Lessons from Four Years (2021–2024) of Klebsiella Pneumoniae Resistance Surveillance Epidemiological Trends in a Romanian Intensive Care Unit
Abstract
1. Introduction
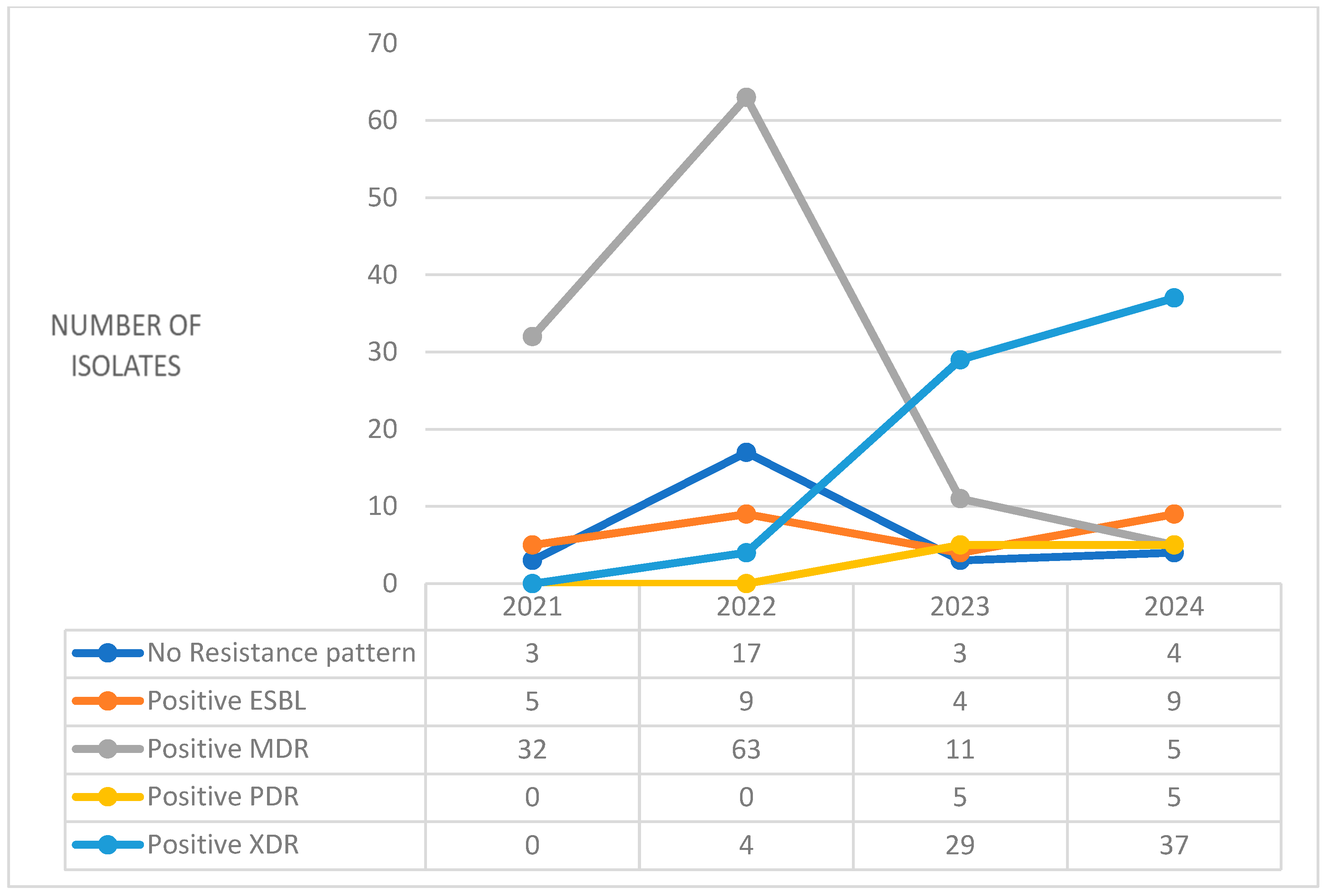
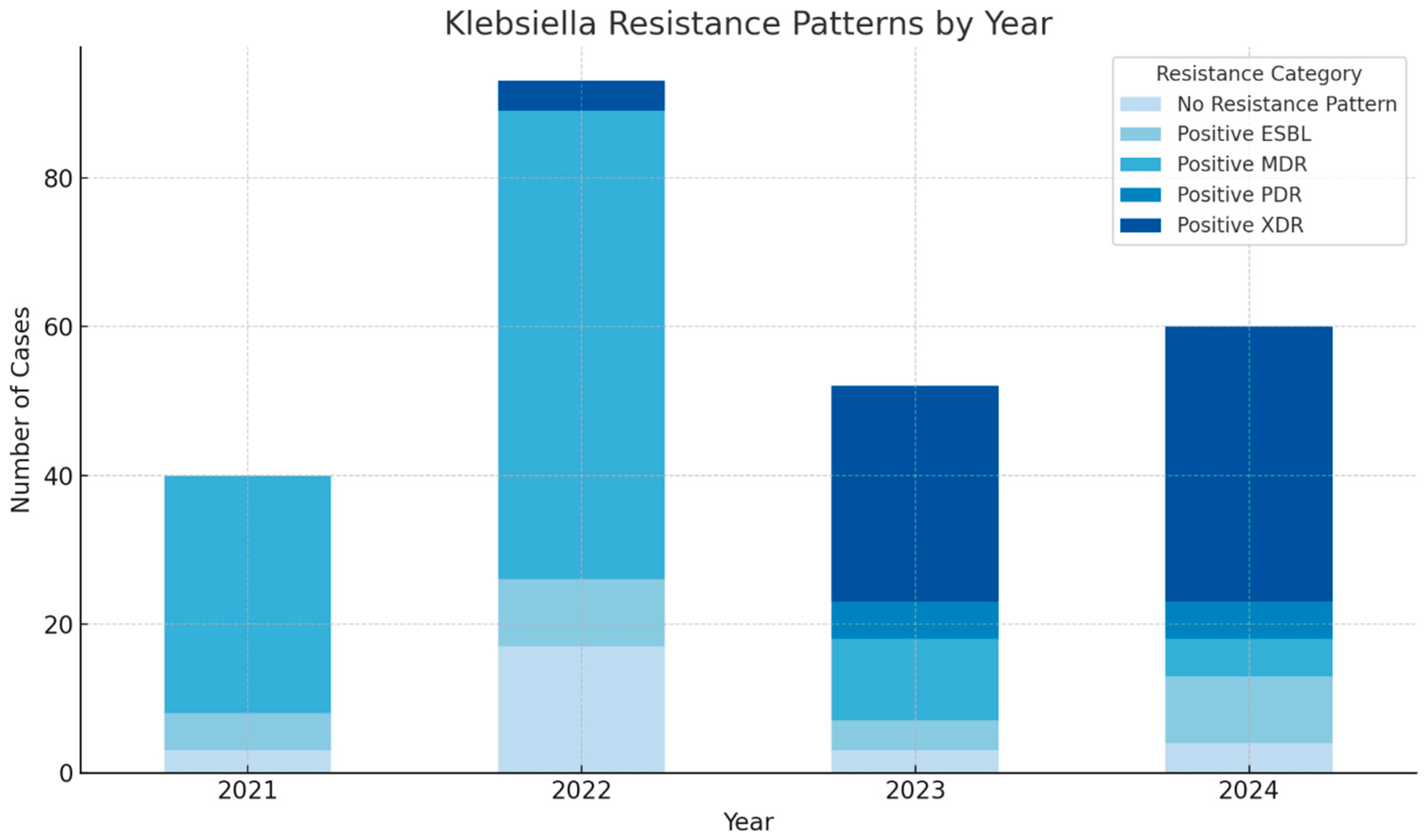
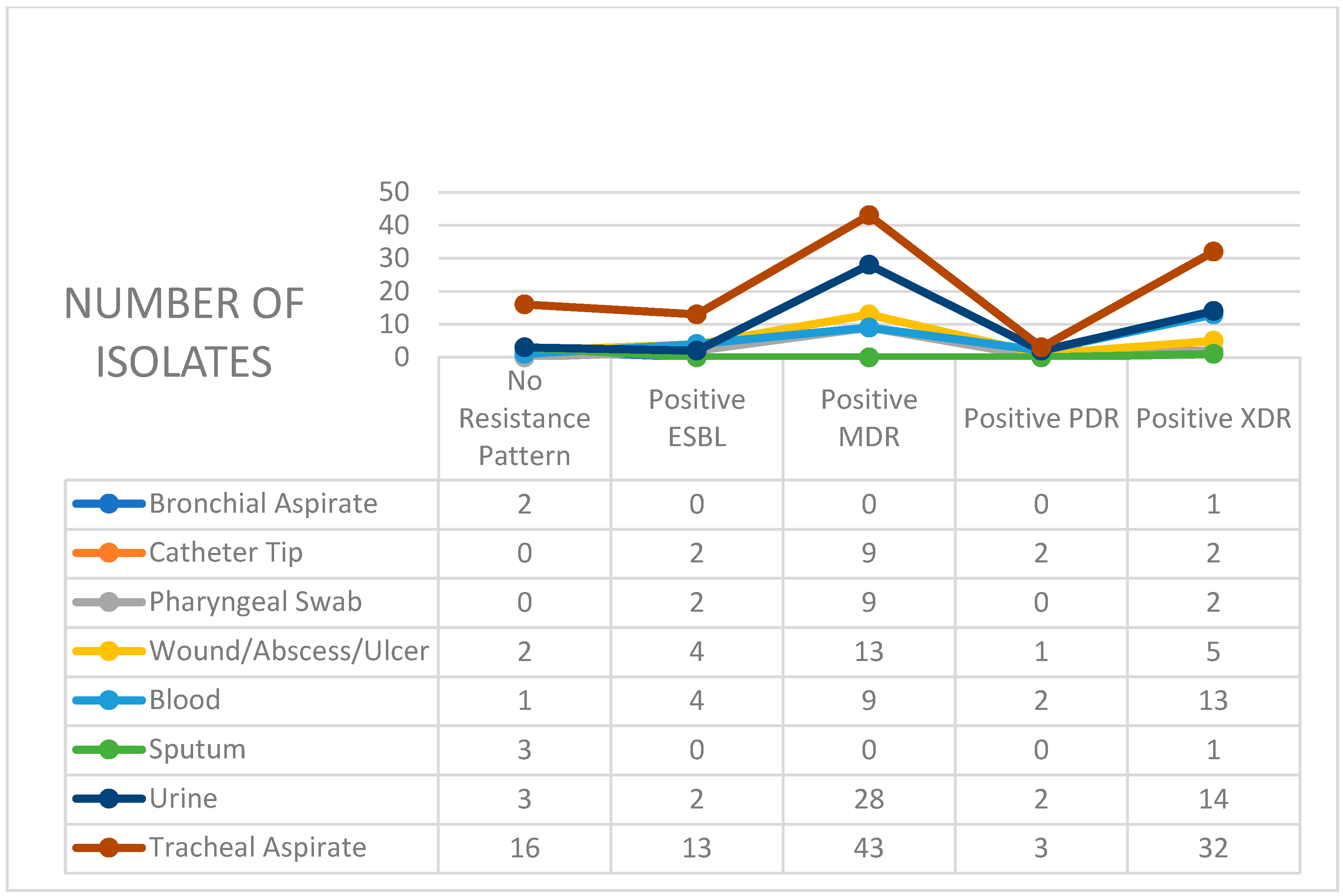
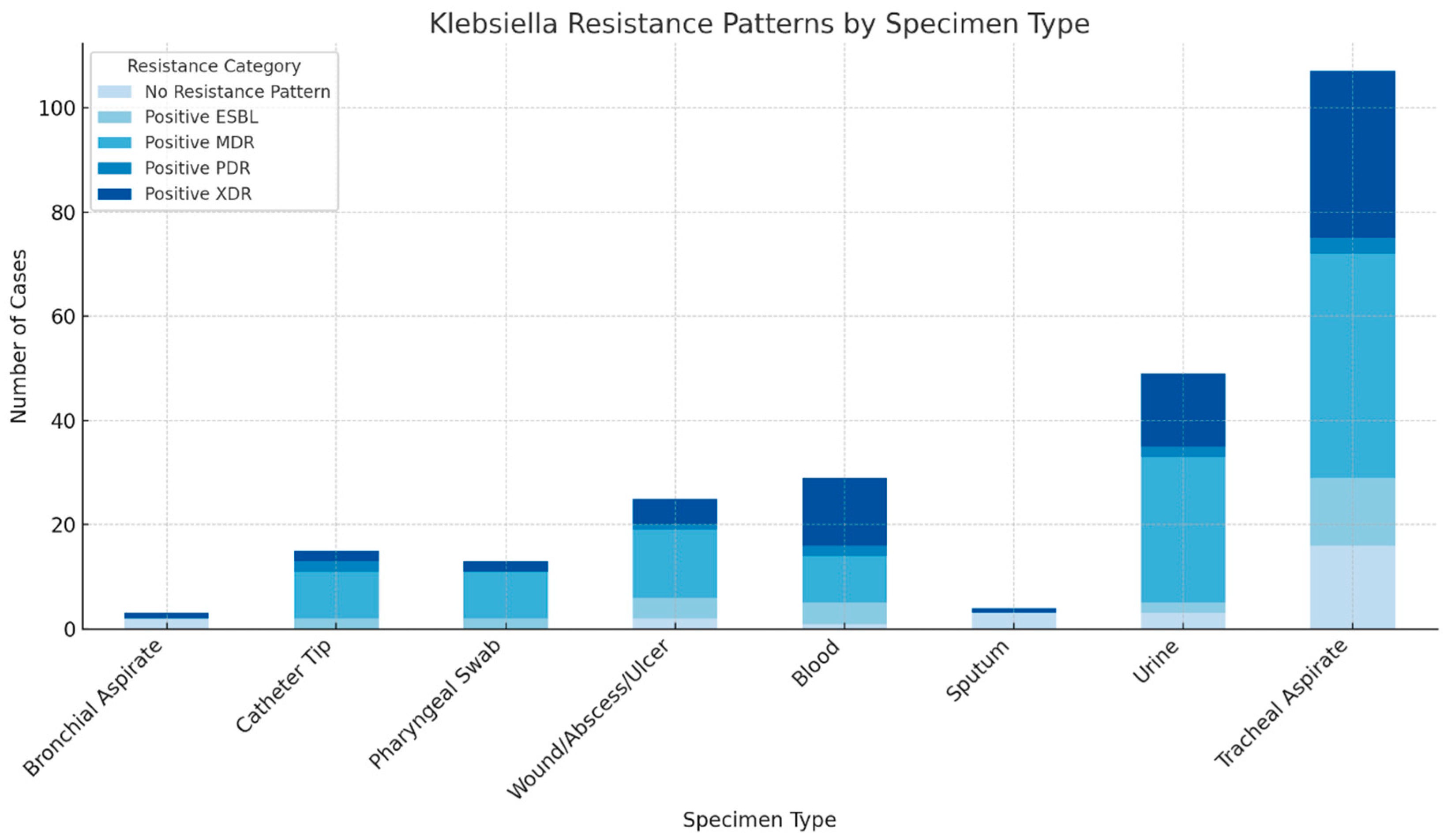
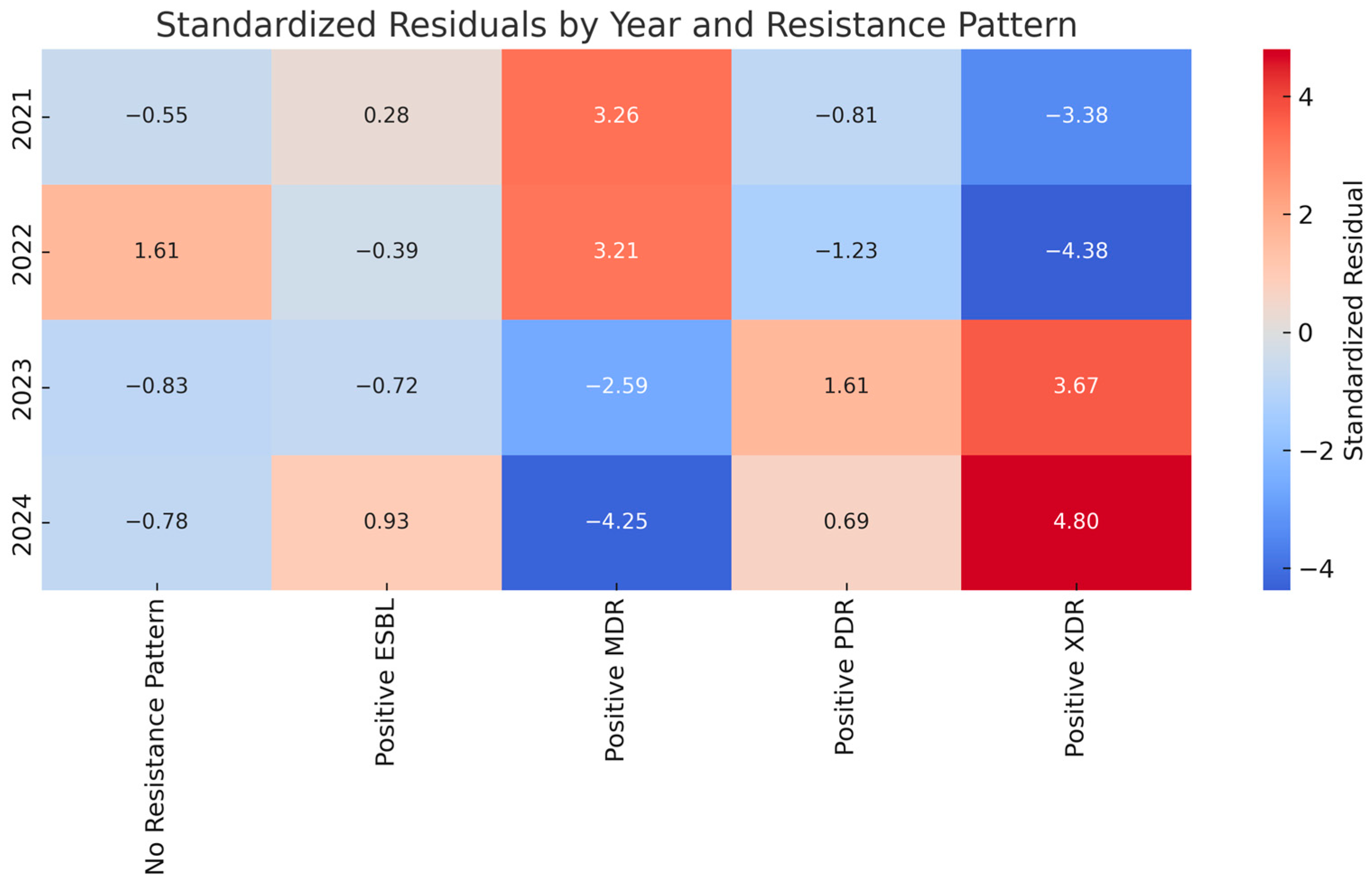
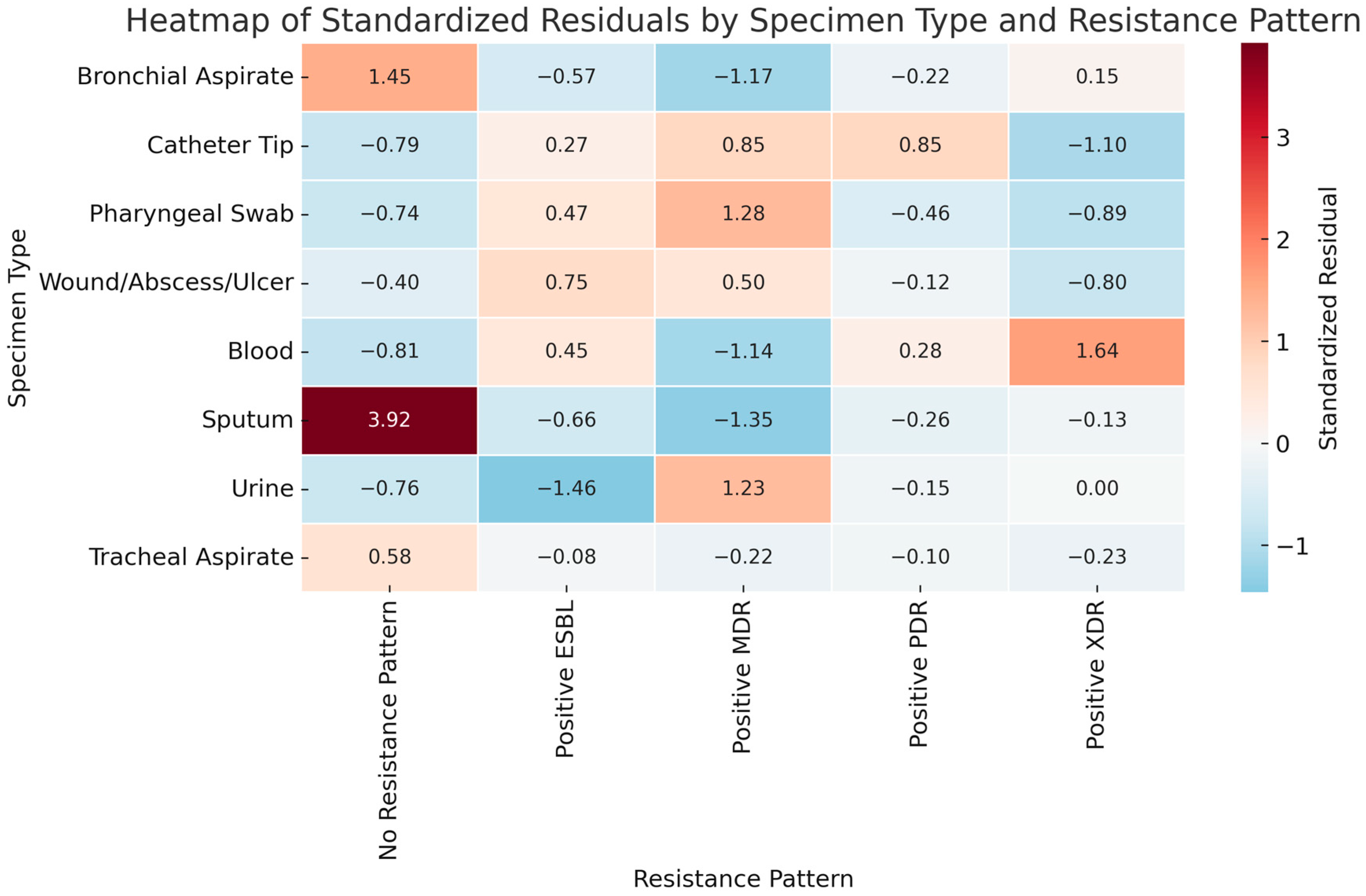
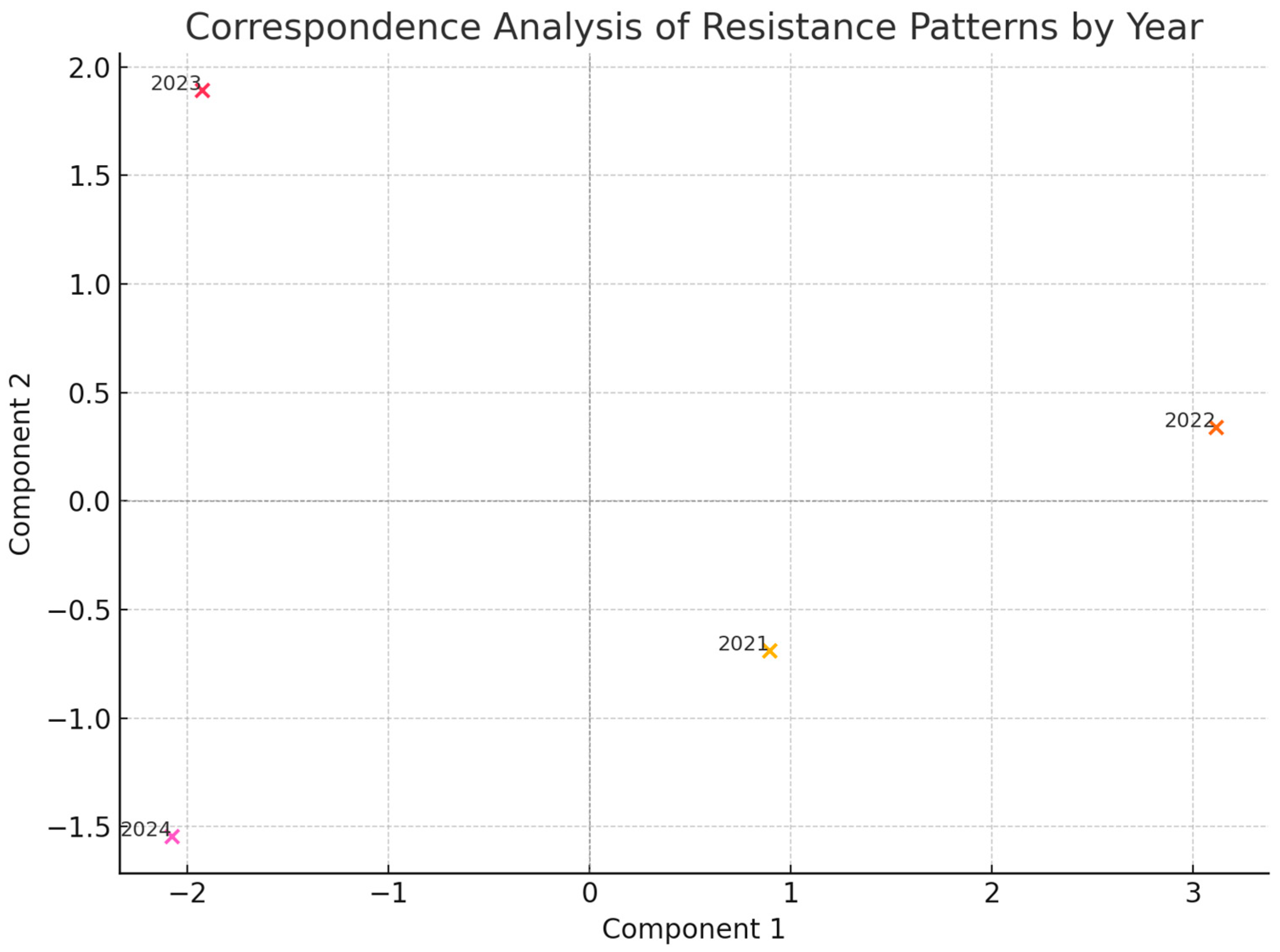
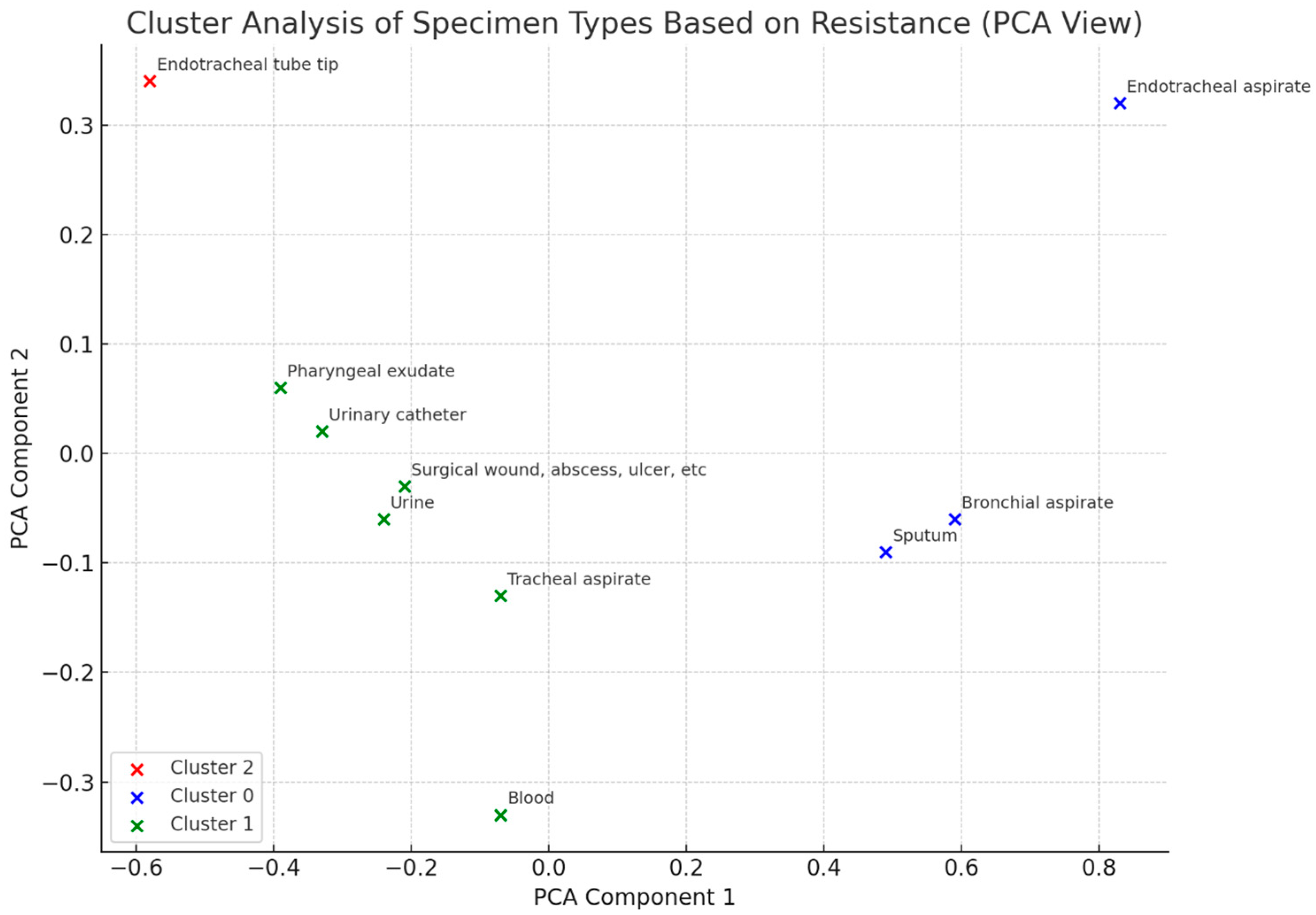
2. Results
3. Discussion
4. Materials and Methods
- -
- Demographic details, specifically patient age and sex;
- -
- Date of sample collection, used to determine the year of isolation;
- -
- Specimen type, categorized based on the anatomical source (e.g., tracheal aspirate, blood, urine, catheter tip, and wound exudate);
- -
- Antimicrobial resistance phenotype of the isolate;
- -
- Type of infection, where explicitly noted in the clinical record (e.g., ventilator-associated pneumonia and bloodstream infection).
- -
- MDR (Multidrug-Resistant): Resistance to at least one agent in three or more antimicrobial classes.
- -
- XDR (Extensively Drug-Resistant): Resistance to all but one or two available antimicrobial categories.
- -
- PDR (Pandrug-Resistant): Resistance to all antibiotics tested.
- -
- ESBL (Extended-Spectrum Beta-Lactamase Producers): Confirmed via phenotypic methods according to standard testing algorithms.
- -
- No Resistance Pattern: Isolates exhibiting full or near-complete susceptibility to tested agents.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Asokan, S.; Jacob, T.; Jacob, J.; AlSosowaa, A.A.; Cherian, T.; Peijnenburg, W.J.G.M.; Vijayan, S. Klebsiella Pneumoniae: A Growing Threat in the Era of Antimicrobial Resistance. Microbe 2025, 7, 100333. [Google Scholar] [CrossRef]
- Suay-García, B.; Pérez-Gracia, M.T. Present and Future of Carbapenem-Resistant Enterobacteriaceae (CRE) Infections. Antibiotics 2019, 8, 435–456. [Google Scholar] [CrossRef]
- Mukherjee, S.; Bhadury, P.; Mitra, S.; Naha, S.; Saha, B.; Dutta, S.; Basu, S. Hypervirulent Klebsiella Pneumoniae Causing Neonatal Bloodstream Infections: Emergence of NDM-1-Producing Hypervirulent ST11-K2 and ST15-K54 Strains Possessing PLVPK-Associated Markers. Microbiol. Spectr. 2023, 11, e04121-22. [Google Scholar] [CrossRef]
- Bereanu, A.S.; Vintilă, B.I.; Bereanu, R.; Codru, I.R.; Hașegan, A.; Olteanu, C.; Săceleanu, V.; Sava, M. TiO2 Nanocomposite Coatings and Inactivation of Carbapenemase-Producing Klebsiella Pneumoniae Biofilm—Opportunities and Challenges. Microorganisms 2024, 12, 684. [Google Scholar] [CrossRef]
- Bassetti, M.; Carnelutti, A.; Peghin, M. Patient Specific Risk Stratification for Antimicrobial Resistance and Possible Treatment Strategies in Gram-Negative Bacterial Infections. Expert Rev. Anti Infect. Ther. 2017, 15, 55–65. [Google Scholar] [CrossRef]
- Ibrahim, M.E. Risk Factors in Acquiring Multidrug-Resistant Klebsiella Pneumoniae Infections in a Hospital Setting in Saudi Arabia. Sci. Rep. 2023, 13, 11626. [Google Scholar] [CrossRef] [PubMed]
- Mihetiu, A.; Bratu, D.G.; Tanasescu, C.; Vintilă, B.I.; Sandu, A.; Sandu, M.; Serban, D.; Sabau, D.; Hasegan, A. Laparoscopic Management of Multiple Liver, Omental, Mesenteric, Peritoneal, and Round Ligament Hydatid Cysts—A Rare Report of a Case and a Systematic Literature Review. J. Pers. Med. 2024, 14, 205. [Google Scholar] [CrossRef]
- Guerra, M.E.S.; Destro, G.; Vieira, B.; Lima, A.S.; Ferraz, L.F.C.; Hakansson, A.P.; Darrieux, M.; Converso, T.R. Klebsiella Pneumoniae Biofilms and Their Role in Disease Pathogenesis. Front. Cell. Infect. Microbiol. 2022, 12, 877995. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Thakur, A.; Thakur, N.; Kumar, V.; Chauhan, A.; Bhardwaj, N. Changing Trend in the Antibiotic Resistance Pattern of Klebsiella Pneumonia Isolated From Endotracheal Aspirate Samples of ICU Patients of a Tertiary Care Hospital in North India. Cureus 2023, 15, e36317. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Simmonds, A.; Uhlemann, A.C. Clinical Implications of Genomic Adaptation and Evolution of Carbapenem-Resistant Klebsiella Pneumoniae. J. Infect. Dis. 2017, 215, S18. [Google Scholar] [CrossRef]
- Howroyd, F.; Chacko, C.; MacDuff, A.; Gautam, N.; Pouchet, B.; Tunnicliffe, B.; Weblin, J.; Gao-Smith, F.; Ahmed, Z.; Duggal, N.A.; et al. Ventilator-Associated Pneumonia: Pathobiological Heterogeneity and Diagnostic Challenges. Nat. Commun. 2024, 15, 6447. [Google Scholar] [CrossRef] [PubMed]
- Aiesh, B.M.; Qashou, R.; Shemmessian, G.; Swaileh, M.W.; Abutaha, S.A.; Sabateen, A.; Barqawi, A.K.; AbuTaha, A.; Zyoud, S.H. Nosocomial Infections in the Surgical Intensive Care Unit: An Observational Retrospective Study from a Large Tertiary Hospital in Palestine. BMC Infect. Dis. 2023, 23, 686. [Google Scholar] [CrossRef] [PubMed]
- Hranjec, T.; Sawyer, R.G. Management of Infections in Critically Ill Patients. Surg. Infect. 2014, 15, 474. [Google Scholar] [CrossRef]
- Gavriliu, L.C.; Benea, O.E.; Benea, S. Antimicrobial Resistance Temporal Trend of Klebsiella Pneumoniae Isolated from Blood. J. Med. Life 2016, 9, 419. [Google Scholar]
- Codru, I.R.; Sava, M.; Vintilă, B.I.; Bereanu, A.S.; Bîrluțiu, V. A Study on the Contributions of Sonication to the Identification of Bacteria Associated with Intubation Cannula Biofilm and the Risk of Ventilator-Associated Pneumonia. Medicina 2023, 59, 1058. [Google Scholar] [CrossRef]
- Codru, I.R.; Vintilă, B.I.; Bereanu, A.S.; Sava, M.; Popa, L.M.; Birlutiu, V. Antimicrobial Resistance Patterns and Biofilm Analysis via Sonication in Intensive Care Unit Patients at a County Emergency Hospital in Romania. Pharmaceuticals 2025, 18, 161. [Google Scholar] [CrossRef]
- Birlutiu, V.; Birlutiu, R.M. Endocarditis Due to Abiotrophia Defectiva, a Biofilm-Related Infection Associated with the Presence of Fixed Braces: A Case Report. Medicine 2017, 96, e8756. [Google Scholar] [CrossRef]
- Li, R.; Zhang, Z.; Wang, Z.; Qian, K.; Fernanda Mojica, M.; Wang, D.; Restrepo Arbelaez, N. Containment of a Carbapenem-Resistant Klebsiella Pneumoniae in an Intensive Care Unit during the COVID-19 Pandemic. Front. Public Health 2025, 13, 1557068. [Google Scholar] [CrossRef]
- Cohen, M.J.; Block, C.; Levin, P.D.; Schwartz, C.; Gross, I.; Weiss, Y.; Moses, A.E.; Benenson, S. Institutional Control Measures to Curtail the Epidemic Spread of Carbapenem-Resistant Klebsiella Pneumoniae: A 4-Year Perspective. Infect. Control Hosp. Epidemiol. 2011, 32, 673–678. [Google Scholar] [CrossRef]
- Herbert, S.; Halvorsen, D.S.; Leong, T.; Franklin, C.; Harrington, G.; Spelman, D. Large Outbreak of Infection and Colonization with Gram-Negative Pathogens Carrying the Metallo-β-Lactamase Gene BlaIMP-4 at a 320-Bed Tertiary Hospital in Australia. Infect. Control 2007, 28, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, L.Y.; Song, W.Q.; Wang, Y.; Dong, F.; Liu, G. Risk Factors for Carbapenem-Resistant K. Pneumoniae Bloodstream Infection and Predictors of Mortality in Chinese Paediatric Patients. BMC Infect. Dis. 2018, 18, 248. [Google Scholar] [CrossRef] [PubMed]
- Chi, X.; Meng, X.; Xiong, L.; Chen, T.; Zhou, Y.; Ji, J.; Zheng, B.; Xiao, Y. Small Wards in the ICU: A Favorable Measure for Controlling the Transmission of Carbapenem-Resistant Klebsiella Pneumoniae. Intensive Care Med. 2022, 48, 1573–1581. [Google Scholar] [CrossRef]
- Perez, F.; Villegas, M.V. The Role of Surveillance Systems in Confronting the Global Crisis of Antibiotic-Resistant Bacteria. Curr. Opin. Infect. Dis. 2015, 28, 375. [Google Scholar] [CrossRef]
- Antibiotic Resistance in Europe: Hospitals Are Part of the Problem. Healthcare-in-Europe.Com. Available online: https://healthcare-in-europe.com/en/news/antibiotic-resistance-in-europe-hospitals-are-part-of-the-problem.html (accessed on 2 July 2025).
- van Duin, D.; Paterson, D.L. Multidrug Resistant Bacteria in the Community: An Update. Infect. Dis. Clin. N. Am. 2020, 34, 709. [Google Scholar] [CrossRef]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable Deaths and Disability-Adjusted Life-Years Caused by Infections with Antibiotic-Resistant Bacteria in the EU and the European Economic Area in 2015: A Population-Level Modelling Analysis. Lancet. Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef]
- Almutairy, B. Extensively and Multidrug-Resistant Bacterial Strains: Case Studies of Antibiotics Resistance. Front. Microbiol. 2024, 15, 1381511. [Google Scholar] [CrossRef]
- Ristori, M.V.; Scarpa, F.; Sanna, D.; Casu, M.; Petrosillo, N.; Longo, U.G.; Lucia, D.F.; Spoto, S.; Chiantia, R.M.; Caserta, A.; et al. Multidrug-Resistant Klebsiella Pneumoniae Strains in a Hospital: Phylogenetic Analysis to Investigate Local Epidemiology. Microorganisms 2024, 12, 2541. [Google Scholar] [CrossRef] [PubMed]
- Assefa, M. Multi-Drug Resistant Gram-Negative Bacterial Pneumonia: Etiology, Risk Factors, and Drug Resistance Patterns. Pneumonia 2022, 14, 4. [Google Scholar] [CrossRef]
- Nazari, M.; Hemmati, J.; Asghari, B. Comprehensive Analysis of Virulence Genes, Antibiotic Resistance, Biofilm Formation, and Sequence Types in Clinical Isolates of Klebsiella Pneumoniae. Can. J. Infect. Dis. Med. Microbiol. 2024, 2024, 1403019. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, R.A.; Bedawy, A.M.; Negm, E.M.; Hassan, T.H.; Ibrahim, D.A.; Elsheikh, S.M.; Amer, R.M. Carbapenem-Resistant Klebsiella Pneumoniae Among Patients with Ventilator-Associated Pneumonia: Evaluation of Antibiotic Combinations and Susceptibility to New Antibiotics. Infect. Drug Resist. 2022, 15, 3537. [Google Scholar] [CrossRef]
- Guo, S.; Xu, J.J.; Wei, Y.S.; Xu, J.H.; Li, Y.; Xue, R. Clinical and Molecular Characteristics of Klebsiella Pneumoniae Ventilator-Associated Pneumonia in Mainland China. BMC Infect. Dis. 2016, 16, 608. [Google Scholar] [CrossRef]
- Oleksy-Wawrzyniak, M.; Junka, A.; Brożyna, M.; Paweł, M.; Kwiek, B.; Nowak, M.; Mączyńska, B.; Bartoszewicz, M. The In Vitro Ability of Klebsiella Pneumoniae to Form Biofilm and the Potential of Various Compounds to Eradicate It from Urinary Catheters. Pathogens 2022, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Huang, H. Risk Factors of Mortality in Bloodstream Infections Caused by Klebsiella Pneumonia: A Single-Center Retrospective Study in China. Medicine 2017, 96, e7924. [Google Scholar] [CrossRef] [PubMed]
- Codru, I.R.; Vintilă, B.I.; Sava, M.; Bereanu, A.S.; Neamțu, S.I.; Bădilă, R.M.; Bîrluțiu, V. Optimizing Diagnosis and Management of Ventilator-Associated Pneumonia: A Systematic Evaluation of Biofilm Detection Methods and Bacterial Colonization on Endotracheal Tubes. Microorganisms 2024, 12, 1966. [Google Scholar] [CrossRef] [PubMed]
- Santella, B.; Boccella, M.; Folliero, V.; Iervolino, D.; Pagliano, P.; Fortino, L.; Serio, B.; Vozzella, E.A.; Schiavo, L.; Galdiero, M.; et al. Antimicrobial Susceptibility Profiles of Klebsiella Pneumoniae Strains Collected from Clinical Samples in a Hospital in Southern Italy. Can. J. Infect. Dis. Med. Microbiol. 2024, 2024, 5548434. [Google Scholar] [CrossRef]
- Hafiz, T.A.; Alanazi, S.; Alghamdi, S.S.; Mubaraki, M.A.; Aljabr, W.; Madkhali, N.; Alharbi, S.R.; Binkhamis, K.; Alotaibi, F. Klebsiella Pneumoniae Bacteraemia Epidemiology: Resistance Profiles and Clinical Outcome of King Fahad Medical City Isolates, Riyadh, Saudi Arabia. BMC Infect. Dis. 2023, 23, 579. [Google Scholar] [CrossRef]
- Hou, G.; Ahmad, S.; Li, Y.; Yan, D.; Yang, S.; Chen, S.; Qiu, Z.; Yu, X.; Li, N.; Li, Y.; et al. Epidemiological, Virulence, and Antibiotic Resistance Analysis of Klebsiella Pneumoniae, a Major Source of Threat to Livestock and Poultry in Some Regions of Xinjiang, China. Animals 2024, 14, 1433. [Google Scholar] [CrossRef]
- Bereanu, A.S.; Bereanu, R.; Mohor, C.; Vintilă, B.I.; Codru, I.R.; Olteanu, C.; Sava, M. Prevalence of Infections and Antimicrobial Resistance of ESKAPE Group Bacteria Isolated from Patients Admitted to the Intensive Care Unit of a County Emergency Hospital in Romania. Antibiotics 2024, 13, 400. [Google Scholar] [CrossRef]
- Seddon, M.M.; Bookstaver, P.B.; Justo, J.A.; Kohn, J.; Rac, H.; Haggard, E.; Mediwala, K.N.; Dash, S.; Al-Hasan, M.N. Role of Early De-Escalation of Antimicrobial Therapy on Risk of Clostridioides Difficile Infection Following Enterobacteriaceae Bloodstream Infections. Clin. Infect. Dis. 2019, 69, 414–420. [Google Scholar] [CrossRef]
- Reese, M.; Bookstaver, P.B.; Kohn, J.; Troficanto, C.; Yongue, E.; Winders, H.R.; Al-Hasan, M.N. Missed Opportunities for Early De-Escalation of Antipseudomonal Beta-Lactam Antimicrobial Therapy in Enterobacterales Bloodstream Infection. Antibiotics 2024, 13, 1031. [Google Scholar] [CrossRef]
- Li, Y.; Kumar, S.; Zhang, L. Mechanisms of Antibiotic Resistance and Developments in Therapeutic Strategies to Combat Klebsiella Pneumoniae Infection. Infect Drug Resist. 2024, 17, 1107–1119. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Antunes, W.; Mota, S.; Madureira-Carvalho, Á.; Dinis-Oliveira, R.J.; Dias da Silva, D. An Overview of the Recent Advances in Antimicrobial Resistance. Microorganisms 2024, 12, 1920. [Google Scholar] [CrossRef] [PubMed]
- WHO. Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. Available online: https://www.ecdc.europa.eu/en/news-events/who-publishes-list-bacteria-which-new-antibiotics-are-urgently-needed (accessed on 2 July 2025).
- Vintila, B.I.; Arseniu, A.M.; Morgovan, C.; Butuca, A.; Bîrluțiu, V.; Dobrea, C.M.; Rus, L.L.; Ghibu, S.; Bereanu, A.S.; Arseniu, R.; et al. A Real-World Study on the Clinical Characteristics, Outcomes, and Relationship between Antibiotic Exposure and Clostridioides Difficile Infection. Antibiotics 2024, 13, 144. [Google Scholar] [CrossRef] [PubMed]

| Disk Diffusion Antibiogram for Aerobic Bacteria (Kirby–Bauer) |
|---|
| Aztreonam |
| Ceftazidime/Avibactam |
| Ceftolozane/Tazobactam |
| Cefiderocol |
| Automated System Antibiogram (Vitek) |
| Amikacin |
| Ampicillin/Sulbactam |
| Ampicillin ORAL |
| Aztreonam |
| Cefepime |
| Ceftazidime |
| Ceftriaxone |
| Chloramphenicol |
| Cefuroxime ORAL |
| Gentamicin |
| Imipenem |
| Piperacillin/Tazobactam |
| Tobramycin |
| Trimethoprim/Sulfamethoxazole |
| Moxifloxacin |
| Levofloxacin |
| Ertapenem |
| Minocycline |
| Tetracycline |
| Tigecycline |
| Ceftazidime/Avibactam |
| Ceftolozane/Tazobactam |
| Cefotaxime |
| Imipenem/Relebactam |
| Meropenem/Vaborbactam |
| Temocillin |
| Amoxicillin/Clavulanic |
| Amoxicillin/Clavulanic IV |
| Cefuroxime |
| Ciprofloxacin |
| Meropenem |
| Broth Microdilution |
| Colistin |
| Carbapenemase Detection Test—Enterobacterales |
| NDM |
| KPC |
| OXA-48-like |
| IMP |
| VIM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sava, M.; Vintila, B.I.; Bereanu, A.S.; Fratila, A.M.; Codru, I.R. Lessons from Four Years (2021–2024) of Klebsiella Pneumoniae Resistance Surveillance Epidemiological Trends in a Romanian Intensive Care Unit. Antibiotics 2025, 14, 825. https://doi.org/10.3390/antibiotics14080825
Sava M, Vintila BI, Bereanu AS, Fratila AM, Codru IR. Lessons from Four Years (2021–2024) of Klebsiella Pneumoniae Resistance Surveillance Epidemiological Trends in a Romanian Intensive Care Unit. Antibiotics. 2025; 14(8):825. https://doi.org/10.3390/antibiotics14080825
Chicago/Turabian StyleSava, Mihai, Bogdan Ioan Vintila, Alina Simona Bereanu, Anca Maria Fratila, and Ioana Roxana Codru. 2025. "Lessons from Four Years (2021–2024) of Klebsiella Pneumoniae Resistance Surveillance Epidemiological Trends in a Romanian Intensive Care Unit" Antibiotics 14, no. 8: 825. https://doi.org/10.3390/antibiotics14080825
APA StyleSava, M., Vintila, B. I., Bereanu, A. S., Fratila, A. M., & Codru, I. R. (2025). Lessons from Four Years (2021–2024) of Klebsiella Pneumoniae Resistance Surveillance Epidemiological Trends in a Romanian Intensive Care Unit. Antibiotics, 14(8), 825. https://doi.org/10.3390/antibiotics14080825







