The Influence of Local Antibiotic Therapy on the Microbiological, Clinical, and Radiological Outcomes Following Minimally Invasive Periodontal Surgery in the Treatment of Intrabony Defects—A Randomized Clinical Trial
Abstract
1. Introduction
2. Results
2.1. Periodontal Clinical Parameters
2.2. Microbiology Findings
2.3. Radiological Findings
3. Discussion
Limitations
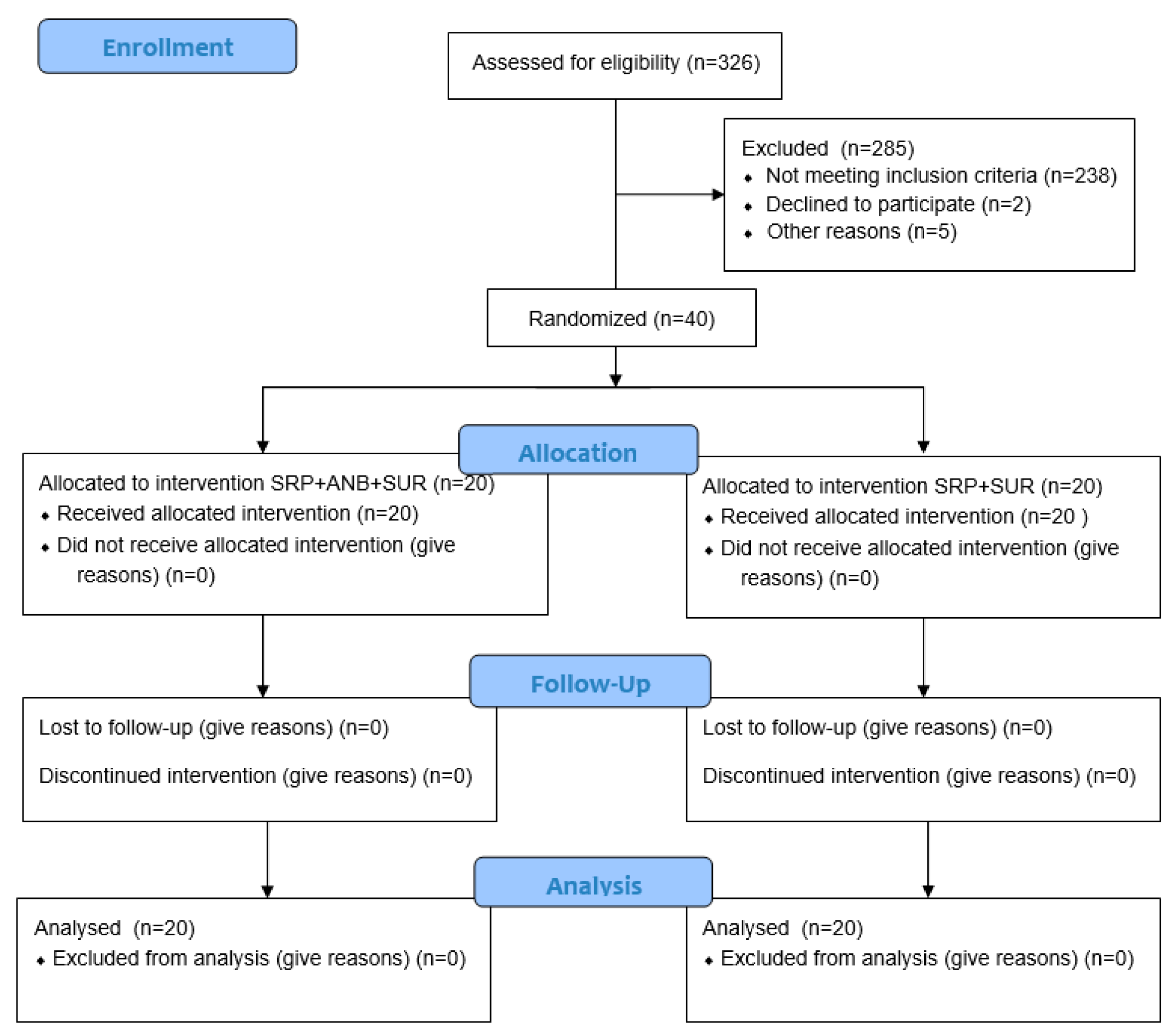
4. Materials and Methods
4.1. Sample Size
4.2. Study Population and Experimental Design
4.3. Clinical Examinations
4.4. Investigator Calibration
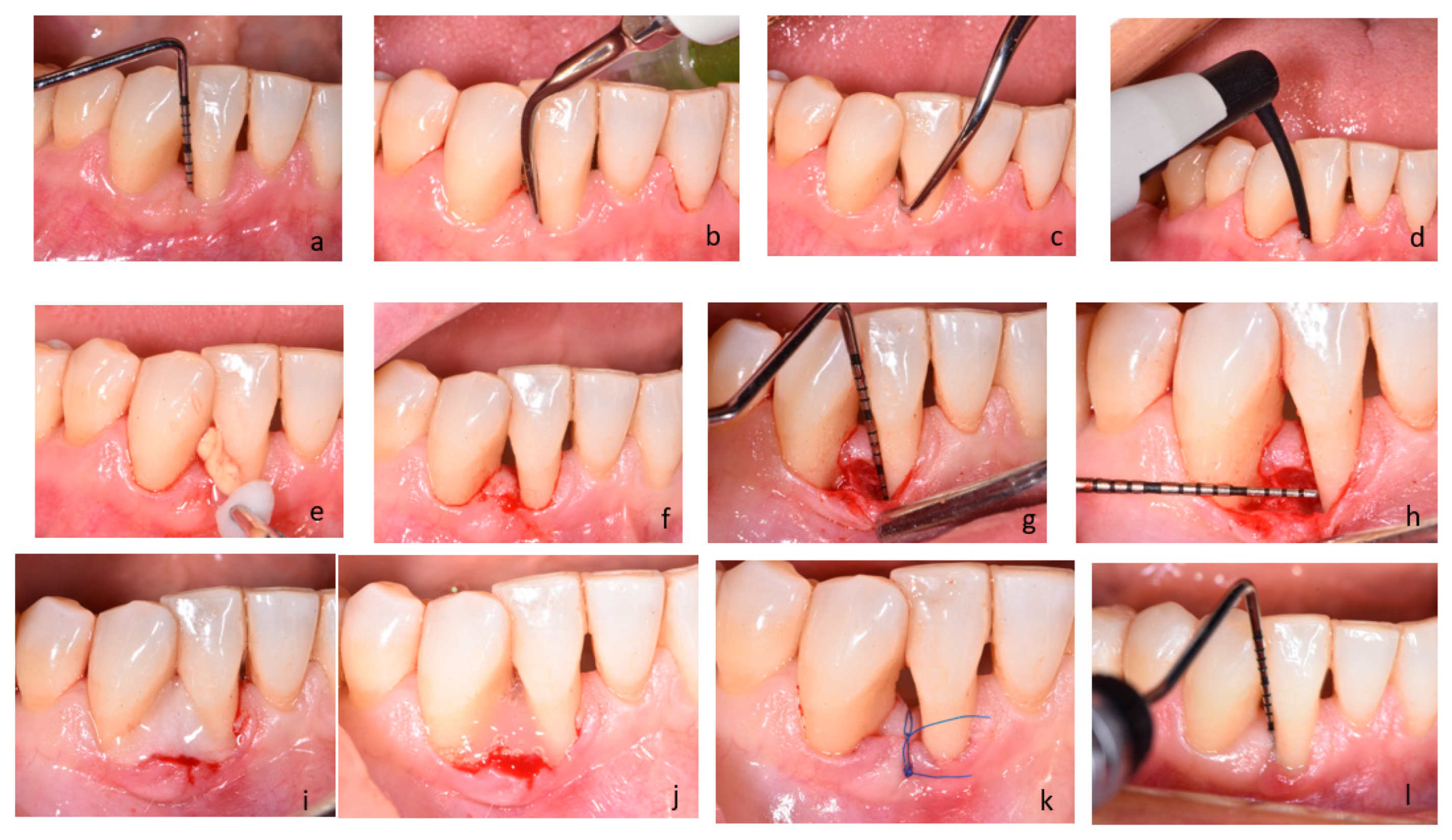
4.5. Clinical Procedure
4.6. Microbiological Evaluation
4.7. Radiological Evaluation
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kassebaum, N.J.; Bernabé, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.L.; Marcenes, W. Global burden of severe periodontitis in 1990–2010: A systematic review and meta-regression. J. Dent. Res. 2014, 93, 1045–1053. [Google Scholar] [CrossRef]
- Nibali, L.; Koidou, V.P.; Nieri, M.; Barbato, L.; Pagliaro, U.; Cairo, F. Periodontal infrabony defects: Systematic review of healing by defect morphology following regenerative surgery. J. Clin. Periodontol. 2021, 48, 101–114. [Google Scholar] [CrossRef]
- Langa, N.P. Focus on intrabony defects—Conservative therapy. Periodontology 2000 2000, 22, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Papapanou, P.N.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Tonetti, M.S. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. S1), S173–S182. [Google Scholar] [CrossRef]
- Xu, W.; Zhou, W.; Wang, H.; Liang, S. Roles of Porphyromonas gingivalis and its virulence factors in periodontitis. Adv. Protein Chem. Struct. Biol. 2020, 120, 45–84. [Google Scholar] [CrossRef] [PubMed]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L., Jr. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef]
- Medina, C.M.A.; Garcés, A.A.A.; Zuluaga, I.C.G.; Ardila, M.; Ariza, G.; Guzman, Z. Coexistence of Porphyromonas gingivalis, Tannerella forsythia and Treponema denticola in the red bacterial complex in chronic periodontitis subjects. Int. J. Odontostomat. 2014, 8, 359–364. [Google Scholar] [CrossRef]
- Potempa, J.; Madej, M.; Scott, D.A. The RagA and RagB proteins of Porphyromonas gingivalis. Mol. Oral Microbiol. 2021, 36, 225–232. [Google Scholar] [CrossRef]
- Suzuki, N.; Yoneda, M.; Hirofuji, T. Mixed red-complex bacterial infection in periodontitis. Int. J. Dent. 2013, 2013, 587279. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Z.; Tang, Z.; Huang, Y.; Huang, M.; Liu, H.; Zhao, J. More than just a periodontal pathogen–the research progress on Fusobacterium nucleatum. Front. Cell. Infect. Microbiol. 2022, 12, 815318. [Google Scholar] [CrossRef]
- Dayakar, M.M.; Bhat, K.S.; Lakshmi, N.B. Prevotella intermedia—An overview and its role in periodontitis. J. Adv. Clin. Res. Insights 2021, 8, 79–82. [Google Scholar]
- Gholizadeh, P.; Pormohammad, A.; Eslami, H.; Shokouhi, B.; Fakhrzadeh, V.; Kafil, H.S. Oral pathogenesis of Aggregatibacter actinomycetemcomitans. Microb. Pathog. 2017, 113, 303–311. [Google Scholar] [CrossRef]
- Lindhe, J.; Meyle, J.; Group D of the European Workshop on Periodontology. Peri-implant diseases: Consensus report of the sixth European Workshop on Periodontology. J. Clin. Periodontol. 2008, 35, 282–285. [Google Scholar] [CrossRef]
- Schmidlin, P.R.; Beuchat, M.; Busslinger, A.; Lehmann, B.; Lutz, F. Tooth substance loss resulting from mechanical, sonic and ultrasonic root instrumentation assessed by liquid scintillation. J. Clin. Periodontol. 2001, 28, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Breininger, D.R.; O’Leary, T.J.; Blumenshine, R.V.H. Comparative effectiveness of ultrasonic and hand scaling for the removal of subgingival plaque and calculus. J. Periodontol. 1987, 58, 9–18. [Google Scholar] [CrossRef]
- Rateitschak-Plüss, E.M.; Schwarz, J.P.; Guggenheim, R.; Duggelin, M.; Rateitschak, K.H. Non-surgical periodontal treatment: Where are the limits? An SEM study. J. Clin. Periodontol. 1992, 19, 240–244. [Google Scholar] [CrossRef]
- Hammami, C.; Nasri, W. Antibiotics in the treatment of periodontitis: A systematic review of the literature. Int. J. Dent. 2021, 2021, 6846074. [Google Scholar] [CrossRef]
- Heta, S.; Robo, I. The side effects of the most commonly used group of antibiotics in periodontal treatments. Med. Sci. 2018, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Budală, D.G.; Luchian, I.; Tatarciuc, M.; Butnaru, O.; Armencia, A.O.; Virvescu, D.I.; Scutariu, M.M.; Rusu, D. Are local drug delivery systems a challenge in clinical periodontology? J. Clin. Med. 2023, 12, 4137. [Google Scholar] [CrossRef]
- Cortellini, P.; Tonetti, M.S. Clinical concepts for regenerative therapy in intrabony defects. Periodontology 2000 2015, 68, 282–307. [Google Scholar] [CrossRef] [PubMed]
- Machtei, E.E.; Romanos, G.; Kang, P.; Travan, S.; Schmidt, S.; Papathanasiou, E.; Kim, D.M. Repeated delivery of chlorhexidine chips for the treatment of peri-implantitis: A multicenter, randomized, comparative clinical trial. J. Periodontol. 2021, 92, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Herrera, D.; Matesanz, P.; Martín, C.; Oud, V.; Feres, M.; Teughels, W. Adjunctive Effect of Locally Delivered Antimicrobials in Periodontitis Therapy: A Systematic Review and Meta-Analysis. J. Clin. Periodontol. 2020, 47 (Suppl. 22), 239–256. [Google Scholar] [CrossRef]
- Salvi, G.E.; Mischler, D.C.; Schmidlin, K.; Matuliene, G.; Pjetursson, B.E.; Brägger, U.; Lang, N.P. Risk factors associated with the longevity of multi-rooted teeth. Long-term outcomes after active and supportive periodontal therapy. J. Clin. Periodontol. 2014, 41, 701–707. [Google Scholar] [CrossRef]
- Polimeni, G.; Xiropaidis, A.V.; Wikesjö, U.M. Biology and principles of periodontal wound healing/regeneration. Periodontology 2000 2006, 41, 30–47. [Google Scholar] [CrossRef]
- Heitz-Mayfield, L.; Tonetti, M.S.; Cortellini, P.; Lang, N.P.; European Research Group on Periodontology (ERGOPERIO). Microbial colonization patterns predict the outcomes of surgical treatment of intrabony defects. J. Clin. Periodontol. 2006, 33, 62–68. [Google Scholar] [CrossRef]
- Silvestri, M.; Sartori, S.; Rasperini, G.; Ricci, G.; Rota, C.; Cattaneo, V. Comparison of infrabony defects treated with enamel matrix derivative versus guided tissue regeneration with a nonresorbable membrane: A multicenter controlled clinical trial. J. Clin. Periodontol. 2003, 30, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Cortellini, P.; Tonetti, M.S. A minimally invasive surgical technique with an enamel matrix derivative in the regenerative treatment of intra-bony defects: A novel approach to limit morbidity. J. Clin. Periodontol. 2007, 34, 87–93. [Google Scholar] [CrossRef]
- Sultan, N.; Jafri, Z.; Sawai, M.; Bhardwaj, A. Minimally invasive periodontal therapy. J. Oral Biol. Craniofac. Res. 2020, 10, 161–165. [Google Scholar] [CrossRef]
- Koop, R.; Merheb, J.; Quirynen, M. Periodontal regeneration with enamel matrix derivative in reconstructive periodontal therapy: A systematic review. J. Periodontol. 2012, 83, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Cortellini, P.; Prato, G.P.; Tonetti, M.S. Periodontal regeneration of human intrabony defects with titanium reinforced membranes: A controlled clinical trial. J. Periodontol. 1995, 66, 797–803. [Google Scholar] [CrossRef]
- Mueller, V.T.; Welch, K.; Bratu, D.C.; Wang, H.L. Early and late studies of EMD use in periodontal intrabony defects. J. Periodontal Res. 2013, 48, 117–125. [Google Scholar] [CrossRef]
- Bosshardt, D.D. Biological mediators and periodontal regeneration: A review of enamel matrix proteins at the cellular and molecular levels. J. Clin. Periodontol. 2008, 35, 87–105. [Google Scholar] [CrossRef]
- Takei, H.H.; Han, T.J.; Carranza, F.A., Jr.; Kenney, E.B.; Lekovic, V. Flap technique for periodontal bone implants: Papilla preservation technique. J. Periodontol. 1985, 56, 204–221. [Google Scholar] [CrossRef]
- Gantes, B.G.; Garrett, S. Coronally displaced flaps in reconstructive periodontal therapy. Dent. Clin. N. Am. 1991, 35, 495–504. [Google Scholar] [CrossRef]
- Harrel, S.K.; Wilson, T.G., Jr.; Nunn, M.E. Prospective assessment of the use of enamel matrix proteins with minimally invasive surgery. J. Periodontol. 2005, 76, 380–384. [Google Scholar] [CrossRef]
- Cortellini, P.; Prato, G.P.; Tonetti, M.S. The simplified papilla preservation flap: A novel surgical approach for the management of soft tissues in regenerative procedures. Int. J. Periodontics Restor. Dent. 1999, 19, 589–599. [Google Scholar]
- Harrel, S.K.; Rees, T.D. Granulation tissue removal in routine and minimally invasive procedures. Compend. Contin. Educ. Dent. 1995, 16, 960–962. [Google Scholar]
- Harrel, S.K.; Nunn, M.E. Longitudinal comparisons of the periodontal status of patients with moderate to severe periodontal disease receiving no treatment, non-surgical treatment, and surgical treatment utilizing individual sites for analysis. J. Periodontol. 2001, 72, 1509–1519. [Google Scholar] [CrossRef]
- Reddy, Y.N.; Deepika, P.C.; Venkatesh, M.P.; Rajeshwari, K.G. Evaluation of moxifloxacin-hydroxyapatite composite graft in the regeneration of intrabony defects: A clinical, radiographic, and microbiological study. Contemp. Clin. Dent. 2016, 7, 357–365. [Google Scholar] [CrossRef]
- Zucchelli, G.; Sforza, N.M.; Clauser, C.; Cesari, C.; De Sanctis, M. Topical and systemic antimicrobial therapy in guided tissue regeneration. J. Periodontol. 1999, 70, 239–247. [Google Scholar] [CrossRef]
- Jung, D.Y.; Park, J.C.; Kim, Y.T.; Yon, J.Y.; Im, G.I.; Kim, B.S.; Kim, C.S. The clinical effect of locally delivered minocycline in association with flap surgery for the treatment of chronic severe periodontitis: A split-mouth design. J. Clin. Periodontol. 2012, 39, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Clementin, M.; Ambrosi, A.; Cicciarelli, V.; De Risi, V.; De Sanctis, M. Clinical performance of minimally invasive periodontal surgery in the treatment of infrabony defects: Systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46, 1236–1253. [Google Scholar] [CrossRef]
- Yusri, S.; Elfana, A.; Elbattawy, W.; Fawzy El-Sayed, K.M. Effect of Locally Delivered Adjunctive Antibiotics during Surgical Periodontal Therapy: A Systematic Review and Meta-Analysis. Clin. Oral Investig. 2021, 25, 219–230. [Google Scholar] [CrossRef]
- Aimetti, M.; Baima, G.; Aliyeva, N.; Lorenzetti, V.; Citterio, F.; Franco, F.; Romano, F. Influence of locally delivered doxycycline on the clinical and molecular inflammatory status of intrabony defects prior to periodontal regeneration: A double-blind randomized controlled trial. J. Periodontal Res. 2023, 58, 1096–1104. [Google Scholar] [CrossRef]
- Murphy, K.G. Postoperative healing complications associated with Gore-Tex Periodontal Material. Part II. Effect of complications on regeneration. Int. J. Periodontics Restor. Dent. 1995, 15, 548–557. [Google Scholar]
- Cortellini, P.; Tonetti, M.S. Improved wound stability with a modified minimally invasive surgical technique in the regenerative treatment of isolated interdental intrabony defects. J. Clin. Periodontol. 2009, 36, 157–163. [Google Scholar] [CrossRef]
- Agarwal, A.; Bhattacharya, H.S.; Srikanth, G.; Singh, A. Comparative evaluation of decalcified freeze dried bone allograft with and without local doxycycline in non-contained human periodontal infrabony defects. J. Indian Soc. Periodontol. 2013, 17, 490–494. [Google Scholar] [CrossRef]
- Cortellini, P.; Tonetti, M.S. Focus on intrabony defects: Guided tissue regeneration. Periodontology 2000 2000, 22, 104–132. [Google Scholar] [CrossRef]
- Sculean, A.; Kiss, A.; Miliauskaite, A.; Schwarz, F.; Arweiler, N.B.; Hannig, M. Ten-year results following treatment of intra-bony defects with enamel matrix proteins and guided tissue regeneration. J. Clin. Periodontol. 2008, 35, 817–824. [Google Scholar] [CrossRef]
- Sculean, A.; Donos, N.; Windisch, P.; Brecx, M.; Gera, I.; Reich, E.; Karring, T. Healing of human intrabony defects following treatment with enamel matrix proteins or guided tissue regeneration. J. Periodontal Res. 1999, 34, 310–322. [Google Scholar] [CrossRef]
- Crea, A.; Dassatti, L.; Hoffmann, O.; Zafiropoulos, G.G.; Deli, G. Treatment of intrabony defects using guided tissue regeneration or enamel matrix derivative: A 3-year prospective randomized clinical study. J. Periodontol. 2008, 79, 2281–2289. [Google Scholar] [CrossRef]
- Sanz, M.; Tonetti, M.S.; Zabalegui, I.; Sicilia, A.; Blanco, J.; Quirynen, M.; Cortellini, P.; Suvan, J.; Lindhe, J. Treatment of intrabony defects with enamel matrix proteins or barrier membranes: Results from a multicenter practice-based clinical trial. J. Periodontol. 2004, 75, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Sculean, A.; Auschill, T.M.; Donos, N.; Brecx, M.; Arweiler, N.B. Effect of an enamel matrix protein derivative (Emdogain®) on ex vivo dental plaque vitality. J. Clin. Periodontol. 2001, 28, 1074–1078. [Google Scholar] [CrossRef] [PubMed]
- Spahr, A.; Lyngstadaas, S.P.; Boeckh, C.; Andersson, C.; Podbielski, A.; Haller, B. Effect of the enamel matrix derivative Emdogain® on the growth of periodontal pathogens in vitro. J. Clin. Periodontol. 2002, 29, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Arweiler, N.; Auschill, T.; Donos, N.; Sculean, A. Antibacterial effect of an enamel matrix protein derivative on in vivo dental biofilm vitality. Clin. Oral Investig. 2002, 6, 205–209. [Google Scholar] [CrossRef]
- Mombelli, A.; Zekeridou, A. Mystery and Misery of Locally-Delivered Drug Therapy in Periodontics: Historical Concepts and Current State. Periodontology 2000 2025, 1–18. [Google Scholar] [CrossRef]
- Ilyes, I.; Boariu, M.; Rusu, D.; Iorio-Siciliano, V.; Vela, O.; Boia, S.; Radulescu, V.; Șurlin, P.; Jentsch, H.; Lodin, A.; et al. Comparative Study of Systemic vs. Local Antibiotics with Subgingival Instrumentation in Stage III–IV Periodontitis: A Retrospective Analysis. Antibiotics 2024, 13, 430. [Google Scholar] [CrossRef]
- Kaldahl, W.B.; Johnson, G.K.; Patil, K.D.; Kalkwarf, K.L. Levels of cigarette consumption and response to periodontal therapy. J. Periodontol. 1996, 67, 675–681. [Google Scholar] [CrossRef]
- St. George, G.; Darbar, U.; Thomas, G. Inflammatory external root resorption following surgical treatment for intra-bony defects: A report of two cases involving Emdogain® and a review of the literature. J. Clin. Periodontol. 2006, 33, 449–454. [Google Scholar] [CrossRef]
- O’Leary, T.J.; Drake, R.B.; Naylor, J.E. The Plaque Control Record. J. Periodontol. 1972, 43, 38. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.P.; Nyman, S.; Senn, C.; Joss, A. Bleeding on probing as it relates to probing pressure and gingival health. J. Clin. Periodontol. 1991, 18, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Cortellini, P.; Bowers, G.M. Periodontal regeneration of intrabony defects: An evidence-based treatment approach. Int. J. Periodontics Restor. Dent. 1995, 15, 115–127. [Google Scholar]

| Control Group | Test Group | Significance | |
|---|---|---|---|
| No. | 20 | 20 | - |
| Gender | 17F/3M | 11F/9M | p * = 0.03 |
| Mean age | 44.7 (5.77) | 49.45 (9.31) | NS (p ** = 0.06) |
| Incisors/canines/ premolars/molars | 7/3/7/3 | 12/0/4/4 | - |
| Surgical access | MPPT n = 13 SPPF n = 7 | MPPT n = 18 SPPF n = 2 | NS (p ** = 0.06) |
| Numbers of walls (1-wall/2-walls/3-walls) | 1/15/4 | 0/16/4 | - |
| Mean intrabony depth (IDD) | 4.6 mm (±1.23) | 4.87 mm (±1.66) | NS (p ** = 0.81) |
| Mean intrabony width (IDW) | 2.7 mm (±0.65) | 2.67 mm (±0.61) | NS (p ** = 0.98) |
| (A) | ||||
| 2 Weeks Pre op. | Baseline | 6 Months Post op. | p * | |
| FMPS | ||||
| Test group | 15.19% (±5.9) | 12.35% (±5.19) | 23.19% (±11.28) | 0.001 |
| Control group | 15.6% (±5.48) | 13.39% (±5.93) | 17.03% (±8.56) | 0.031 |
| p ** | NS (p ** = 0.86) | NS (p ** = 0.35) | NS (p ** = 0.054) | - |
| FMBOP | ||||
| Test group | 13.66% (±5.28%) | 12.14% (±5.32) | 21.02% (±9.61) | NS |
| Control group | 16.69% (±4.36%) | 16.95% (±4.3) | 19.78% (±12.42) | NS |
| p ** | NS (p ** = 0.061) | 0.003 | NS (p ** = 0.473) | - |
| PI | ||||
| Test group | 23.75% (±20.63) | 15% (±14.95) | 27.5% (±22.79) | 0.022 |
| Control group | 17.5% (±16.42) | 15% (±18.84) | 16.25% (±23.33) | NS |
| p ** | NS (p ** = 0.343) | NS (p ** = 0.821) | NS (p ** = 0.108) | - |
| BOP | ||||
| Test group | 40% (±18.84) | 30% (±17.39) | 27.5% (±21.3) | NS |
| Control group | 45% (±22.36) | 33.75% (±18.62) | 32.5% (±28.21) | 0.047 |
| p ** | NS (p ** = 0.506) | NS (p ** = 0.446) | NS (p ** = 0.792) | - |
| (B) | ||||
| 2 Weeks Pre op. | Baseline | 6 Months Post op. | p * | |
| PD | ||||
| Test group | 6.8 mm (±0.95) | 6.7 mm (±0.86) | 3.8 mm (±0.69) | <0.001 |
| Control group | 6.95 mm (±0.88) | 6.85% (±0.67) | 3.95 mm (±1.09) | <0.001 |
| p ** | NS (p ** = 0.526) | NS (p ** = 0.414) | NS (p ** = 0.745) | |
| CAL | ||||
| Test group | 8.2 mm (±1.98) | 8.1 mm (±1.91) | 5.85 mm (±1.98) | <0.001 |
| Control group | 7.5 mm (±1.14) | 7.4 mm (±0.99) | 4.55 mm (±1.79) | <0.001 |
| p ** | NS (p ** = 0.361) | NS (p ** = 0.335) | NS (p ** = 0.05) | |
| GR | ||||
| Test group | 1.4 mm (±1.56) | 1.4 mm (±1.56) | 2.05 mm (±1.9) | <0.001 |
| Control group | 0.55 mm (±0.82) | 0.55 mm (±0.82) | 0.75 mm (±0.85) | NS |
| p ** | NS (p ** = 0.078) | NS (p ** = 0.078) | 0.021 | |
| KT | ||||
| Test group | 4.5 mm (±2.16) | 4.6 mm (±2.13) | 4.3 mm (±2.05) | NS |
| Control group | 4.65 mm (±1.87) | 4.65 mm (±1.87) | 4.75 mm (±2.12) | NS |
| p ** | NS (p ** = 0.857) | NS (p ** = 0.989) | NS (p ** = 0.563) | |
| 2 Weeks Pre Op. | Baseline | p *** | 3 Months Post Op. | p * | ||
|---|---|---|---|---|---|---|
| Treponema denticola | Test group | 3.99 × 105 (±9.36 × 105) | 1.17 × 104 (±6.41 × 106) | <0.001 | 9.93 × 105 (±2.55 × 106) | <0.001 |
| Control group | 3.99 × 105 (±9.36 × 105) | 2.28 × 105 (±8.51 × 105) | NS | 7.69 × 105 (±1.94 × 106) | NS | |
| p ** | NS | NS | - | NS | - | |
| Fusobacterium nucleatum | Test group | 3.27 × 105 (±8.83 × 105) | 2.68 × 104 (±6.38 × 104) | 0.002 | 2.49 × 106 (±1.07 × 107) | 0.001 |
| Control group | 1.65 × 105 (±3.77 × 105) | 7.38 × 104 (±1.01 × 105) | NS | 8.7 × 104 (±2.23 × 105) | NS | |
| p ** | NS | 0.019 | - | NS | - | |
| Tannerella forsythia | Test group | 5.9 × 105 (±1.49 × 106) | 1.17 × 104 (±4.01 × 104) | 0.001 | 1.38 × 105 (±3.59 × 105) | 0.021 |
| Control group | 4.48 × 105 (±8.18 × 105) | 2.33 × 105 (±8.51 × 105) | 0.032 | 1.4 × 105 (±4.36 × 105) | 0.018 | |
| p ** | NS | 0.026 | - | NS | - | |
| Porphyromonas gingivalis | Test group | 1.99 × 106 (±4.95 × 106) | 1.12 × 105 (±3.8 × 105) | <0.001 | 9.37 × 105 (±2.78 × 106) | 0.001 |
| Control group | 1.31 × 106 (±4.26 × 106) | 6.83 × 105 (±2.43 × 106) | 0.008 | 1.68 × 105 (±7.35 × 105) | NS | |
| p ** | NS | NS | - | NS | - | |
| Prevotella intermedia | Test group | 7.15 × 103 (±2.73 × 104) | 1.98 × 102 (±5.65 × 102) | 0.009 | 9.86 × 104 (±4.13 × 105) | 0.019 |
| Control group | 2.11 × 105 (±7.65 × 105) | 3.34 × 105 (±1.26 × 106) | NS | 5.98 × 105 (±2.57 × 106) | NS | |
| p ** | NS | 0.03 | - | NS | - | |
| Aggregatibacter actinomycetemcomitans | Test group | 1.12 × 107 (±3.47 × 107) | 2.92 × 106 (±1.3 × 107) | NS | 3.96 × 106 (±1.77 × 107) | NS |
| Control group | 2.69 × 105 (±8.59 × 105) | 9.41 × 105 (±4.13 × 106) | NS | 1.38 × 105 (±3.75 × 105) | NS | |
| p ** | NS | NS | - | 0.002 | - |
| 2 Weeks Pre Op. | 6 Months Post Op. | p *** (2 Weeks Pre Op–6 Months Post Op) | |
|---|---|---|---|
| R IDD (mm) | |||
| Test group | 3.53 (±0.92) | 1.65 (±0.78) | <0.00 |
| Control group | 3.43 (±0.57) | 1.57 (±1.05) | <0.00 |
| p ** | NS (p ** = 0.807) | NS (p ** = 0.87) | - |
| R IDW (mm) | |||
| Test group | 2.14 (±0.68) | 1.77 (±0.84) | <0.00 |
| Control group | 1.96 (±0.76) | 1.25 (±1.06) | <0.00 |
| p ** | NS (p ** = 0.506) | NS (p ** = 0.082) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skurska, A.; Baczewska, A.; Milewski, R.; Majewski, P.; Charkiewicz, R. The Influence of Local Antibiotic Therapy on the Microbiological, Clinical, and Radiological Outcomes Following Minimally Invasive Periodontal Surgery in the Treatment of Intrabony Defects—A Randomized Clinical Trial. Antibiotics 2025, 14, 850. https://doi.org/10.3390/antibiotics14090850
Skurska A, Baczewska A, Milewski R, Majewski P, Charkiewicz R. The Influence of Local Antibiotic Therapy on the Microbiological, Clinical, and Radiological Outcomes Following Minimally Invasive Periodontal Surgery in the Treatment of Intrabony Defects—A Randomized Clinical Trial. Antibiotics. 2025; 14(9):850. https://doi.org/10.3390/antibiotics14090850
Chicago/Turabian StyleSkurska, Anna, Amelia Baczewska, Robert Milewski, Piotr Majewski, and Radosław Charkiewicz. 2025. "The Influence of Local Antibiotic Therapy on the Microbiological, Clinical, and Radiological Outcomes Following Minimally Invasive Periodontal Surgery in the Treatment of Intrabony Defects—A Randomized Clinical Trial" Antibiotics 14, no. 9: 850. https://doi.org/10.3390/antibiotics14090850
APA StyleSkurska, A., Baczewska, A., Milewski, R., Majewski, P., & Charkiewicz, R. (2025). The Influence of Local Antibiotic Therapy on the Microbiological, Clinical, and Radiological Outcomes Following Minimally Invasive Periodontal Surgery in the Treatment of Intrabony Defects—A Randomized Clinical Trial. Antibiotics, 14(9), 850. https://doi.org/10.3390/antibiotics14090850







