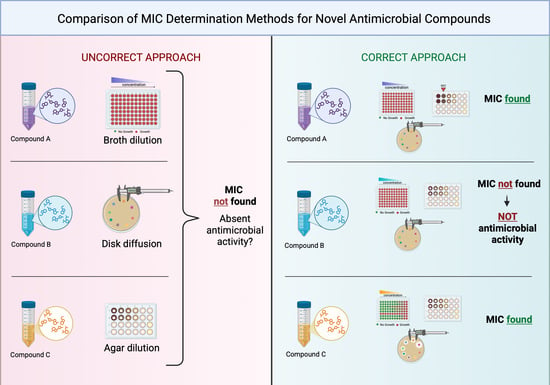
Beyond One-Size-Fits-All: Addressing Methodological Constraints in Novel Antimicrobials Discovery
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Materials
Microbial Strains and Culture Conditions
4.2. Methods
4.2.1. Mechanosynthesis of Natural Extract/SiO2 Nanocomposites
4.2.2. Extraction Procedure
4.2.3. Disk Diffusion
4.2.4. Broth Dilution
4.2.5. Agar Dilution
4.2.6. Literature Search
4.2.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | Amoxicillin/clavulanic acid |
| AM | Arnica montana extract |
| AMR | Antimicrobial resistance |
| AST | Antimicrobial susceptibility testing |
| BmimPF6 | 1-Butyl-3-methylimidazolium hexa-fluorophosphate |
| CLSI | Clinical and Laboratory Standards Institute |
| ECOFFs | Clinical breakpoints and epidemiological cut-off values |
| ER | Erythromycin |
| EUCAST | European Committee on Antimicrobial Susceptibility Testing |
| GE | Grapefruit Extract |
| HP | Harpagophytum procumbens extract |
| HP/SiO2 | Harpagophytum procumbens extract/SiO2 |
| HmimTFS | 1-Decyl-3-methylimidazolium bis (trifluoromethylsulfonyl)imide |
| IHME | Institute for Health Metrics and Evaluation |
| KS | Kanamycin sulfate |
| MBC | Minimum Bactericidal Concentration |
| MHA | Muller Hinton Agar |
| MIC | Minimum Inhibitory Concentration |
| OF | Ofloxacin |
| OOO | Ozonated olive oil |
| OSO | Ozonated sunflower oil |
| PE | Polyphenol Emma extract |
| PS | Polyphenol Solgar extract |
| RC | Rosa canina extract |
| RC/SiO2 | Rosa canina extract/SiO2 |
| RF | Rifampicin |
| RX | Rifaximin |
References
- Antibacterial Agents in Clinical and Preclinical Development: An Overview and Analysis. 2021. Available online: https://www.who.int/publications/i/item/9789240047655 (accessed on 4 August 2025).
- Walsh, C. Where Will New Antibiotics Come From? Nat. Rev. Microbiol. 2003, 1, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Iskandar, K.; Molinier, L.; Hallit, S.; Sartelli, M.; Catena, F.; Coccolini, F.; Craig Hardcastle, T.; Roques, C.; Salameh, P. Drivers of Antibiotic Resistance Transmission in Low- and Middle-Income Countries from a “One Health” Perspective—A Review. Antibiotics 2020, 9, 372. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance (AMR). Available online: https://www.healthdata.org/research-analysis/health-topics/antimicrobial-resistance-amr (accessed on 4 August 2025).
- Boi, S.; Puxeddu, S.; Delogu, I.; Farci, D.; Piano, D.; Manzin, A.; Ceccarelli, M.; Angius, F.; Scorciapino, M.A.; Milenkovic, S. Seeking Correlation Among Porin Permeabilities and Minimum Inhibitory Concentrations Through Machine Learning: A Promising Route to the Essential Molecular Descriptors. Molecules 2025, 30, 1224. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y. Search. Available online: https://onlinelibrary.wiley.com/authored-by/Wang/Yu (accessed on 4 August 2025).
- Castangia, I.; Marongiu, F.; Manca, M.L.; Pompei, R.; Angius, F.; Ardu, A.; Fadda, A.M.; Manconi, M.; Ennas, G. Combination of Grape Extract-Silver Nanoparticles and Liposomes: A Totally Green Approach. Eur. J. Pharm. Sci. 2017, 97, 62–69. [Google Scholar] [CrossRef]
- Fulgheri, F.; Angius, F.; Perra, M.; Delogu, I.; Puxeddu, S.; Georgiev, M.I.; Novotná, R.; Franková, J.; Lobina, M.; Manzin, A.; et al. Liposomal Delivery of a Biotechnological Lavandula Angustifolia Miller Extract Rich in Rosmarinic Acid for Topical Herpes Simplex Therapy. Antioxidants 2025, 14, 811. [Google Scholar] [CrossRef]
- Kamble, M.G.; Singh, A.; Singh, S.V.; Kamble, M.G.; Sagar, N.A.; Rani, N. Nanotechnology for Encapsulation of Bioactive Components: A Review. Discov. Food 2025, 5, 116. [Google Scholar] [CrossRef]
- Hu, H.; Hua, S.Y.; Lin, X.; Lu, F.; Zhang, W.; Zhou, L.; Cui, J.; Wang, R.; Xia, J.; Xu, F.; et al. Hybrid Biomimetic Membrane Coated Particles-Mediated Bacterial Ferroptosis for Acute MRSA Pneumonia. ACS Nano 2023, 17, 11692–11712. [Google Scholar] [CrossRef]
- Zhou, Z.; Kai, M.; Wang, S.; Wang, D.; Peng, Y.; Yu, Y.; Gao, W.; Zhang, L. Emerging Nanoparticle Designs against Bacterial Infections. WIREs Nanomed. Nanobiotechnol. 2023, 15, e1881. [Google Scholar] [CrossRef]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Micol, V. Tackling Antibiotic Resistance with Compounds of Natural Origin: A Comprehensive Review. Biomedicines 2020, 8, 405. [Google Scholar] [CrossRef]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Antibacterial Plant Compounds, Extracts and Essential Oils: An Updated Review on Their Effects and Putative Mechanisms of Action. Phytomedicine 2021, 90, 153626. [Google Scholar] [CrossRef]
- Vojtovič, D.; Luhová, L.; Petřivalský, M. Something Smells Bad to Plant Pathogens: Production of Hydrogen Sulfide in Plants and Its Role in Plant Defence Responses. J. Adv. Res. 2020, 27, 199–209. [Google Scholar] [CrossRef]
- Chojnacka, K.; Witek-Krowiak, A.; Skrzypczak, D.; Mikula, K.; Młynarz, P. Phytochemicals Containing Biologically Active Polyphenols as an Effective Agent against COVID-19-Inducing Coronavirus. J. Funct. Foods 2020, 73, 104146. [Google Scholar] [CrossRef]
- Guzman, J.D. Natural Cinnamic Acids, Synthetic Derivatives and Hybrids with Antimicrobial Activity. Molecules 2014, 19, 19292–19349. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Hossain, M.E.; Mannan Mithi, F.; Ahmed, M.; Saldías, M.; Akkol, E.K.; Sobarzo-Sánchez, E. Multifunctional Therapeutic Potential of Phytocomplexes and Natural Extracts for Antimicrobial Properties. Antibiotics 2021, 10, 1076. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, S. Phytochemicals for Bacterial Resistance--Strengths, Weaknesses and Opportunities. Planta Med. 2008, 74, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Thakur, L.; Ghodasra, U.; Patel, N.; Dabhi, M. Novel Approaches for Stability Improvement in Natural Medicines. Pharmacogn. Rev. 2011, 5, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Kumar, V.; Yadav, S.K. Nanotechnology: A Tool to Enhance Therapeutic Values of Natural Plant Products. ScienceAlert 2012, 7, 34–42. Available online: https://scialert.net/abstract/?doi=tmr.2012.34.42&utm (accessed on 4 August 2025).
- Manconi, M.; Marongiu, F.; Castangia, I.; Manca, M.L.; Caddeo, C.; Tuberoso, C.I.G.; D’hallewin, G.; Bacchetta, G.; Fadda, A.M. Polymer-Associated Liposomes for the Oral Delivery of Grape Pomace Extract. Colloids Surf. B Biointerfaces 2016, 146, 910–917. [Google Scholar] [CrossRef]
- Scano, A.; Ebau, F.; Manca, M.L.; Cabras, V.; Cesare Marincola, F.; Manconi, M.; Pilloni, M.; Fadda, A.M.; Ennas, G. Novel Drug Delivery Systems for Natural Extracts: The Case Study of Vitis Vinifera Extract-SiO2 Nanocomposites. Int. J. Pharm. 2018, 551, 84–96. [Google Scholar] [CrossRef]
- Xue, H.; Zhao, J.; Wang, Y.; Shi, Z.; Xie, K.; Liao, X.; Tan, J. Factors Affecting the Stability of Anthocyanins and Strategies for Improving Their Stability: A Review. Food Chem. X 2024, 24, 101883. [Google Scholar] [CrossRef]
- Nagalingam, A. Chapter 15—Drug Delivery Aspects of Herbal Medicines. In Japanese Kampo Medicines for the Treatment of Common Diseases: Focus on Inflammation; Arumugam, S., Watanabe, K., Eds.; Academic Press: San Diego, CA, USA, 2017; pp. 143–164. ISBN 978-0-12-809398-6. [Google Scholar]
- Fernandes, M.M.; Carvalho, E.O.; Correia, D.M.; Esperança, J.M.S.S.; Padrão, J.; Ivanova, K.; Hoyo, J.; Tzanov, T.; Lanceros-Mendez, S. Ionic Liquids as Biocompatible Antibacterial Agents: A Case Study on Structure-Related Bioactivity on Escherichia Coli. ACS Appl. Bio Mater. 2022, 5, 5181–5189. [Google Scholar] [CrossRef]
- Hafeez, S.; Rasool, Z.; Hafeez, S.; Paracha, R.Z.; Iqbal, M.; Khan, D.; Adnan, F. Imidazolium, Pyridinium and Pyrazinium Based Ionic Liquids with Octyl Side Chains as Potential Antibacterial Agents against Multidrug Resistant Uropathogenic E. coli. Heliyon 2024, 10, e39829. [Google Scholar] [CrossRef]
- Novello, E.; Scalzo, G.; D’Agata, G.; Raucci, M.G.; Ambrosio, L.; Soriente, A.; Tomasello, B.; Restuccia, C.; Parafati, L.; Consoli, G.M.L.; et al. Synthesis, Characterisation, and In Vitro Evaluation of Biocompatibility, Antibacterial and Antitumor Activity of Imidazolium Ionic Liquids. Pharmaceutics 2024, 16, 642. [Google Scholar] [CrossRef]
- Ferraz, R.; Silva, D.; Dias, A.R.; Dias, V.; Santos, M.M.; Pinheiro, L.; Prudêncio, C.; Noronha, J.P.; Petrovski, Ž.; Branco, L.C. Synthesis and Antibacterial Activity of Ionic Liquids and Organic Salts Based on Penicillin G and Amoxicillin Hydrolysate Derivatives against Resistant Bacteria. Pharmaceutics 2020, 12, 221. [Google Scholar] [CrossRef] [PubMed]
- Puxeddu, S.; Scano, A.; Scorciapino, M.A.; Delogu, I.; Vascellari, S.; Ennas, G.; Manzin, A.; Angius, F. Physico-Chemical Investigation and Antimicrobial Efficacy of Ozonated Oils: The Case Study of Commercial Ozonated Olive and Sunflower Seed Refined Oils. Molecules 2024, 29, 679. [Google Scholar] [CrossRef] [PubMed]
- M07|Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. Available online: https://clsi.org/shop/standards/m07/ (accessed on 4 August 2025).
- Eucast: MIC Determination. Available online: https://www.eucast.org/ast_of_bacteria/mic_determination (accessed on 4 August 2025).
- M26|Methods for Determining Bactericidal Activity of Antimicrobial Agents. Available online: https://clsi.org/shop/standards/m26/ (accessed on 4 August 2025).
- ISO 20776-1:2020; Susceptibility Testing of Infectious Agents and Evaluation of Performance of Antimicrobial Susceptibility Test Devices—Part 1: Broth Micro-Dilution Reference Method for Testing the In Vitro Activity of Antimicrobial Agents Against Rapidly Growing Aerobic Bacteria Involved in Infectious Diseases. International Organization for Standardization (ISO): Geneva, Switzerland, 2020.
- Hattab, S.; Ma, A.H.; Tariq, Z.; Vega Prado, I.; Drobish, I.; Lee, R.; Yee, R. Rapid Phenotypic and Genotypic Antimicrobial Susceptibility Testing Approaches for Use in the Clinical Laboratory. Antibiotics 2024, 13, 786. [Google Scholar] [CrossRef]
- Eucast: Warnings! Available online: https://www.eucast.org/ast-of-bacteria/warnings?utm (accessed on 4 August 2025).
- Eucast: MIC and Zone Distributions and ECOFFs. Available online: https://www.eucast.org/mic_and_zone_distributions_and_ecoffs (accessed on 4 August 2025).
- M100|Performance Standards for Antimicrobial Susceptibility Testing. Available online: https://clsi.org/shop/standards/m100/ (accessed on 4 August 2025).
- Eloff, J. A Sensitive and Quick Microplate Method to Determine the Minimal Inhibitory Concentration of Plant Extracts for Bacteria. Planta Med. 1998, 64, 711–713. [Google Scholar] [CrossRef]
- Muraina, I.A.; Picard, J.; Eloff, J.N. Development of a Reproducible Method to Determine Minimum Inhibitory Concentration (MIC) of Plant Extract against a Slow-Growing Mycoplasmas Organism. Phytomedicine 2009, 16, 262–264. [Google Scholar] [CrossRef][Green Version]
- Golus, J.; Sawicki, R.; Widelski, J.; Ginalska, G. The Agar Microdilution Method—A New Method for Antimicrobial Susceptibility Testing for Essential Oils and Plant Extracts. J. Appl. Microbiol. 2016, 121, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- Barbarossa, A.; Rosato, A.; Corbo, F.; Clodoveo, M.L.; Fracchiolla, G.; Carrieri, A.; Carocci, A. Non-Antibiotic Drug Repositioning as an Alternative Antimicrobial Approach. Antibiotics 2022, 11, 816. [Google Scholar] [CrossRef]
- Foletto, V.S.; da Rosa, T.F.; Serafin, M.B.; Bottega, A.; Hörner, R. Repositioning of non-antibiotic drugs as an alternative to microbial resistance: A systematic review. Int. J. Antimicrob. Agents 2021, 58, 106380. [Google Scholar] [CrossRef] [PubMed]
- Boyd, N.K.; Teng, C.; Frei, C.R. Brief Overview of Approaches and Challenges in New Antibiotic Development: A Focus on Drug Repurposing. Frontiers 2021, 11, 684515. [Google Scholar] [CrossRef]
- Bonev, B.; Hooper, J.; Parisot, J. Principles of Assessing Bacterial Susceptibility to Antibiotics Using the Agar Diffusion Method. J. Antimicrob. Chemother. 2008, 61, 1295–1301. [Google Scholar] [CrossRef]
- Scano, A.; Mereu, E.; Cabras, V.; Mannias, G.; Garau, A.; Pilloni, M.; Orrù, G.; Scano, A.; Ennas, G. Green Preparation of Antimicrobial 1D-Coordination Polymers: [Zn(4,4′-Bipy)Cl2]∞ and [Zn(4,4′-Bipy)2(OAc)2]∞ by Ultrasonication of Zn(II) Salts and 4,4′-Bipyridine. Molecules 2022, 27, 6677. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in Vitro Evaluating Antimicrobial Activity: A Review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Cos, P.; Vlietinck, A.J.; Berghe, D.V.; Maes, L. Anti-Infective Potential of Natural Products: How to Develop a Stronger in Vitro ‘Proof-of-Concept’. J. Ethnopharmacol. 2006, 106, 290–302. [Google Scholar] [CrossRef]
- Reller, L.B.; Weinstein, M.; Jorgensen, J.H.; Ferraro, M.J. Antimicrobial Susceptibility Testing: A Review of General Principles and Contemporary Practices. Clin. Infect. Dis. 2009, 49, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Štumpf, S.; Hostnik, G.; Primožič, M.; Leitgeb, M.; Salminen, J.-P.; Bren, U. The Effect of Growth Medium Strength on Minimum Inhibitory Concentrations of Tannins and Tannin Extracts against E. coli. Molecules 2020, 25, 2947. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and Broth Dilution Methods to Determine the Minimal Inhibitory Concentration (MIC) of Antimicrobial Substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Abbasi, F.; Hashemi, H.; Samaei, M.R.; SavarDashtaki, A.; Azhdarpoor, A.; Fallahi, M.J. The Synergistic Interference Effect of Silica Nanoparticles Concentration and the Wavelength of ELISA on the Colorimetric Assay of Cell Toxicity. Sci. Rep. 2021, 11, 15133. [Google Scholar] [CrossRef]
- Zu, B.; Li, W.; Lan, L.; Liu, Y.; Zhang, Y.; Li, J.; Mei, X. Adsorption of Tylosin and Tetracycline onto Microplastics: Behavior and Effects of Adsorbents and Salinity. Water Air Soil Pollut. 2023, 234, 582. [Google Scholar] [CrossRef]
- Hossain, T.J. Methods for Screening and Evaluation of Antimicrobial Activity: A Review of Protocols, Advantages, and Limitations. Eur. J. Microbiol. Immunol. 2024, 14, 97–115. [Google Scholar] [CrossRef] [PubMed]
- Salam, M.A.; Al-Amin, M.Y.; Pawar, J.S.; Akhter, N.; Lucy, I.B. Conventional Methods and Future Trends in Antimicrobial Susceptibility Testing. Saudi J. Biol. Sci. 2023, 30, 103582. [Google Scholar] [CrossRef]
- Colacino, E.; Delogu, F.; Hanusa, T. Advances in Mechanochemistry. ACS Sustain. Chem. Eng. 2021, 9, 10662–10663. [Google Scholar] [CrossRef]
- Solares-Briones, M.; Coyote-Dotor, G.; Páez-Franco, J.C.; Zermeño-Ortega, M.R.; de la O Contreras, C.M.; Canseco-González, D.; Avila-Sorrosa, A.; Morales-Morales, D.; Germán-Acacio, J.M. Mechanochemistry: A Green Approach in the Preparation of Pharmaceutical Cocrystals. Pharmaceutics 2021, 13, 790. [Google Scholar] [CrossRef]
- Reynes, J.F.; Leon, F.; García, F. Mechanochemistry for Organic and Inorganic Synthesis. ACS Org. Inorg. 2024, 4, 432–470. [Google Scholar] [CrossRef]
- Baláž, M.; Bedlovičová, Z.; Daneu, N.; Siksa, P.; Sokoli, L.; Tkáčiková, Ľ.; Salayová, A.; Džunda, R.; Kováčová, M.; Bureš, R.; et al. Mechanochemistry as an Alternative Method of Green Synthesis of Silver Nanoparticles with Antibacterial Activity: A Comparative Study. Nanomaterials 2021, 11, 1139. [Google Scholar] [CrossRef]
- Pilloni, M.; Kumar, V.B.; Ennas, G.; Porat, Z.; Scano, A.; Cabras, V.; Gedanken, A. Formation of metallic silver and copper in non-aqueous media by ultrasonic radiation. Ultrason. Sonochem. 2018, 47, 108–113. [Google Scholar] [CrossRef]
- Suslick, K.S.; Didenko, Y.; Fang, M.M.; Hyeon, T.; Kolbeck, K.J.; McNamara, W.B.; Millan, M.M.; Wong, M. Acoustic cavitation and its chemical consequences. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Eng. Sci. 1999, 357, 335–353. [Google Scholar] [CrossRef]
- Suslick, K.S.; Price, G.J. Applications of ultrasound to materials chemistry. Annu. Rev. Mater. Sci. 1999, 29, 295–326. [Google Scholar] [CrossRef]
- Leong, T.; Ashokkumar, M.; Kentish, S. The fundamentals of power ultrasound—A review. Acoust. Aust. 2011, 39, 54–63. [Google Scholar]

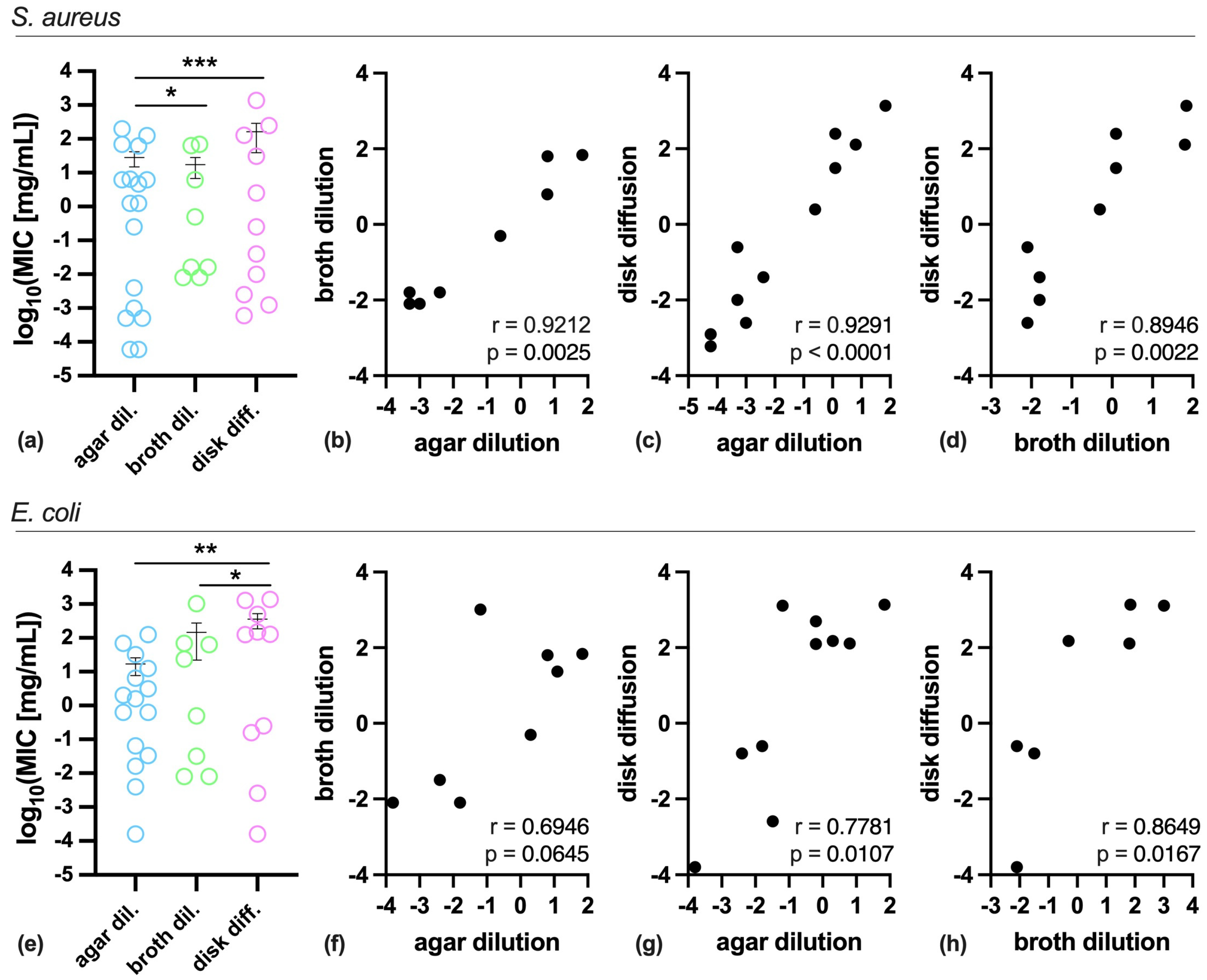
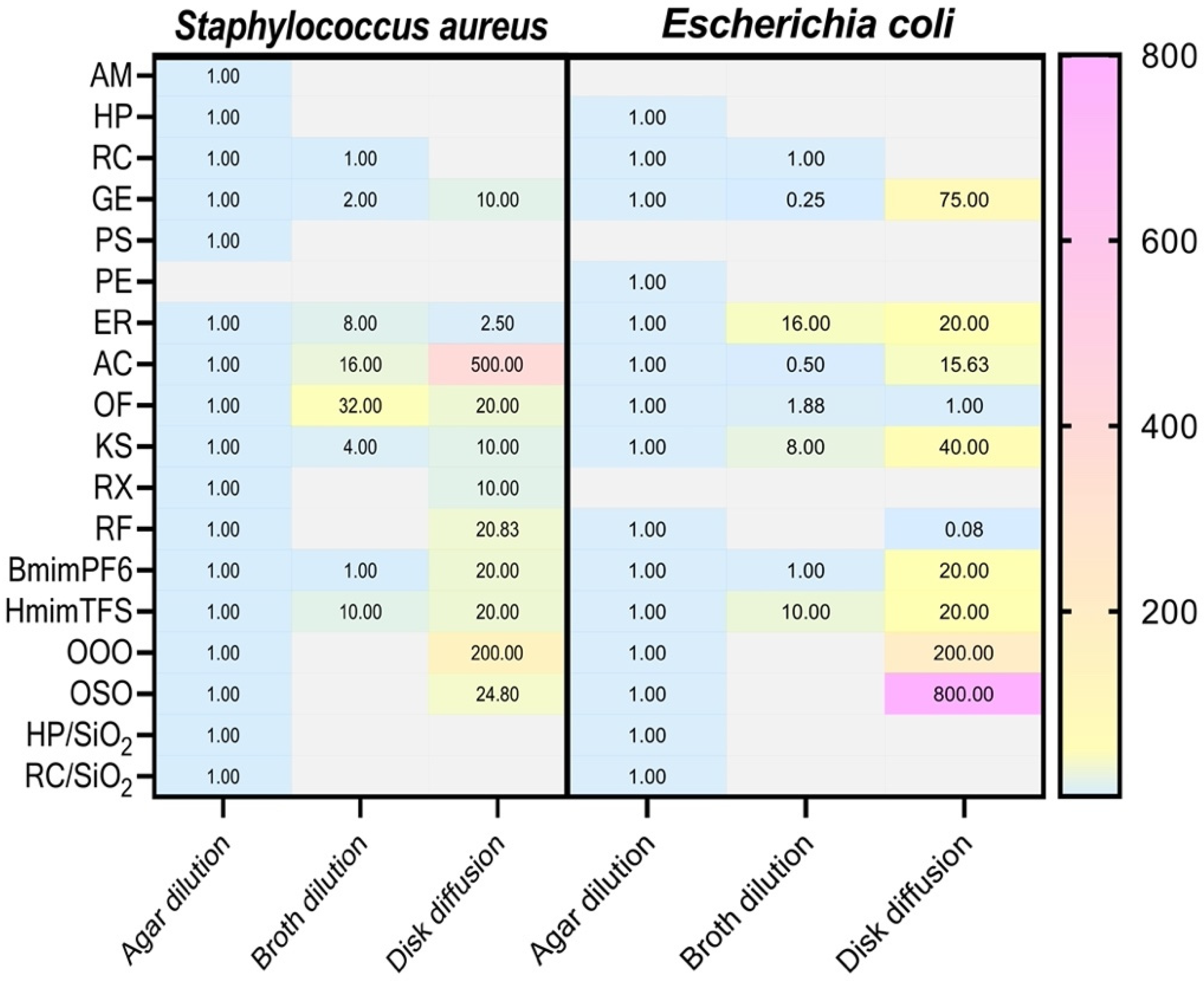

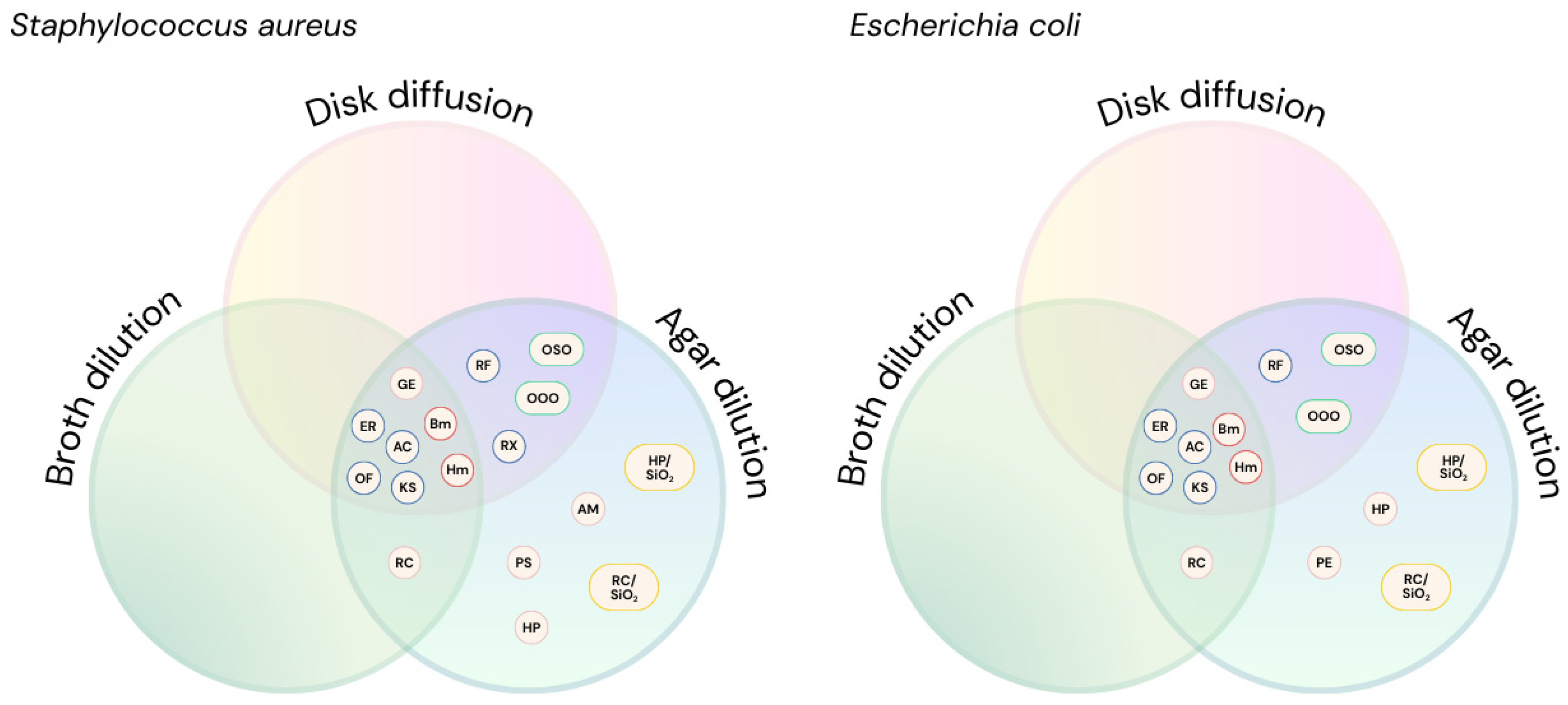
| Substance | S. aureus | E. coli | |||||
|---|---|---|---|---|---|---|---|
| Agar Dilution | Broth Dilution | Disk Diffusion | Agar Dilution | Broth Dilution | Disk Diffusion | ||
| Arnica montana extract | AM | 200.00 ± 52.11 | >200 | >200 | >200 | >200 | >200 |
| Harpagophytum procumbens extract | HP | 8.33 ± 3.61 | >200 | >200 | 4.16 ± 1.81 | >200 | >200 |
| Rosa canina extract | RC | 8.33 ± 3.61 | 6.25 ± 2.18 | >200 | 10.42 ± 3.61 | 12.5 ± 4.37 | >200 |
| Grapefruit extract | GE | 0.25 ± 0.08 | 0.50 ± 0.16 | 2.50 ± 0.87 | 2.00 ± 0.07 | 0.67 ± 0.29 | 150.00 ± 52.53 |
| Polyphenols Solgar extract | PS | 4.65 ± 1.62 | >9.3 | >186 | >9.3 | >9.3 | >186 |
| Polyphenols EMMA extract | PE | >1.6 | >1.6 | >32 | 1.60 ± 0.56 | >1.6 | >32 |
| Erythromycin | ER | 0.0008 ± 0.0003 | 0.008 ± 0.0028 | 0.0038 ± 0.0018 | 0.086 ± 0.037 | 0.77 ± 0.36 | 1.28 ± 0.44 |
| Amoxicillin/clavulanic acid | AC | 0.0005 ± 0.0002 | 0.0067 ± 0.0023 | 0.25 ± 0.08 | 0.021 ± 0.009 | 0.008 ± 0.0028 | 0.21 ± 0.07 |
| Ofloxacin | OF | 0.00042 ± 0.00014 | 0.016 ± 0.0056 | 0.0083 ± 0.0029 | 0.000012 ± 0.000006 | 0.00003 ± 0.00001 | 0.00013 ± 0.00005 |
| Kanamycin sulfate | KS | 0.004 ± 0.0014 | 0.19 ± 0.09 | 0.040 ± 0.014 | 0.010 ± 0.008 | 0.043 ± 0.018 | 0.24 ± 0.11 |
| Rifaximin | RX | 0.00006 ± 0.00002 | >0.128 | 0.0006 ± 0.0002 | >1.024 | >0.128 | >20.48 |
| Rifampicin | RF | 0.000093 ± 0.000032 | >0.128 | 0.00125 ± 0.00043 | 0.048 ± 0.023 | >0.128 | 1.92 ± 0.91 |
| 1-Butyl-3-methylimidazolium hexa-fluorophosphate | BmimPF6 | 69.00 ± 24.15 | 69.00 ± 24.15 | 1380.00 ± 438.00 | 69.00 ± 24.15 | 69.00 ± 24.15 | 1380.00 ± 438.00 |
| 1-Decyl-3-methyl imidazolium bis (trifluoromethylsulfonyl)imide | HmimTFS | 6.40 ± 2.24 | 64.00 ± 22.40 | 128.00 ± 44.80 | 6.40 ± 2.24 | 64.00 ± 22.40 | 128.00 ± 44.80 |
| Ozonated olive oil | OOO | 1.25 ± 0.43 | >50 | 208.33 ± 72.17 | 0.83 ± 0.36 | >50 | 125.00 ± 43.75 |
| Ozonated sunflower oil | OSO | 1.25 ± 0.43 | >50 | 25.83 ± 8.95 | 0.83 ± 0.36 | >50 | 500.00 ± 175.00 |
| Harpagophytum procumbens extract/SiO2 | HP/SiO2 | 125.00 ± 43.75 | >125 | >125 | 47.25 ± 21.57 | >125 | >125 |
| Rosa canina extract/SiO2 | RC/SiO2 | 93.75 ± 44.19 | >125 | >125 | 93.75 ± 44.19 | >125 | >125 |
| Substance | S. aureus | E. coli | |
|---|---|---|---|
| Arnica montana extract | AM | >200 | >200 |
| Harpagophytum procumbens extract | HP | >200 | >200 |
| Rosa canina extract | RC | 12.50 ± 4.37 | 20.83 ± 7.21 |
| Grapefruit Extract | GE | 0.83 ± 0.28 | 1.00 ± 0.35 |
| Polyphenols Solgar extract | PS | >9.3 | >9.3 |
| Polyphenols EMMA extract | PE | >1.6 | >1.6 |
| Erythromycin | ER | 0.016 ± 0.015 | >1.024 |
| Amoxicillin/clavulanic acid | AC | 0.008 ± 0.028 | 0.008 ± 0.028 |
| Ofloxacin | OF | 0.016 ± 0.005 | 0.000125 ± 0.000043 |
| Kanamycin sulfate | KS | 0.032 ± 0.011 | 0.032 ± 0.011 |
| Rifaximin | RX | >1.024 | >1.024 |
| Rifampicin | RF | >0.128 | >1.024 |
| 1-Butyl-3-methylimidazolium hexa-fluorophosphate | BmimPF6 | 69.00 ± 24.15 | 69.00 ± 24.15 |
| 1-Decyl-3-methyl imidazolium bis (trifluoromethylsulfonyl)imide | HmimTFS | 64.00 ± 22.4 | 53.33 ± 18.47 |
| Ozonated olive oil | OOO | 13.33 ± 5.77 | 13.33 ± 4.61 |
| Ozonated sunflower oil | OSO | 25.00 ± 8.75 | 30.00 ± 10.5 |
| Harpagophytum procumbens extract/SiO2 | HP/SiO2 | >125 | >125 |
| Rosa canina extract/SiO2 | RC/SiO2 | >125 | >125 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puxeddu, S.; Canton, S.; Scano, A.; Delogu, I.; Pibiri, A.; Cabriolu, C.; Vascellari, S.; Pettinau, F.; Pivetta, T.; Ennas, G.; et al. Beyond One-Size-Fits-All: Addressing Methodological Constraints in Novel Antimicrobials Discovery. Antibiotics 2025, 14, 848. https://doi.org/10.3390/antibiotics14080848
Puxeddu S, Canton S, Scano A, Delogu I, Pibiri A, Cabriolu C, Vascellari S, Pettinau F, Pivetta T, Ennas G, et al. Beyond One-Size-Fits-All: Addressing Methodological Constraints in Novel Antimicrobials Discovery. Antibiotics. 2025; 14(8):848. https://doi.org/10.3390/antibiotics14080848
Chicago/Turabian StylePuxeddu, Silvia, Serena Canton, Alessandra Scano, Ilenia Delogu, Andrea Pibiri, Cristiana Cabriolu, Sarah Vascellari, Francesca Pettinau, Tiziana Pivetta, Guido Ennas, and et al. 2025. "Beyond One-Size-Fits-All: Addressing Methodological Constraints in Novel Antimicrobials Discovery" Antibiotics 14, no. 8: 848. https://doi.org/10.3390/antibiotics14080848
APA StylePuxeddu, S., Canton, S., Scano, A., Delogu, I., Pibiri, A., Cabriolu, C., Vascellari, S., Pettinau, F., Pivetta, T., Ennas, G., Manzin, A., & Angius, F. (2025). Beyond One-Size-Fits-All: Addressing Methodological Constraints in Novel Antimicrobials Discovery. Antibiotics, 14(8), 848. https://doi.org/10.3390/antibiotics14080848