014. J. Infect. 2018, 77, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Khawcharoenporn, T.; Kanoktipakorn, P. Effectiveness of appropriate antibiotic prophylaxis for transurethral resection of the prostate in the era of antibiotic resistance. Infect. Control Hosp. Epidemiol. 2022, 43, 1693–1697. [Google Scholar] [CrossRef] [PubMed]
- Korinek, A.M.; Baugnon, T.; Golmard, J.L.; van Effenterre, R.; Coriat, P.; Puybasset, L. Risk factors for adult nosocomial meningitis after craniotomy: Role of antibiotic prophylaxis. Neurosurgery 2006, 59, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Kuo, F.C.; Chang, Y.H.; Huang, T.W.; Chen, D.W.; Tan, T.L.; Lee, M.S. Post-operative prophylactic antibiotics in aseptic revision hip and knee arthroplasty: A propensity score matching analysis. Sci. Rep. 2022, 12, 18319. [Google Scholar] [CrossRef]
- Kusachi, S.; Sumiyama, Y.; Nagao, J.; Arima, Y.; Yoshida, Y.; Tanaka, H.; Nakamura, Y.; Saida, Y.; Watanabe, M.; Watanabe, R.; et al. Prophylactic antibiotics given within 24 hours of surgery, compared with antibiotics given for 72 hours perioperatively, increased the rate of methicillin-resistant Staphylococcus aureus isolated from surgical site infections. J. Infect. Chemother. 2008, 14, 44–50. [Google Scholar] [CrossRef]
- Lee, D.G.; Choi, S.M.; Choi, J.H.; Yoo, J.H.; Park, Y.H.; Kim, Y.J.; Lee, S.; Min, C.K.; Kim, H.J.; Kim, D.W.; et al. Selective bowel decontamination for the prevention of infection in acute myelogenous leukemia: A prospective randomized trial. Korean J. Intern. Med. 2002, 17, 38–44. [Google Scholar] [CrossRef]
- Lee, J.K.; Lee, S.; Hong, S.K.; Byun, S.S.; Lee, S.E. Clinical importance of the antibiotic regimen in transrectal ultrasound-guided biopsy: Quinolone versus cephalosporin. BMC Urol. 2016, 16, 51. [Google Scholar] [CrossRef]
- Lee, S.S.F.; Fulford, A.E.; Quinn, M.A.; Seabrook, J.; Rajakumar, I. Levofloxacin for febrile neutropenia prophylaxis in acute myeloid leukemia patients associated with reduction in hospital admissions. Support. Care Cancer 2018, 26, 1499–1504. [Google Scholar] [CrossRef]
- Lista, F.; Redondo, C.; Meilán, E.; García-Tello, A.; de Fata, F.R.; Angulo, J.C. Efficacy and safety of Fosfomycin-trometamol in the prophylaxis for transrectal prostate biopsy. Prospective randomized comparison with ciprofloxacin. Actas Urol. Esp. 2014, 38, 391–396. [Google Scholar] [CrossRef]
- Liu, C.; Kakis, A.; Nichols, A.; Ries, M.D.; Vail, T.P.; Bozic, K.J. Targeted Use of Vancomycin as Perioperative Prophylaxis Reduces Periprosthetic Joint Infection in Revision TKA. Clin. Orthop. Relat. Res. 2014, 472, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Longtin, Y.; Gervais, P.; Birnie, D.H.; Wang, J.; Alings, M.; Philippon, F.; Parkash, R.; Manlucu, J.; Angaran, P.; Rinne, C.; et al. Impact of Choice of Prophylaxis on the Microbiology of Cardiac Implantable Electronic Device Infections: Insights From the Prevention of Arrhythmia Device Infection Trial (PADIT). Open Forum Infect. Dis. 2021, 8, ofab513. [Google Scholar] [CrossRef] [PubMed]
- Lopez, W.Y.; Rider, S.M.; Nwosu, K.; Kazarian, E.R.; Blucher, J.A.; Schoenfeld, E.M.; Simpson, A.K.; Kang, J.D.; Schoenfeld, A.J. The Impact of Vancomycin and Cefazolin as Standard Preoperative Antibiotic Prophylaxis on Surgical Site Infections Following Instrumented Spinal Fusion. Spine 2019, 44, E366–E371. [Google Scholar] [CrossRef] [PubMed]
- Macesic, N.; Morrissey, C.O.; Cheng, A.C.; Spencer, A.; Peleg, A.Y. Changing microbial epidemiology in hematopoietic stem cell transplant recipients: Increasing resistance over a 9-year period. Transpl. Infect. Dis. 2014, 16, 887–896. [Google Scholar] [CrossRef]
- Mahida, N.; Boswell, T. Fluoroquinolone prophylaxis in haematopoietic bone marrow transplantation: A driver for antimicrobial resistance. J. Hosp. Infect. 2018, 98, 241–242. [Google Scholar] [CrossRef]
- Manecksha, R.P.; Nason, G.J.; Cullen, I.M.; Fennell, J.P.; McEvoy, E.; McDermott, T.; Flynn, R.J.; Grainger, R.; Thornhill, J.A. Prospective study of antibiotic prophylaxis for prostate biopsy involving >1100 men. Sci. World J. 2012, 2012, 650858. [Google Scholar] [CrossRef]
- Marigi, I.M.; Yu, K.; Nieboer, M.; Marigi, E.M.; Sperling, J.W.; Sanchez-Sotelo, J.; Barlow, J.D. After Primary Shoulder Arthroplasty Appropriate Vancomycin Antibiotic Prophylaxis Does Not Lead to Increased Infectious Complications When Compared to Cefazolin. J. Shoulder Elb. Surg. 2024, 33, 2612–2618. [Google Scholar] [CrossRef]
- Martino, R.; Subira, M.; Altes, A.; Lopez, R.; Sureda, A.; Domingo-Albos, A.; Pericas, R.; Brunet, S. Effect of discontinuing prophylaxis with norfloxacin in patients with hematologic malignancies and severe neutropenia—A matched case-control study of the effect on infectious morbidity. Acta Haematol. 1998, 99, 206–211. [Google Scholar] [CrossRef]
- Mathur, P.; Trikha, V.; Farooque, K.; Sharma, V.; Jain, N.; Bhardwaj, N.; Sharma, S.; Misra, M.C. Implementation of a short course of prophylactic antibiotic treatment for prevention of postoperative infections in clean orthopaedic surgeries. Indian J. Med. Res. 2013, 137, 111–116. [Google Scholar] [PubMed]
- McCullough, M.C.; Chu, C.K.; Duggal, C.S.; Losken, A.; Carlson, G.W. Antibiotic Prophylaxis and Resistance in Surgical Site Infection After Immediate Tissue Expander Reconstruction of the Breast. Ann. Plast. Surg. 2016, 77, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Merrer, J.; Desbouchages, L.; Serazin, V.; Razafimamonjy, J.; Pauthier, F.; Leneveu, M. Comparison of routine prophylaxis with vancomycin or cefazolin for femoral neck fracture surgery: Microbiological and clinical outcomes. Infect. Control Hosp. Epidemiol. 2006, 27, 1366–1371. [Google Scholar] [CrossRef] [PubMed]
- Mestrallet, P.; Yanni, A.; Roman, A.; Rodriguez, A.; Bouland, C.; Javadian, R.; Konopnicki, D.; Dequanter, D. Antibiotic use in patients undergoing complex clean-contaminated head and neck surgery: A prospective study. J. Int. Soc. Prev. Community Dent. 2023, 13, 349–355. [Google Scholar] [CrossRef]
- Mohri, Y.; Tonouchi, H.; Kobayashi, M.; Nakai, K.; Kusunoki, M.; Mie Surgical Infection Research, G. Randomized clinical trial of single- versus multiple-dose antimicrobial prophylaxis in gastric cancer surgery. Br. J. Surg. 2007, 94, 683–688. [Google Scholar] [CrossRef]
- Munoz, L.; Martino, R.; Subira, M.; Brunet, S.; Sureda, A.; Sierra, J. Intensified prophylaxis of febrile neutropenia with ofloxacin plus rifampin during severe short-duration neutropenia in patients with lymphoma. Leuk. Lymphoma 1999, 34, 585–589. [Google Scholar] [CrossRef]
- Newman, T.H.; Stroman, L.; Hadjipavlou, M.; Haque, A.; Rusere, J.; Chan, K.; Ioannides, D.; Di Benedetto, A.; Pinczes, T.; Popert, R.; et al. EXIT from TRansrectal prostate biopsies (TREXIT): Sepsis rates of transrectal biopsy with rectal swab culture guided antimicrobials versus freehand transperineal biopsy. Prostate Cancer Prostatic Dis. 2022, 25, 283–287. [Google Scholar] [CrossRef]
- Nguyen, A.V.; Coggins, W.S.; Jain, R.R.; Branch, D.W.; Allison, R.Z.; Maynard, K.; Oliver, B.; Lall, R.R. Cefazolin versus vancomycin for neurosurgical operative prophylaxis—A single institution retrospective cohort study. Clin. Neurol. Neurosurg. 2019, 182, 152–157. [Google Scholar] [CrossRef]
- Nucci, M.; Pulcheri, W.; Spector, N.; Bueno, A.; Silveira, S.; Marangoni, D.; Ferreira, R.; Deoliveira, H. Quinolone prophylaxis in neutropenic patients—Efficacy versus resistance. Oncol. Rep. 1994, 1, 1101–1105. [Google Scholar] [CrossRef]
- Nutman, A.; Temkin, E.; Harbarth, S.; Carevic, B.; Ris, F.; Fankhauser-Rodriguez, C.; Radovanovic, I.; Dubinsky-Pertzov, B.; Cohen-Percia, S.; Kariv, Y.; et al. Personalized Ertapenem Prophylaxis for Carriers of Extended-spectrum β-Lactamase-producing Enterobacteriaceae Undergoing Colorectal Surgery. Clin. Infect. Dis. 2020, 70, 1891–1897. [Google Scholar] [CrossRef]
- Ongun, S.; Aslan, G.; Avkan-Oguz, V. The effectiveness of single-dose fosfomycin as antimicrobial prophylaxis for patients undergoing transrectal ultrasound-guided biopsy of the prostate. Urol. Int. 2012, 89, 439–444. [Google Scholar] [CrossRef]
- Pace, G.; Carmignani, L.; Marenghi, C.; Mombelli, G.; Bozzini, G. Cephalosporins periprostatic injection: Are really effective on infections following prostate biopsy? Int. Urol. Nephrol. 2012, 44, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- Patrick, S.; James, C.; Ali, A.; Lawson, S.; Mary, E.; Modak, A. Vascular surgical antibiotic prophylaxis study (VSAPS). Vasc. Endovasc. Surg. 2010, 44, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Peel, T.N.; Astbury, S.; Cheng, A.C.; Paterson, D.L.; Buising, K.L.; Spelman, T.; An, T.D.; Adie, S.; Boyce, G.; McDougall, C.; et al. Trial of Vancomycin and Cefazolin as Surgical Prophylaxis in Arthroplasty. N. Engl. J. Med. 2023, 389, 1488–1498. [Google Scholar] [CrossRef] [PubMed]
- Prentice, H.G.; Hann, I.M.; Nazareth, B.; Paterson, P.; Bhamra, A.; Kibbler, C.C. Oral ciprofloxacin plus colistin: Prophylaxis against bacterial infection in neutropenic patients. A strategy for the prevention of emergence of antimicrobial resistance. Br. J. Haematol. 2001, 115, 46–52. [Google Scholar] [CrossRef]
- Qin, T.J.; Mi, Y.C.; Feng, S.Z.; Li, D.P.; Wei, J.L.; Yang, D.L.; Han, M.Z.; Wang, J.X.; Bian, S.G. Clinical study on fluoroquinolone prophylaxis in neutropenia patients with acute leukemia. Zhonghua Yi Xue Za Zhi 2007, 87, 1389–1393. [Google Scholar]
- Rubinstein, E.; Findler, G.; Amit, P.; Shaked, I. Perioperative prophylactic cephazolin in spinal surgery. A double-blind placebo-controlled trial. J. Bone Jt. Surg. Br. 1994, 76, 99–102. [Google Scholar] [CrossRef]
- Saito, T.; Yoshioka, S.; Iinuma, Y.; Takakura, S.; Fujihara, N.; Ichinohe, T.; Ishikawa, T.; Uchiyama, T.; Ichiyama, S. Effects on spectrum and susceptibility patterns of isolates causing bloodstream infection by restriction of fluoroquinolone prophylaxis in a hematology-oncology unit. Eur. J. Clin. Microbiol. Infect. Dis. 2008, 27, 209–216. [Google Scholar] [CrossRef]
- Satlin, M.J.; Chen, L.; Douglass, C.; Hovan, M.; Davidson, E.; Soave, R.; La Spina, M.; Gomez-Arteaga, A.; van Besien, K.; Mayer, S.; et al. Colonization With Fluoroquinolone-Resistant Enterobacterales Decreases the Effectiveness of Fluoroquinolone Prophylaxis in Hematopoietic Cell Transplant Recipients. Clin. Infect. Dis. 2021, 73, 1257–1265. [Google Scholar] [CrossRef]
- Satlin, M.J.; Vardhana, S.; Soave, R.; Shore, T.B.; Mark, T.M.; Jacobs, S.E.; Walsh, T.J.; Gergis, U. Impact of Prophylactic Levofloxacin on Rates of Bloodstream Infection and Fever in Neutropenic Patients with Multiple Myeloma Undergoing Autologous Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2015, 21, 1808–1814. [Google Scholar] [CrossRef]
- Schroeder, M.; Schadeck-Gressel, C.; Selbach, J.; Westerhausen, M. Antibiotic prophylaxis with gyrase inhibitors during cytostatically induced granulocytopenias in patients with solid tumors: A double-blind prospective randomized study. Onkologie 1992, 15, 476–479. [Google Scholar] [CrossRef]
- Sewick, A.; Makani, A.; Wu, C.; O’Donnell, J.; Baldwin, K.D.; Lee, G.C. Does dual antibiotic prophylaxis better prevent surgical site infections in total joint arthroplasty? Clin. Orthop. Relat. Res. 2012, 470, 2702–2707. [Google Scholar] [CrossRef] [PubMed]
- Signorelli, J.; Zimmer, A.; Liewer, S.; Shostrom, V.K.; Freifeld, A. Incidence of Febrile Neutropenia in Autologous Hematopoietic Stem Cell Transplant (HSCT) Recipients on levofloxacin prophylaxis. Transpl. Infect. Dis. 2020, 22, e13225. [Google Scholar] [CrossRef]
- Simondsen, K.A.; Reed, M.P.; Mably, M.S.; Zhang, Y.; Longo, W.L. Retrospective analysis of fluoroquinolone prophylaxis in patients undergoing allogeneic hematopoietic stem cell transplantation. J. Oncol. Pharm. Pract. 2013, 19, 291–297. [Google Scholar] [CrossRef]
- Sinkó, J.; Cser, V.; Konkoly Thege, M.; Masszi, T. Gram-negative bacteremia in neutropenic patients with hematologic disorders. Experiences with prophylactic use of fluoroquinolones. Orv. Hetil. 2011, 152, 1063–1067. [Google Scholar] [CrossRef]
- Slavin, M.A.; Grigg, A.P.; Schwarer, A.P.; Szer, J.; Spencer, A.; Sainani, A.; Thursky, K.A.; Roberts, A.W. A randomized comparison of empiric or pre-emptive antibiotic therapy after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2007, 40, 157–163. [Google Scholar] [CrossRef]
- Sohn, B.S.; Yoon, D.H.; Kim, S.; Lee, K.; Kang, E.H.; Park, J.S.; Lee, D.H.; Kim, S.H.; Huh, J.; Suh, C. The role of prophylactic antimicrobials during autologous stem cell transplantation: A single-center experience. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 1653–1661. [Google Scholar] [CrossRef]
- Sojo, J.F.; Massana, M.B.; Morgades, M.; Polo, S.V.; Quesada, M.D.; Santasusana, J.M.R. Comparative study on the usefulness of antibacterial prophylaxis with levofloxacin in patients submitted to hematopoietic stem cell transplantation. Med. Clin. 2016, 146, 16–19. [Google Scholar] [CrossRef]
- Stallard, S.; Savioli, F.; McConnachie, A.; Norrie, J.; Dudman, K.; Morrow, E.S.; Romics, L. Antibiotic prophylaxis in breast cancer surgery (PAUS trial): Randomised clinical double-blind parallel-group multicentre superiority trial. Br. J. Surg. 2022, 109, 1224–1231. [Google Scholar] [CrossRef]
- Tang, B.; Liu, X.; Xing, F.; Wang, C.; Jia, C.; Peng, S.; Zhao, Y.; Dai, C.; Xu, F. Single Dose Based Ertapenem Prophylaxis Reduces Surgical Site Infection after Selective Hepatectomy of Hepatocellular Carcinoma: A Propensity Score Matching Study. Biomed. Res. Int. 2018, 2018, 2520191. [Google Scholar] [CrossRef]
- Timmers, G.J.; Simoons-Smit, A.M.; Leidekker, M.E.; Janssen, J.; Vandenbroucke-Grauls, C.; Huijgens, P.C. Levofloxacin vs. ciprofloxacin plus phenethicillin for the prevention of bacterial infections in patients with haematological malignancies. Clin. Microbiol. Infect. 2007, 13, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Trecarichi, E.M.; Giuliano, G.; Cattaneo, C.; Ballanti, S.; Criscuolo, M.; Candoni, A.; Marchesi, F.; Laurino, M.; Dargenio, M.; Fanci, R.; et al. Bloodstream infections caused by Escherichia coli in onco-haematological patients: Risk factors and mortality in an Italian prospective survey. PLoS ONE 2019, 14, e0224465. [Google Scholar] [CrossRef] [PubMed]
- Ugarte-Torres, A.; Villasis-Keever, A.; Hernandez-Bribiesca, M.E.; Crespo-Solis, E.; Ruiz-Palacios, G.M.; Sifuentes-Osornio, J.; Ponce-de-Leon-Garduno, A. Fluoroquinolone prophylaxis utility during chemoradiation induced severe neutropenia in patients with acute leukemia, with fluoroquinolone resistance high prevalence, in a reference hospital in Mexico City. Rev. Investig. Clin.-Clin. Transl. Investig. 2006, 58, 547–554. [Google Scholar]
- Urbino, I.; Frairia, C.; Busca, A.; Corcione, S.; D’Ardia, S.; Dellacasa, C.M.; Giai, V.; Secreto, C.; Freilone, R.; De Rosa, F.G.; et al. Levofloxacin Prophylaxis Versus no Prophylaxis in Acute Myeloid Leukemia During Post-Induction Aplasia: A Single Center Study. Mediterr. J. Hematol. Infect. Dis. 2023, 15, e2023022. [Google Scholar] [CrossRef]
- Verlinden, A.; Jansens, H.; Goossens, H.; van de Velde, A.L.; Schroyens, W.A.; Berneman, Z.N.; Gadisseur, A.P. Clinical and microbiological impact of discontinuation of fluoroquinolone prophylaxis in patients with prolonged profound neutropenia. Eur. J. Haematol. 2014, 93, 302–308. [Google Scholar] [CrossRef]
- Ward, T.T.; Thomas, R.G.; Fye, C.L.; Arbeit, R.; Coltman, C.A.; Craig, W.; Dana, B.W.; Finegold, S.M.; Lentino, J.; Penn, R.L. Trimethoprim-sulfamethoxazole prophylaxis in granulocytopenic patients with acute leukemia: Evaluation of serum antibiotic levels in a randomized, double-blind, placebo-controlled Department of Veterans Affairs Cooperative Study. Clin. Infect. Dis. 1993, 17, 323–332. [Google Scholar] [CrossRef]
- Wilson, S.E.; Turpin, R.S.; Kumar, R.N.; Itani, K.M.; Jensen, E.H.; Pellissier, J.M.; Abramson, M.A. Comparative costs of ertapenem and cefotetan as prophylaxis for elective colorectal surgery. Surg. Infect. 2008, 9, 349–356. [Google Scholar] [CrossRef]
- Wolska, A.; Robak, T.; Szmigielska-Kaplon, A.; Pluta, A.; Kopka, P.; Wierzbowska, A. Ciprofloxacin prophylaxis for patients undergoing high-dose chemotherapy and autologous stem cell transplantation (ASCT)—A single-center experience. Adv. Med. Sci. 2012, 57, 118–123. [Google Scholar] [CrossRef]
- Yang, C.H.; Chew, K.Y.; Solomkin, J.S.; Lin, P.Y.; Chiang, Y.C.; Kuo, Y.R. Surgical Site Infections Among High-Risk Patients in Clean-Contaminated Head and Neck Reconstructive Surgery Concordance With Preoperative Oral Flora. Ann. Plast. Surg. 2013, 71, S55–S60. [Google Scholar] [CrossRef]
- Zaidi, Y.; Hastings, M.; Murray, J.; Hassan, R.; Kurshid, M.; Mahendra, P. Quinolone resistance in neutropenic patients: The effect of prescribing policy in the UK and Pakistan. Clin. Lab. Haematol. 2001, 23, 39–42. [Google Scholar] [CrossRef]
- Zavrelova, A.; Paterova, P.; Gabalec, F.; Zak, P.; Radocha, J. Ciprofloxacin prophylaxis during autologous stem cell transplantation for multiple myeloma in patients with a high rate of fluoroquinolone-resistant gram-negative bacteria colonization. Biomed. Pap. 2019, 163, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Mohyuddin, G.R.; Aziz, M.; McClune, B.; Abdallah, A.O.; Qazilbash, M. Antibiotic prophylaxis for patients with newly diagnosed multiple myeloma: Systematic review and meta-analysis. Eur. J. Haematol. 2020, 104, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Classen, A.-Y.; Henze, L.; von Lilienfeld-Toal, M.; Maschmeyer, G.; Sandherr, M.; Graeff, L.D.; Alakel, N.; Christopeit, M.; Krause, S.; Mayer, K.; et al. Primary prophylaxis of bacterial infections and Pneumocystis jirovecii pneumonia in patients with hematologic malignancies and solid tumors: 2020 updated guidelines of the Infectious Diseases Working Party of the German Society of Hematology and Medical Oncology (AGIHO/DGHO). Ann. Hematol. 2021, 100, 1603–1620. [Google Scholar] [CrossRef] [PubMed]
- Cruciani, M.; Malena, M.; Bosco, O.; Nardi, S.; Serpelloni, G.; Mengoli, C. Reappraisal with meta-analysis of the addition of Gram-positive prophylaxis to fluoroquinolone in neutropenic patients. J. Clin. Oncol. 2003, 21, 4127–4137. [Google Scholar] [CrossRef]
- Allegranzi, B.; Zayed, B.; Bischoff, P.; Kubilay, N.Z.; de Jonge, S.; de Vries, F.; Gomes, S.M.; Gans, S.; Wallert, E.D.; Wu, X.; et al. New WHO recommendations on intraoperative and postoperative measures for surgical site infection prevention: An evidence-based global perspective. Lancet Infect. Dis. 2016, 16, e288–e303. [Google Scholar] [CrossRef]
- Berríos-Torres, S.I.; Umscheid, C.A.; Bratzler, D.W.; Leas, B.; Stone, E.C.; Kelz, R.R.; Reinke, C.E.; Morgan, S.; Solomkin, J.S.; Mazuski, J.E.; et al. Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection, 2017. JAMA Surg. 2017, 152, 784–791. [Google Scholar] [CrossRef]
- Bratzler, D.W.; Dellinger, E.P.; Olsen, K.M.; Perl, T.M.; Auwaerter, P.G.; Bolon, M.K.; Fish, D.N.; Napolitano, L.M.; Sawyer, R.G.; Slain, D.; et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Surg. Infect. 2013, 14, 73–156. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Systematic Review and Evidence-Based Guidance on Perioperative Prophylaxis; European Centre for Disease Prevention and Control: Solna, Stockholm, 2013. [Google Scholar]
- Chiesa-Estomba, C.M.; Lechien, J.R.; Fakhry, N.; Melkane, A.; Calvo-Henriquez, C.; de Siati, D.; Gonzalez-Garcia, J.A.; Fagan, J.J.; Ayad, T. Systematic review of international guidelines for perioperative antibiotic prophylaxis in Head & Neck Surgery. A YO-IFOS Head & Neck Study Group Position Paper. Head Neck 2019, 41, 3434–3456. [Google Scholar] [CrossRef]
- Iocca, O.; Copelli, C.; Ramieri, G.; Zocchi, J.; Savo, M.; Di Maio, P. Antibiotic prophylaxis in head and neck cancer surgery: Systematic review and Bayesian network meta-analysis. Head Neck 2022, 44, 254–261. [Google Scholar] [CrossRef]
- Mottet, N.; Cornford, P.; van der Bergh, R.C.N.; Briers, E.; Eberli, D.; De Meerlerr, G.; De Santis, M.; Gillessen, S.; Grummet, J.; Henry, A.M.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef]
- Righi, E.; Mutters, N.T.; Guirao, X.; Del Toro, M.D.; Eckmann, C.; Friedrich, A.W.; Giannella, M.; Kluytmans, J.; Presterl, E.; Christaki, E.; et al. ESCMID/EUCIC clinical practice guidelines on perioperative antibiotic prophylaxis in patients colonized by multidrug-resistant Gram-negative bacteria before surgery. Clin. Microbiol. Infect. 2023, 29, 463–479. [Google Scholar] [CrossRef]
- Freedberg, D.E.; Zhou, M.J.; Cohen, M.E.; Annavajhala, M.K.; Khan, S.; Moscoso, D.I.; Brooks, C.; Whittier, S.; Chong, D.H.; Uhlemann, A.C.; et al. Pathogen colonization of the gastrointestinal microbiome at intensive care unit admission and risk for subsequent death or infection. Intensive Care Med. 2018, 44, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Ziakas, P.D.; Thapa, R.; Rice, L.B.; Mylonakis, E. Trends and significance of VRE colonization in the ICU: A meta-analysis of published studies. PLoS ONE 2013, 8, e75658. [Google Scholar] [CrossRef] [PubMed]
- Teillant, A.; Gandra, S.; Barter, D.; Morgan, D.J.; Laxminarayan, R. Potential burden of antibiotic resistance on surgery and cancer chemotherapy antibiotic prophylaxis in the USA: A literature review and modelling study. Lancet Infect. Dis. 2015, 15, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Laxminarayan, R. The overlooked pandemic of antimicrobial resistance. Lancet 2022, 399, 606–607. [Google Scholar] [CrossRef]
- Naylor, N.R.; Evans, S.; Pouwels, K.B.; Troughton, R.; Lamagni, T.; Muller-Pebody, B.; Knight, G.M.; Atun, R.; Robotham, J.V. Quantifying the primary and secondary effects of antimicrobial resistance on surgery patients: Methods and data sources for empirical estimation in England. Front. Public Health 2022, 10, 803943. [Google Scholar] [CrossRef]
- Savoldi, A.; Mutters, N.T.; Tacconelli, E. Personalized infection prevention and control: A concept whose time has arrived. Antimicrob. Steward. Healthc. Epidemiol. 2023, 3, e151. [Google Scholar] [CrossRef]
- Temkin, E.; Margalit, I.; Nutman, A.; Carmeli, Y. Surgical antibiotic prophylaxis in patients colonized with multidrug-resistant Gram-negative bacteria: Practical and conceptual aspects. J. Antimicrob. Chemother. 2021, 76, i40–i46. [Google Scholar] [CrossRef]

| Study Characteristics | Hemato-Oncology (N = 55) | Surgery (N = 54) |
|---|---|---|
| Median number of patients included per study (IQR) | 210 (104–341) | 672 (281–1183) |
| Year of publication | ||
| 1991–2000 | 10 (18%) | 2 (4%) |
| 2001–2010 | 13 (24%) | 13 (24%) |
| 2011–2024 | 32 (58%) | 39 (72%) |
| Year of start of study period | ||
| 1984–2000 | 21 (38%) | 8 (15%) |
| 2001–2010 | 21 (38%) | 29 (54%) |
| 2011–2024 | 13 (24%) | 17 (31%) |
| Geographical distribution | ||
| Africa | 0 (0%) | 1 (2%) |
| Asia | 7 (13%) | 18 (33%) |
| Australia | 2 (4%) | 1 (2%) |
| Europe | 25 (45%) | 16 (30%) |
| North America | 14 (25%) | 14 (26%) |
| South America | 5 (9%) | 0 (0%) |
| Intercontinental | 2 (4%) | 4 (7%) |
| Antibiotic prophylaxis | ||
| Cephalosporin | 1 (2%) | 24 (44%) |
| Cephalosporin in combination | 0 (0%) | 4 (7%) |
| Quinolone | 47 (85%) | 9 (17%) |
| Quinolone in combination | 4 (7%) | 1 (2%) |
| Penicillin | 0 (0%) | 3 (6%) |
| Penicillin + beta-lactamase inhibitor | 0 (0%) | 6 (11%) |
| Carbapenem | 0 (0%) | 4 (7%) |
| Others * | 3 (5%) | 3 (6%) |
| Study design | ||
| Retrospective cohort study | 22 (40%) | 17 (32%) |
| Prospective cohort study | 8 (15) | 12 (22%) |
| Randomized controlled trial | 8 (15%) | 15 (28%) |
| Prospective before–after study | 2 (4%) | 1 (2%) |
| Retrospective before–after study | 11 (20%) | 4 (7%) |
| Mixed-cohort study | 4 (7%) | 2 (4%) |
| Hemato-oncological treatment | ||
| Stem cell transplantation | 18 (33%) | |
| Chemotherapy | 39 (71%) | |
| Type of surgery | - | |
| Abdominal surgery | 15 (28%) | |
| Trauma surgery | 11 (20%) | |
| Urological surgery | 12 (22%) | |
| Plastic surgery | 2 (4%) | |
| Heart and thoracic surgery | 4 (7%) | |
| Gynecological surgery | 2 (4%) | |
| Neurosurgery | 4 (7%) | |
| Head and neck surgery | 3 (6%) | |
| All or unspecific surgeries | 1 (2%) | |
| Type of infection ** | ||
| All or unspecific infections | 39 (71%) | 22 (41%) |
| Bacteremia | 48 (87%) | 9 (17%) |
| Surgical site infection | 0 (0%) | 36 (67%) |
| Urinary tract infection | 1 (2%) | 9 (17%) |
| Abdominal infection | 0 (0%) | 1 (2%) |
| Fever/febrile neutropenia | 3 (5%) | 0 (0%) |
| Pneumonia | 0 (0%) | 1 (2%) |
| Central nervous system | 0 (0%) | 2 (4%) |
| Invasive medical device infection | 0 (0%) | 4 (7%) |
| Outcome measure *** | ||
| Breakthrough infections (total, including AMR) | 45 (82%) | 52 (96%) |
| Antimicrobial resistance in infections | 49 (89%) | 46 (85%) |
| Length of hospital stay | 8 (15%) | 9 (17%) |
| Mortality | 23 (42%) | 7 (13%) |
| Healthcare costs | 1 (2%) | 3 (6%) |
| Category | Ns (No) * | Relative Risk (95% CI) | p-Value | I-Square | p-Value for Heterogeneity |
|---|---|---|---|---|---|
| Reported causative bacteria (as reported in the study) | |||||
| All bacteria | 27 (42) | 0.86 (0.82–0.90) | <0.001 | 80.0% | <0.001 |
| Causative bacteria classified as Gram-positive/Gram-negative bacteria | |||||
| Gram-positive bacteria | 10 (10) | 1.06 (0.95–1.19) | 0.284 | 78.0% | <0.001 |
| Gram-negative bacteria | 16 (16) | 0.51 (0.45–0.59) | <0.001 | 62.0% | <0.001 |
| Type of infection | |||||
| All | 12 (18) | 0.96 (0.90–1.03) | 0.279 | 66.0% | <0.001 |
| Bacteremia | 25 (28) | 0.81 (0.76–0.87) | <0.001 | 79.0% | <0.001 |
| Prophylactic antibiotic studied | |||||
| Fluoroquinolone | 28 (44) | 0.86 (0.82–0.90) | <0.001 | 79.0% | <0.001 |
| Stem cell transplantation | |||||
| No | 4 (5) | 0.71 (0.51–0,99) | 0.044 | 0.0% | 0.54 |
| Partly | 9 (16) | 0.92 (0.86–0.97) | 0.005 | 80.0% | <0.001 |
| Yes | 11 (18) | 0.75 (0.69–0.81) | <0.001 | 84.0% | <0.001 |
| Geographical location | |||||
| Europe | 13 (19) | 0.92 (0.86–0.99) | 0.024 | 66.0% | <0.001 |
| North America | 8 (15) | 0.63 (0.56–0.7) | <0.001 | 80.0% | <0.001 |
| South America | 5 (8) | 0.90 (0.83–0.98) | 0.017 | 89.0% | <0.001 |
| Publication year | |||||
| 1991–2000 | 6 (10) | 0.77 (0.64–0.94) | 0.009 | 73.0% | <0.001 |
| 2001–2010 | 7 (10) | 0.93 (0.79–1.08) | 0.329 | 82.0% | <0.001 |
| 2011–2024 | 17 (28) | 0.85 (0.81–0.90) | <0.001 | 80.0% | <0.001 |
| Category | Ns (No) * | Relative Risk (95% CI) | p-Value | I-Square | p-Value for Heterogeneity |
|---|---|---|---|---|---|
| Reported causative bacteria (as reported in the study) | |||||
| All bacteria | 4 (4) | 1.97 (1.02–3.79) | 0.044 | 39.0% | 0.178 |
| Enterobacterales | 4 (4) | 2.93 (2.36–3.63) | <0.001 | 0.0% | 0.598 |
| Escherichia coli | 9 (11) | 1.87 (1.66–2.10) | <0.001 | 56.0% | 0.012 |
| Gram-negative bacteria | 7 (9) | 2.27 (1.87–2.77) | <0.001 | 73.0% | <0.001 |
| Gram-positive bacteria | 3 (3) | 1.01 (0.77–1.31) | 0.966 | 0.0% | 0.475 |
| Causative bacteria classified as Gram-positive/Gram-negative bacteria | |||||
| Gram-positive bacteria | 4 (4) | 1.13 (0.87–1.46) | 0.353 | 54.0% | 0.091 |
| Gram-negative bacteria | 18 (24) | 2.14 (1.95–2.34) | <0.001 | 66.0% | <0.001 |
| Type of infection | |||||
| Any or unspecific infections | 4 (6) | 2.52 (1.62–3.92) | <0.001 | 65.0% | 0.013 |
| bacteremia | 17 (19) | 2.05 (1.86–2.26) | <0.001 | 78.0% | <0.001 |
| Prophylactic antibiotic studied | |||||
| Fluoroquinolone | 19 (23) | 2.04 (1.87–2.22) | <0.001 | 73.0% | <0.001 |
| Stem cell transplantation | |||||
| No | 3 (3) | 2.27 (1.57–3.28) | <0.001 | 56.0% | 0.101 |
| Partly | 8 (11) | 1.93 (1.73–2.15) | <0.001 | 56.0% | 0.011 |
| Yes | 7 (9) | 2.54 (2.14–3.01) | <0.001 | 66.0% | 0.003 |
| Geographical location | |||||
| Asia | 4 (5) | 1.29 (1.02–1.63) | 0.032 | 81.0% | <0.001 |
| Europe | 9 (11) | 2.56 (2.18–3.01) | <0.001 | 62.0% | 0.004 |
| North America | 4 (5) | 1.86 (1.64–2.11) | <0.001 | 2.0% | 0.396 |
| Publication year | |||||
| 1991–2000 | 3 (4) | 7.4 (2.77–19.76) | <0.001 | 19.0% | 0.295 |
| 2001–2010 | 5 (6) | 2.33 (1.61–3.37) | <0.001 | 74.0% | 0.002 |
| 2011–2024 | 13 (16) | 1.98 (1.81–2.16) | <0.001 | 78.0% | <0.001 |
| Study | Setting | Patients | Patient Groups Being Compared/Antibiotic Prophylaxis | Outcome Studied | Risk of Infection | Unadjusted Relative Risk (95% CI) * |
|---|---|---|---|---|---|---|
| Colonized versus non-colonized patients | ||||||
| Satlin (2021) [113] | Hemato-oncological | Stem cell transplantation patients | Not colonized/fluoroquinolone (levofloxacin) vs. colonization with fluoroquinolone-resistant Enterobacterales/fluoroquinolone (levofloxacin) | Proportion of BSIs caused by FQ-resistant Gram-negative bacteria | 1/80 vs. 16/54 | 23.3 (3.2–173.5) |
| Akhmedov (2023) [28] | Hemato-oncological | Stem cell transplantation patients | Colonization with resistant Gram-negative bacteria/no prophylaxis vs. not colonized/no prophylaxis vs. not colonized/fluoroquinolone prophylaxis | General BSI rate | 43/147 vs. 9/32 vs. 28/98 | 1.0 (0.5–1.8) § |
| Dubinsky-Pertzov (2019) [51] | Surgical | Patients with elective colorectal surgery | Not colonized/cephalosporin + metronidazole vs. colonization with ESBL-producing Enterobacterales/cephalosporin + metronidazole | Proportion of SSIs caused by ESBL-PE | 7/440 vs. 16/222 | 4.5 (1.9–10.9) |
| Yang (2013) [133] | Surgical | Patients with surgeries in high-risk head and neck cancer patients | Pre-surgical colonization/pre-prophylaxis vs. surgical site infection/clindamycin + gentamicin | Proportion of clindamycin resistance among Gram-positive bacteria | 82/171 vs. 26/31 | 1.75 (1.40–2.18) |
| Targeted prophylaxis for colonized patients | ||||||
| Nutman (2020) [104] | Surgical | Patients with elective colorectal surgery | Colonization with ESBL-producing Enterobacterales/adjusted using ertapenem vs. colonization with ESBL-producing Enterobacterales/cephalosporin + metronidazole | Proportion of SSIs caused by ESBL-PE | 4/269 vs. 15/209 | 0.21 (0.07–0.62) |
| De Pastena (2021) [49] | Surgical | Patients with pancreatic surgery | Colonization with ESBL-producing Enterobacterales/piperacillin–tazobactam vs. colonization with ESBL-producing Enterobacterales/ampicillin–sulbactam | General rate of hospital-acquired infections | 11/29 vs. 30/47 | 0.59 (0.36–0.99) |
| Newman (2022) [101] | Surgical | Patients with transrectal prostate biopsies | Known colonization status/targeted prophylaxis vs. unknown colonization status/empirical prophylaxis | General BSI rate | 9/403 vs. 12/609 | 1.1 (0.48–2.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rink, M.; Gladstone, B.P.; Nikolai, L.A.; Bitzer, M.; Tacconelli, E.; Göpel, S. The Impact of Antibiotic Prophylaxis on Antibiotic Resistance, Clinical Outcomes, and Costs in Adult Hemato-Oncological and Surgical Patients: A Systematic Review and Meta-Analysis. Antibiotics 2025, 14, 853. https://doi.org/10.3390/antibiotics14090853
Rink M, Gladstone BP, Nikolai LA, Bitzer M, Tacconelli E, Göpel S. The Impact of Antibiotic Prophylaxis on Antibiotic Resistance, Clinical Outcomes, and Costs in Adult Hemato-Oncological and Surgical Patients: A Systematic Review and Meta-Analysis. Antibiotics. 2025; 14(9):853. https://doi.org/10.3390/antibiotics14090853
Chicago/Turabian StyleRink, Marissa, Beryl Primrose Gladstone, Lea Ann Nikolai, Michael Bitzer, Evelina Tacconelli, and Siri Göpel. 2025. "The Impact of Antibiotic Prophylaxis on Antibiotic Resistance, Clinical Outcomes, and Costs in Adult Hemato-Oncological and Surgical Patients: A Systematic Review and Meta-Analysis" Antibiotics 14, no. 9: 853. https://doi.org/10.3390/antibiotics14090853
APA StyleRink, M., Gladstone, B. P., Nikolai, L. A., Bitzer, M., Tacconelli, E., & Göpel, S. (2025). The Impact of Antibiotic Prophylaxis on Antibiotic Resistance, Clinical Outcomes, and Costs in Adult Hemato-Oncological and Surgical Patients: A Systematic Review and Meta-Analysis. Antibiotics, 14(9), 853. https://doi.org/10.3390/antibiotics14090853






