Antibiotic Prophylaxis in Instrumented Lumbar Spine Surgery: Cefazolin Outperforms Clindamycin Regardless of Duration
Abstract
1. Introduction
2. Results
2.1. Patients Data and Characteristics
2.2. Antibiotic Prophylaxis Regimens and the Prevalence of SSIs
2.3. Effect of Single Dose Antibiotic Prophylaxis—1 g Cefazolin, 2 g Cefazolin, and 0.6 g Clindamycin—On Rate of SSIs
2.4. Effect of Single Dose Versus 72 h Antibiotic Prophylaxis on SSI Occurance Between 2023 and 2024
3. Discussion
4. Patients and Methods
4.1. Patients Selection
4.2. Antibiotic Prophylaxis Protocols
4.3. SSI Definition, Variables Analyzed, and Exclusions
4.4. Statistic Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | body mass index |
| DM | diabetes mellitus |
| MISS | minimal invasive spine surgery |
| N/A | not available |
| OR | odds ratio |
| qu | quartile |
| SD | standard deviation |
| SSI | surgical site infection |
References
- Pull ter Gunne, A.F.; Cohen, D.B. Incidence, prevalence, and analysis of risk factors for surgical site infection following adult spinal surgery. Spine 2009, 34, 1422–1428. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.Q.; Sun, C.G.; Fei, Z.G.; Zhou, Q.J. Risk Factors for Surgical Site Infection After Spinal Surgery: A Systematic Review and Meta-Analysis Based on Twenty-Seven Studies. World Neurosurg. 2019, 123, e318–e329. [Google Scholar] [CrossRef] [PubMed]
- Fei, Q.; Li, J.; Lin, J.; Li, D.; Wang, B.; Meng, H.; Wang, Q.; Su, N.; Yang, Y. Risk Factors for Surgical Site Infection After Spinal Surgery: A Meta-Analysis. World Neurosurg. 2016, 95, 507–515. [Google Scholar] [CrossRef]
- Reisener, M.J.; Pumberger, M.; Shue, J.; Girardi, F.P.; Hughes, A.P. Trends in lumbar spinal fusion-a literature review. J. Spine Surg. 2020, 6, 752–761. [Google Scholar] [CrossRef]
- Sheikh, S.R.; Thompson, N.R.; Benzel, E.; Steinmetz, M.; Mroz, T.; Tomic, D.; Machado, A.; Jehi, L. Can We Justify It? Trends in the Utilization of Spinal Fusions and Associated Reimbursement. Neurosurgery 2020, 86, E193–E202. [Google Scholar] [CrossRef]
- Shawky Abdelgawaad, A.; El Sadik, M.H.M.; Hassan, K.M.; El-Sharkawi, M. Perioperative antibiotic prophylaxis in spinal surgery. SICOT-J 2021, 7, 31. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, R.; Huo, X.; Xiong, W.; Kang, L.; Xue, Y. Incidence of Surgical Site Infection After Spine Surgery: A Systematic Review and Meta-analysis. Spine 2020, 45, 208–216. [Google Scholar] [CrossRef]
- Freire-Archer, M.; Sarraj, M.; Koziarz, A.; Thornley, P.; Alshaalan, F.; Alnemari, H.; Kachur, E.; Bhandari, M.; Oitment, C. Incidence and Recurrence of Deep Spine Surgical Site Infections: A Systematic Review and Meta-analysis. Spine 2023, 48, E269–E285. [Google Scholar] [CrossRef]
- Zhang, T.; Lian, X.; Chen, Y.; Cai, B.; Xu, J. Clinical outcome of postoperative surgical site infections in patients with posterior thoracolumbar and lumbar instrumentation. J. Hosp. Infect. 2022, 128, 26–35. [Google Scholar] [CrossRef]
- Schimmel, J.J.; Horsting, P.P.; de Kleuver, M.; Wonders, G.; van Limbeek, J. Risk factors for deep surgical site infections after spinal fusion. Eur. Spine J. 2010, 19, 1711–1719. [Google Scholar] [CrossRef] [PubMed]
- Mangram, A.J.; Horan, T.C.; Pearson, M.L.; Silver, L.C.; Jarvis, W.R. Guideline for Prevention of Surgical Site Infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am. J. Infect. Control. 1999, 27, 97–132; quiz 133–134; discussion 196. [Google Scholar] [CrossRef]
- Mangram, A.J. A brief overview of the 1999 CDC Guideline for the Prevention of Surgical Site Infection. Centers for Disease Control and Prevention. J. Chemother. 2001, 13, 35–39. [Google Scholar] [CrossRef]
- Garcia, M.M.; Maya, I.O.; Lopez, J.L.; Dubois, J.G.; Cuevas, D.M.; Cruz, J.I.A.; Salvatierra, L.A.; Diaz, I.A.; Ovejero, A.M.H. Effects of Extended Oral Antibiotic Prophylaxis on Surgical Site Infections After Instrumented Spinal Fusion: A Cohort Study of 901 Patients With a Minimum Follow-up of 1 Year. Acta orthop. 2023, 94, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Bratzler, D.W.; Dellinger, E.P.; Olsen, K.M.; Perl, T.M.; Auwaerter, P.G.; Bolon, M.K.; Fish, D.N.; Napolitano, L.M.; Sawyer, R.G.; Slain, D.; et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Surg. Infect. 2013, 14, 73–156. [Google Scholar] [CrossRef] [PubMed]
- North American Spine Society Clinical Guidelines Committee. Evidence Based Vlinical Guidelines for Multidisciplinary Spine Surgery. 2013. Available online: https://www.spine.org/Research/Clinical-Guidelines (accessed on 12 August 2025).
- Marimuthu, C.; Abraham, V.T.; Ravichandran, M.; Achimuthu, R. Antimicrobial Prophylaxis in Instrumented Spinal Fusion Surgery: A Comparative Analysis of 24-Hour and 72-Hour Dosages. Asian Spine J. 2016, 10, 1018–1022. [Google Scholar] [CrossRef]
- Maciejczak, A.; Wolan-Nieroda, A.; Walaszek, M.; Kolpa, M.; Wolak, Z. Antibiotic prophylaxis in spine surgery: A comparison of single-dose and 72-hour protocols. J. Hosp. Infect. 2019, 103, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Pomares, P.F.; Santana, P.D.; Mateo, F.M.; Palleck, C.L.M.; Bermejo, F.T.; Galovich, L.Á. Comparison of Surgical Site Infection After Instrumented Spine Surgery in Patients With High Risk of Infection According to Different Antibiotic Prophylaxis Protocols: A Cohort Study of 132 Patients With a Minimum Follow-Up of 1 year. Glob. Spine. J. 2025, 15, 1890–1894. [Google Scholar] [CrossRef]
- Norvell, M.R.; Porter, M.; Ricco, M.H.; Koonce, R.C.; Hogan, C.A.; Basler, E.; Wong, M.; Jeffres, M.N. Cefazolin vs Second-line Antibiotics for Surgical Site Infection Prevention After Total Joint Arthroplasty Among Patients With a Beta-lactam Allergy. Open Forum Infect Dis. 2023, 10, ofad224. [Google Scholar] [CrossRef]
- Zastrow, R.K.; Huang, H.H.; Galatz, L.M.; Saunders-Hao, P.; Poeran, J.; Moucha, C.S. Characteristics of Antibiotic Prophylaxis and Risk of Surgical Site Infections in Primary Total Hip and Knee Arthroplasty. J. Arthroplasty. 2020, 35, 2581–2589. [Google Scholar] [CrossRef]
- Todd, B. New CDC Guideline for the Prevention of Surgical Site Infection. Am. J. Nurs. 2017, 117, 17. [Google Scholar] [CrossRef]
- Parvizi, J.; Shohat, N.; Gehrke, T. Prevention of periprosthetic joint infection: New guidelines. Bone Jt. J. 2017, 99-B, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.C.; Rainone, G.J.; Woodhouse, C.J.; Kramer, D.E.; Whiting, A.C. Post-operative antibiotic prophylaxis in spine surgery patients with thoracolumbar drains: A meta analysis. World Neurosurg. X 2024, 23, 100373. [Google Scholar] [CrossRef] [PubMed]
- Phillips, B.T.; Sheldon, E.S.; Orhurhu, V.; Ravinsky, R.A.; Freiser, M.E.; Asgarzadeh, M.; Viswanath, O.; Kaye, A.D.; Roguski, M. Preoperative Versus Extended Postoperative Antimicrobial Prophylaxis of Surgical Site Infection During Spinal Surgery: A Comprehensive Systematic Review and Meta-Analysis. Adv. Ther. 2020, 37, 2710–2733. [Google Scholar] [CrossRef] [PubMed]
- Burger, J.; Palmowski, Y.; Pumberger, M. Comprehensive treatment algorithm of postoperative spinal implant infection. J. Spine Surg. 2020, 6, 793–799. [Google Scholar] [CrossRef]
- Rohrer, F.; Maurer, A.; Noetzli, H.; Gahl, B.; Limacher, A.; Hermann, T.; Bruegger, J. Prolonged antibiotic prophylaxis use in elective orthopaedic surgery—A cross-sectional analysis. BMC Musculoskelet. Disord. 2021, 22, 420. [Google Scholar] [CrossRef]
- Horan, T.C.; Andrus, M.; Dudeck, M.A. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am. J. Infect. Control. 2008, 36, 309–332. [Google Scholar] [CrossRef]
- Parvizi, J.; Tan, T.L.; Goswami, K.; Higuera, C.; Della Valle, C.; Chen, A.F.; Shohat, N. The 2018 Definition of Periprosthetic Hip and Knee Infection: An Evidence-Based and Validated Criteria. J. Arthroplasty. 2018, 33, 1309–1314.E2. [Google Scholar] [CrossRef] [PubMed]
- Christensen, R.H.B. Ordinal: Regression Models for Ordinal Data. Available online: https://CRAN.R-project.org/package=ordinal (accessed on 12 August 2025).
- Lenth, V.R.; Banfai, B.; Bolker, B.; Buerkner, P.; Giné-Vázquez, I.; Herve, M.; Jung, M.; Love, J.; Miguez, F.; Piaskowski, J.; et al. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. Available online: https://rvlenth.github.io/emmeans/ (accessed on 12 August 2025).
- R_Core_Team. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 12 August 2025).


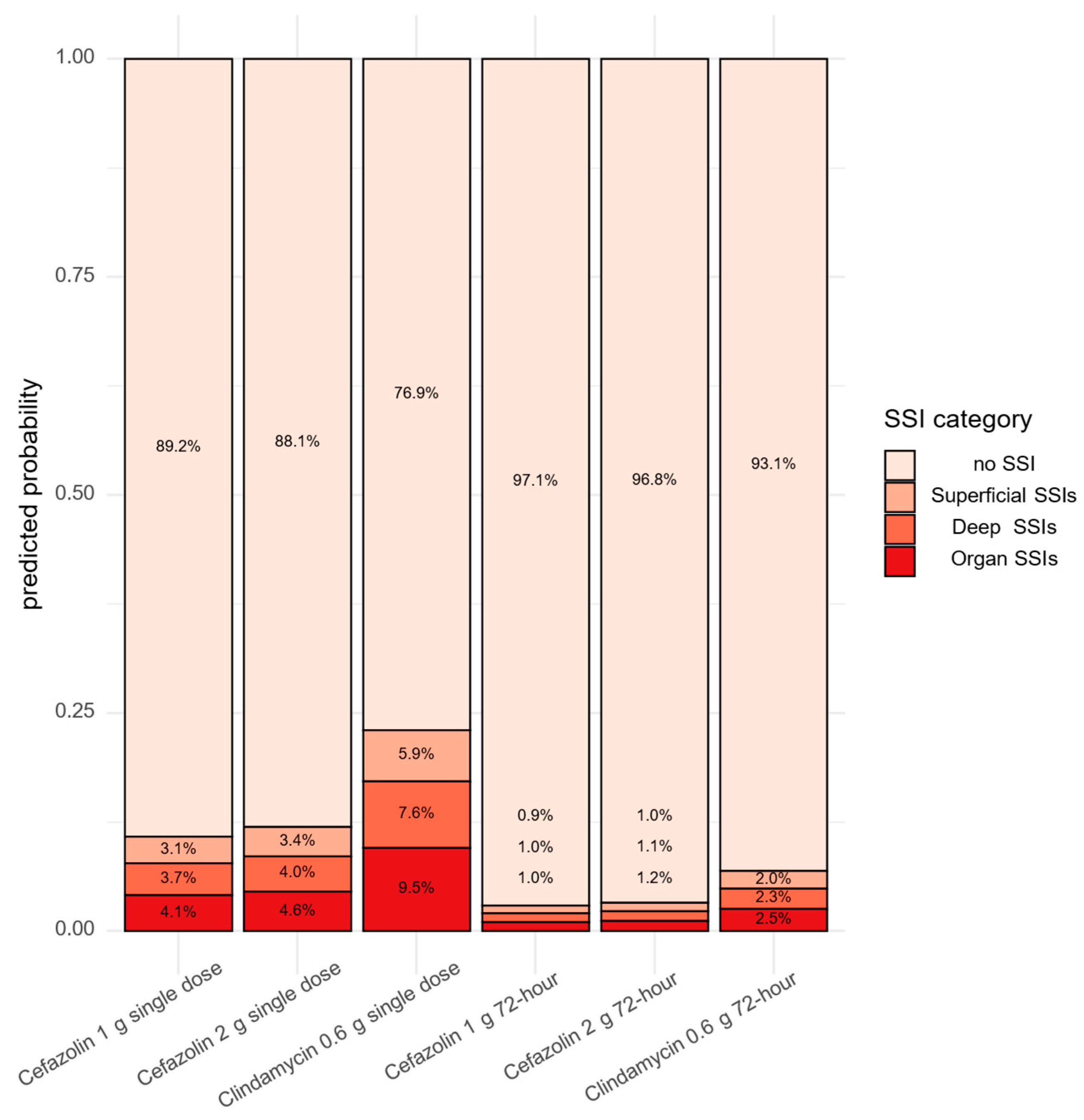
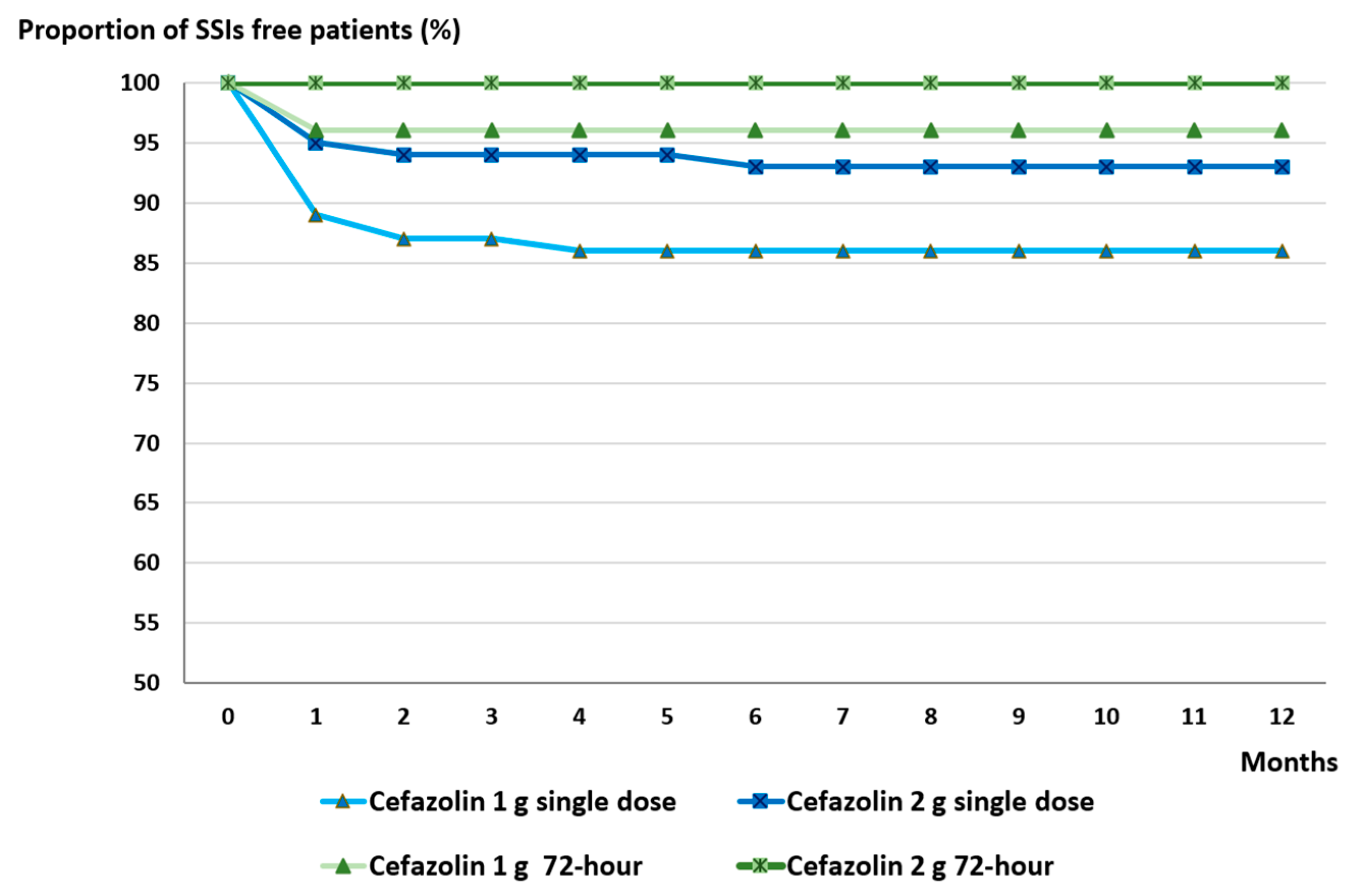
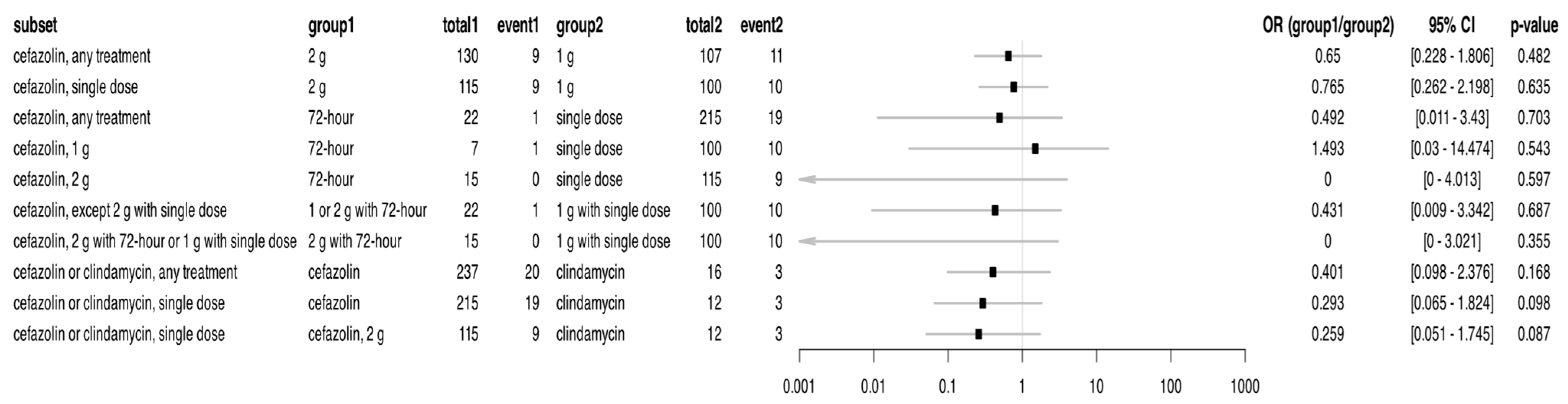
| Characteristics of the Patients | Non-Infected Cases (807) | SSI Cases (107) | p-Value |
|---|---|---|---|
| Age (years), mean (SD 1) | 63.4 (12.2) | 65.5 (11.6) | 0.0890 (Welch) |
| min; 1st qu. 2; median; 3rd qu.; max | 14.2; 56.6; 65.7; 72.3; 87.6 | 21.5; 60.6; 67.5; 73.6; 83.6 | |
| Male (%) | 330 (40.9%) | 36 (33.6%) | 0.1724 (Fisher) |
| Diabetes mellitus (%) | 169 (21.0%) (N/A 3 = 1) | 28 (26.2%) | 0.2136 (Fisher) |
| BMI 4, mean (SD) min; 1st qu.; median; 3rd qu.; max | 29.1 (5.1) (N/A = 5) 16.7; 25.5; 28.9; 32.4; 48.3 | 30.9 (6.4) 17.6; 26.7; 30.5; 34.3; 58.2 | 0.0081 (Welch) |
| MISS 5 (%) | 160 (19.8%) | 16 (15.0%) | 0.2959 (Fisher) |
| Segments (%) | (N/A = 1) | 0.0005 (Fisher) | |
| 1–2 | 698 (86.6%) | 71 (66.4%) | |
| 3–7 | 106 (13.2%) | 36 (33.6%) | |
| ≥8 | 2 (0.2%) | 0 (0%) | |
| SSIs 6 (%) | |||
| Superficial SSIs | N/A | 30 (28.0%) | |
| Deep SSIs | N/A | 36 (33.6%) | |
| Organ SSIs | N/A | 41 (38.3%) |
| Antibiotics | Dosage | Duration | Time Interval |
|---|---|---|---|
| Cefazolin | 1 g | Single dose | 2016–2024 |
| Cefazolin | 1 g | 72 h | 2023–2024 |
| Cefazolin | 2 g | Single dose | 2016–2024 |
| Cefazolin | 2 g | 72 h | 2023–2024 |
| Clindamycin | 0.6 g | Single dose | 2016–2024 |
| Clindamycin | 0.6 g | 72 h | 2023–2024 |
| SSIs | Total n = 253 | Single Dose Antibiotic n = 227 | 72 h Antibiotic n = 26 |
|---|---|---|---|
| None | 230 | 205 | 25 |
| Cefazolin 1 g = 90 | Cefazolin 1 g = 6 | ||
| Cefazolin 2 g = 106 | Cefazolin 2 g = 15 | ||
| Clindamycin 0.6 g = 9 | Clindamycin 0.6 g = 4 | ||
| Superficial | 2 | 2 | 0 |
| Cefazolin 1 g = 2 | |||
| Deep | 11 | 10 | 1 |
| Cefazolin 1 g = 4 | Cefazolin 1 g | ||
| Cefazolin 2 g = 6 | |||
| Organ | 10 | 10 | 0 |
| Cefazolin 1 g = 4 | |||
| Cefazolin 2 g = 3 | |||
| Clindamycin 0.6 g = 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagy, Z.; Szabó, D.; Agócs, G.; Szilágyi, K.; Rojcsik, Z.; Budai, J.; Papp, Z.; Padányi, C.; Erőss, L.; Sipos, L.; et al. Antibiotic Prophylaxis in Instrumented Lumbar Spine Surgery: Cefazolin Outperforms Clindamycin Regardless of Duration. Antibiotics 2025, 14, 830. https://doi.org/10.3390/antibiotics14080830
Nagy Z, Szabó D, Agócs G, Szilágyi K, Rojcsik Z, Budai J, Papp Z, Padányi C, Erőss L, Sipos L, et al. Antibiotic Prophylaxis in Instrumented Lumbar Spine Surgery: Cefazolin Outperforms Clindamycin Regardless of Duration. Antibiotics. 2025; 14(8):830. https://doi.org/10.3390/antibiotics14080830
Chicago/Turabian StyleNagy, Zoltán, Dóra Szabó, Gergely Agócs, Konrád Szilágyi, Zsanett Rojcsik, József Budai, Zoltán Papp, Csaba Padányi, Loránd Erőss, László Sipos, and et al. 2025. "Antibiotic Prophylaxis in Instrumented Lumbar Spine Surgery: Cefazolin Outperforms Clindamycin Regardless of Duration" Antibiotics 14, no. 8: 830. https://doi.org/10.3390/antibiotics14080830
APA StyleNagy, Z., Szabó, D., Agócs, G., Szilágyi, K., Rojcsik, Z., Budai, J., Papp, Z., Padányi, C., Erőss, L., Sipos, L., & Banczerowski, P. (2025). Antibiotic Prophylaxis in Instrumented Lumbar Spine Surgery: Cefazolin Outperforms Clindamycin Regardless of Duration. Antibiotics, 14(8), 830. https://doi.org/10.3390/antibiotics14080830





