Protective Effects of Luteolin on Glaesserella parasuis-Induced Injury: An In Vitro Study with Porcine Vascular Endothelial Cells
Abstract
1. Introduction
2. Results
2.1. Dose-Dependent Cytotoxicity of Lut on PIECs
2.2. GPS Increased Vascular Endothelial Permeability in PIECs
2.3. GPS Increased the Expression of Vascular-Endothelial-Permeability-Related Proteins in PIECs
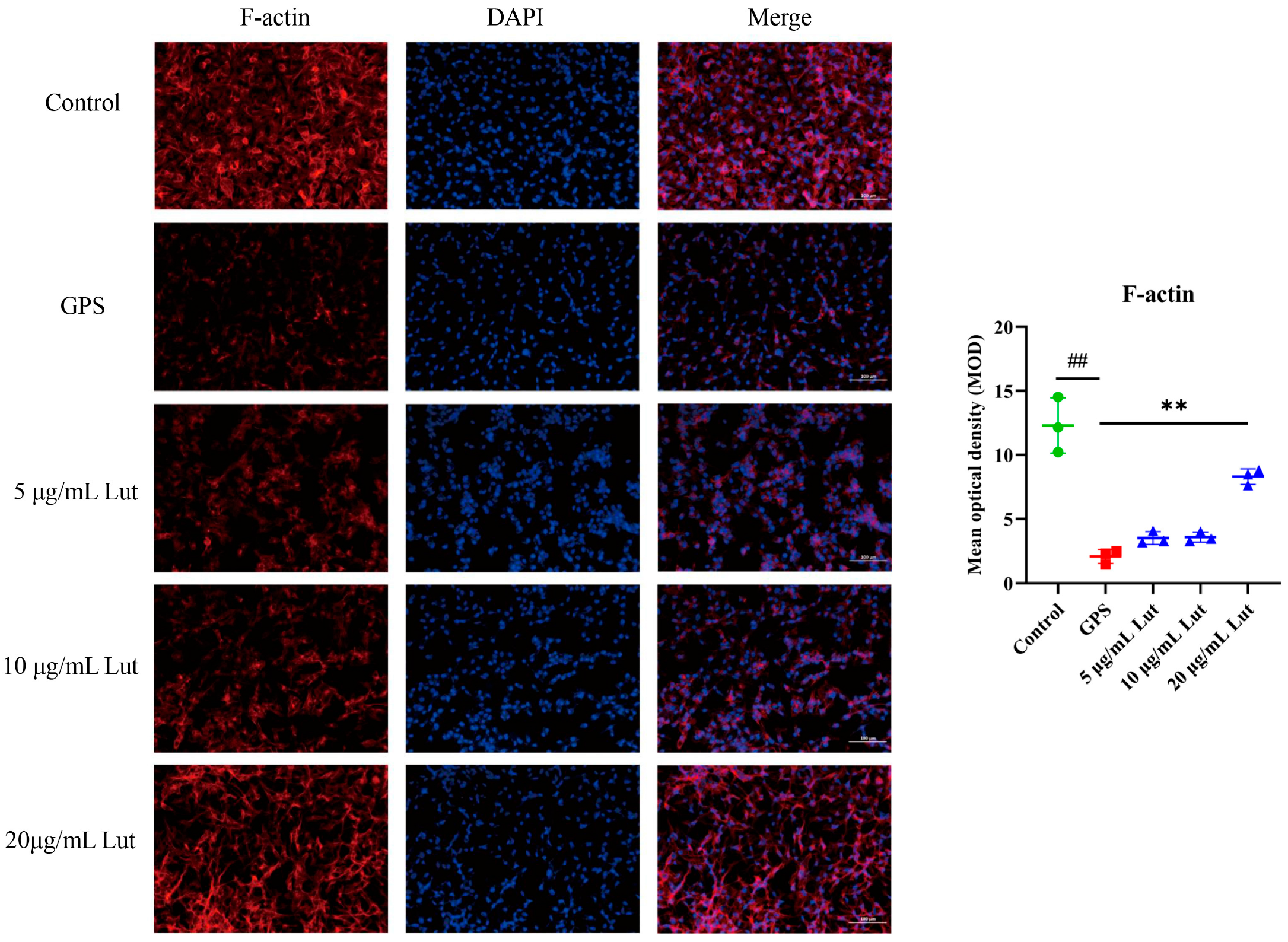
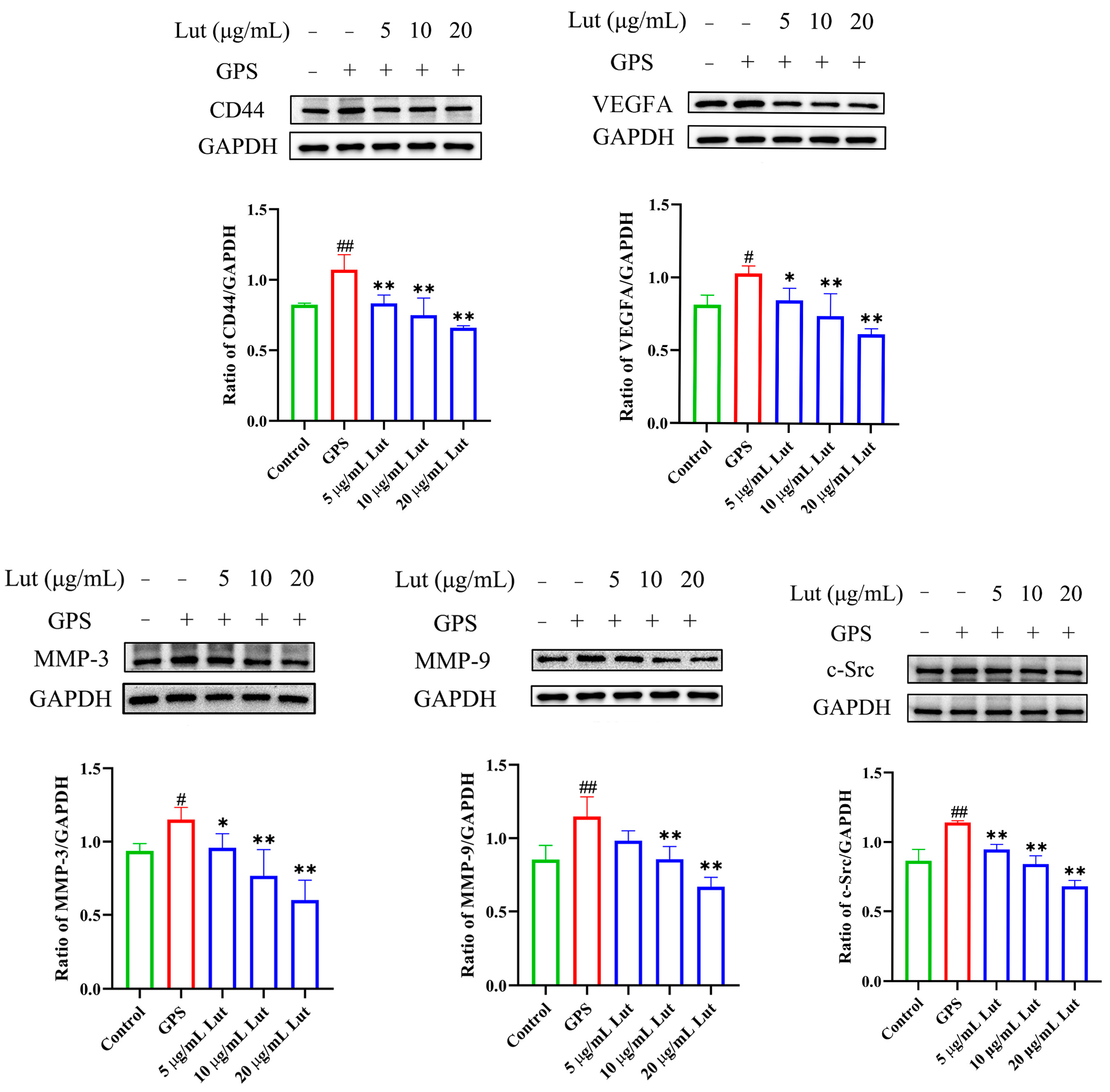
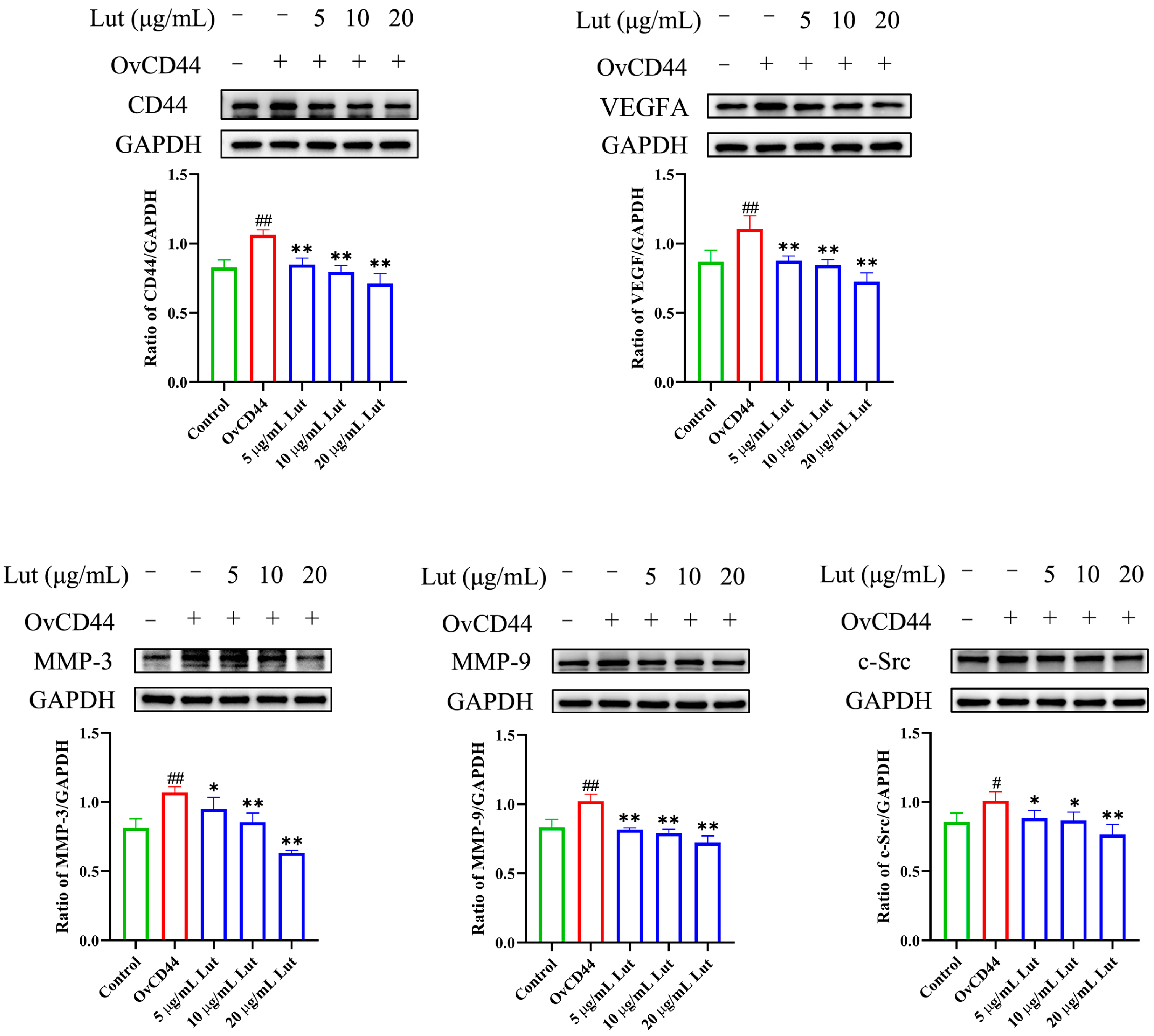
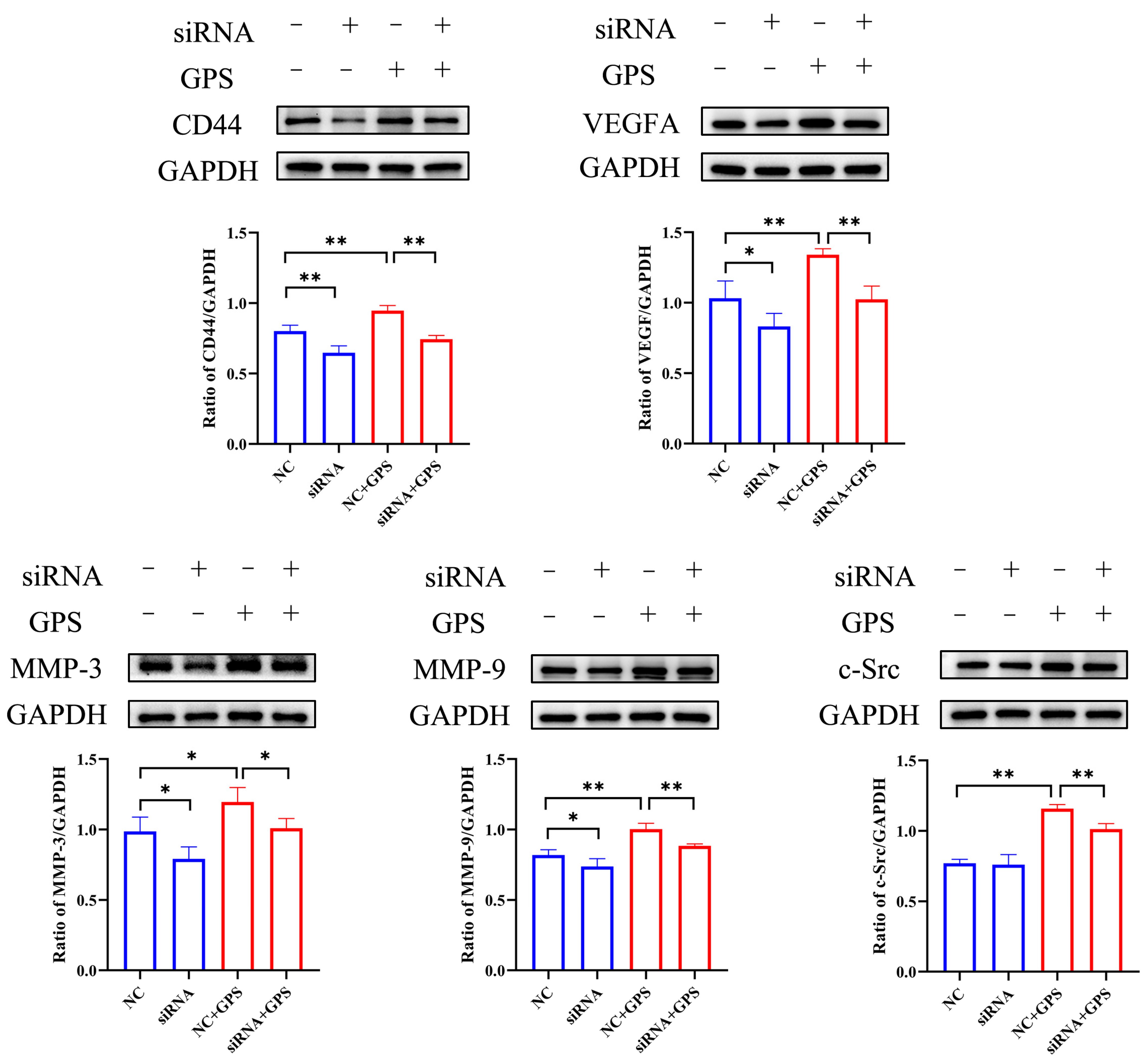
2.4. Lut Attenuated the GPS-Induced Increase in Vascular Endothelial Permeability by Suppressing the CD44 Pathway in PIECs
3. Discussion
4. Materials and Methods
4.1. Reagents and Chemicals
4.2. Cell and Bacterial Culture
4.3. Cell Viability Assay
4.4. RNA Isolation and Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR)
4.5. Western Blot
4.6. Immunofluorescence
4.7. Cell Transfection
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| c-Src | SRC proto-oncogene, non-receptor tyrosine kinase |
| CCK8 | Cell counting kit-8 |
| CD44 | Cluster of differentiation 44 |
| DMSO | Dimethyl sulfoxide |
| ECL | Enhanced chemiluminescence |
| F-actin | Filamentous actin |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| GPS | Glaesserella parasuis |
| HUVEC | Human umbilical vein endothelial cell |
| IL-1β | Interleukin-1beta |
| IL-6 | Interleukin 6 |
| IL-8 | Interleukin 8 |
| LPS | Lipopolysaccharide |
| Lut | Luteolin |
| MMP-3 | MatrixmetalloProteinase-3 |
| MMP-9 | MatrixmetalloProteinase-9 |
| NC | Negative control siRNA |
| OvCD44 | CD44 overexpression |
| PIECs | Porcine iliac artery endothelial cells |
| RNAi | RNA interference |
| RT-qPCR | Reverse transcription quantitative polymerase chain reaction |
| siRNA | Small interfering RNA |
| TNF | Tumor necrosis factor |
| VEGFA | Vascular endothelial growth factor |
References
- Huang, A.; Huang, X.; Zhang, Z.; Yuan, Z.; Huang, L.; Wang, Y.; Tao, Y.; Chen, D.; Liu, Z.; Hao, H. Dosing regimen of aditoprim and sulfamethoxazole combination for the Glaesserella parasuis containing resistance and virulence genes. Pharmaceutics 2022, 14, 2058. [Google Scholar] [CrossRef]
- Fu, S.; Yin, R.; Zuo, S.; Liu, J.; Zhang, Y.; Guo, L.; Qiu, Y.; Ye, C.; Liu, Y.; Wu, Z.; et al. The effects of baicalin on piglets challenged with Glaesserella parasuis. Vet. Res. 2020, 51, 102. [Google Scholar] [CrossRef]
- Wang, Z.; Gu, J.; Xiao, K.; Zhu, W.; Lin, Y.; Wen, S.; He, Q.; Xu, X.; Cai, X. Glaesserella parasuis autotransporters EspP1 and EspP2 are novel IgA-specific proteases. Front. Microbiol. 2022, 13, 1041774. [Google Scholar] [CrossRef]
- Ni, H.B.; Gong, Q.L.; Zhao, Q.; Li, X.Y.; Zhang, X.X. Prevalence of Haemophilus parasuis “Glaesserella parasuis” in pigs in China: A systematic review and meta-analysis. Prev. Vet. Med. 2020, 182, 105083. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Liu, J.; Xu, J.; Zuo, S.; Zhang, Y.; Guo, L.; Qiu, Y.; Ye, C.; Liu, Y.; Wu, Z.; et al. The effect of baicalin on microRNA expression profiles in porcine aortic vascular endothelial cells infected by Haemophilus parasuis. Mol. Cell Biochem. 2020, 472, 45–56. [Google Scholar] [CrossRef]
- Claesson-Welsh, L. Vascular permeability–the essentials. Ups. J. Med. Sci. 2015, 120, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Wass, L.; Grankvist, A.; Bell-Sakyi, L.; Bergström, M.; Ulfhammer, E.; Lingblom, C.; Wennerås, C. Cultivation of the causative agent of human neoehrlichiosis from clinical isolates identifies vascular endothelium as a target of infection. Emerg. Microbes Infect. 2019, 8, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Hellenthal, K.E.M.; Brabenec, L.; Wagner, N.M. Regulation and dysregulation of endothelial permeability during systemic inflammation. Cells 2022, 11, 1935. [Google Scholar] [CrossRef]
- Flynn, K.M.; Michaud, M.; Canosa, S.; Madri, J.A. CD44 regulates vascular endothelial barrier integrity via a PECAM-1 dependent mechanism. Angiogenesis 2013, 16, 689–705. [Google Scholar] [CrossRef]
- Sugita, S.; Naito, Y.; Zhou, L.; He, H.; Hao, Q.; Sakamoto, A.; Lee, J.W. Hyaluronic acid restored protein permeability across injured human lung microvascular endothelial cells. FASEB Bioadv. 2022, 4, 619–631. [Google Scholar] [CrossRef]
- Yang, X.; Meegan, J.E.; Jannaway, M.; Coleman, D.C.; Yuan, S.Y. A disintegrin and metalloproteinase 15-mediated glycocalyx shedding contributes to vascular leakage during inflammation. Cardiovasc. Res. 2018, 114, 1752–1763. [Google Scholar] [CrossRef]
- Sun, J.; Law, G.P.; Bridges, C.C.; McKallip, R.J. CD44 as a novel target for treatment of staphylococcal enterotoxin B-induced acute inflammatory lung injury. Clin. Immunol. 2012, 144, 41–52. [Google Scholar] [CrossRef]
- Singleton, P.A.; Salgia, R.; Moreno-Vinasco, L.; Moitra, J.; Sammani, S.; Mirzapoiazova, T.; Garcia, J.G. CD44 regulates hepatocyte growth factor-mediated vascular integrity. Role of c-Met, Tiam1/Rac1, dynamin 2, and cortactin. J. Biol. Chem. 2007, 282, 30643–30657. [Google Scholar] [CrossRef]
- Rungsung, S.; Singh, T.U.; Perumalraja, K.; Mahobiya, A.; Sharma, M.; Lingaraju, M.C.; Parida, S.; Sahoo, M.; Kumar, D. Luteolin alleviates vascular dysfunctions in CLP-induced polymicrobial sepsis in mice. Pharmacol. Rep. 2022, 74, 1054–1068. [Google Scholar] [CrossRef]
- Qin, X.; Qin, H.; Li, Z.; Xue, S.; Huang, B.; Liu, X.; Wang, D. Luteolin alleviates ischemia/reperfusion injury-induced no-reflow by regulating Wnt/β-catenin signaling in rats. Microvasc. Res. 2022, 139, 104266. [Google Scholar] [CrossRef]
- Aziz, N.; Kim, M.Y.; Cho, J.Y. Anti-inflammatory effects of luteolin: A review of in vitro, in vivo, and in silico studies. J. Ethnopharmacol. 2018, 225, 342–358. [Google Scholar] [CrossRef]
- Li, L.; Luo, W.; Qian, Y.; Zhu, W.; Qian, J.; Li, J.; Jin, Y.; Xu, X.; Liang, G. Luteolin protects against diabetic cardiomyopathy by inhibiting NF-κB-mediated inflammation and activating the Nrf2-mediated antioxidant responses. Phytomedicine 2019, 59, 152774. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Rauf, A.; Abu-Izneid, T.; Nadeem, M.; Shariati, M.A.; Khan, I.A.; Imran, A.; Orhan, I.E.; Rizwan, M.; Atif, M.; et al. Luteolin, a flavonoid, as an anticancer agent: A review. Biomed. Pharmacother. 2019, 112, 108612. [Google Scholar] [CrossRef] [PubMed]
- Kempuraj, D.; Thangavel, R.; Kempuraj, D.D.; Ahmed, M.E.; Selvakumar, G.P.; Raikwar, S.P.; Zaheer, S.A.; Iyer, S.S.; Govindarajan, R.; Chandrasekaran, P.N.; et al. Neuroprotective effects of flavone luteolin in neuroinflammation and neurotrauma. Biofactors 2021, 47, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Che, S.; Zhang, L.; Ruan, Z. Reparative effects of ethanol-induced intestinal barrier injury by flavonoid luteolin via MAPK/NF-κB/MLCK and Nrf2 signaling pathways. J. Agric. Food Chem. 2021, 69, 4101–4110. [Google Scholar] [CrossRef]
- Zhang, J.X.; Xing, J.G.; Wang, L.L.; Jiang, H.L.; Guo, S.L.; Liu, R. Luteolin inhibits fibrillary β-Amyloid(1-40)-induced inflammation in a human blood-brain barrier model by suppressing the p38 MAPK-mediated NF-κB signaling pathways. Molecules 2017, 22, 334. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Yeh, C.H.; Yang, M.L.; Kuan, Y.H. Luteolin suppresses inflammatory mediator expression by blocking the Akt/NFκB pathway in acute lung injury induced by lipopolysaccharide in mice. Evid. Based Complement. Alternat. Med. 2012, 2012, 383608. [Google Scholar] [CrossRef]
- Frandoloso, R.; Pivato, M.; Martínez-Martínez, S.; Rodríguez-Ferri, E.F.; Kreutz, L.C.; Martín, C.B. Differences in Haemophilus parasuis adherence to and invasion of AOC-45 porcine aorta endothelial cells. BMC Vet. Res. 2013, 9, 207. [Google Scholar] [CrossRef]
- Barton-Pai, A.; Feleder, C.; Johnson, A. Tumor necrosis factor-α induces increased lung vascular permeability: A role for GSK3α/β. Eur. J. Pharmacol. 2011, 657, 159–166. [Google Scholar] [CrossRef][Green Version]
- Kunimura, K.; Miki, S.; Takashima, M.; Suzuki, J.I. S-1-propenylcysteine improves TNF-α-induced vascular endothelial barrier dysfunction by suppressing the GEF-H1/RhoA/Rac pathway. Cell Commun. Signal. 2021, 19, 17. [Google Scholar] [CrossRef] [PubMed]
- Friedl, J.; Puhlmann, M.; Bartlett, D.L.; Libutti, S.K.; Turner, E.N.; Gnant, M.F.; Alexander, H.R. Induction of permeability across endothelial cell monolayers by tumor necrosis factor (TNF) occurs via a tissue factor-dependent mechanism: Relationship between the procoagulant and permeability effects of TNF. Blood 2002, 100, 1334–1339. [Google Scholar] [CrossRef]
- Petreaca, M.L.; Yao, M.; Liu, Y.; Defea, K.; Martins-Green, M. Transactivation of vascular endothelial growth factor receptor-2 by interleukin-8 (IL-8/CXCL8) is required for IL-8/CXCL8-induced endothelial permeability. Mol. Biol. Cell 2007, 18, 5014–5023. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, X.; Pan, Z.; Liu, S.; Han, H.; Zhao, C.; Tang, X. The role of IL-8/CXCR2 signaling in microcystin-LR triggered endothelial cell activation and increased vascular permeability. Chemosphere 2018, 194, 43–48. [Google Scholar] [CrossRef]
- Sheikpranbabu, S.; Kalishwaralal, K.; Venkataraman, D.; Eom, S.H.; Park, J.; Gurunathan, S. Silver nanoparticles inhibit VEGF-and IL-1beta-induced vascular permeability via Src dependent pathway in porcine retinal endothelial cells. J. Nanobiotechnol. 2009, 7, 8. [Google Scholar] [CrossRef]
- Yang, Y.C.; Fu, H.; Zhang, B.; Wu, Y.B. Interleukin-6 downregulates the expression of vascular endothelial-cadherin and increases permeability in renal glomerular endothelial cells via the trans-signaling pathway. Inflammation 2022, 45, 2544–2558. [Google Scholar] [CrossRef]
- Fu, P.; Birukova, A.A.; Xing, J.; Sammani, S.; Murley, J.S.; Garcia, J.G.; Grdina, D.J.; Birukov, K.G. Amifostine reduces lung vascular permeability via suppression of inflammatory signalling. Eur. Respir. J. 2009, 33, 612–624. [Google Scholar] [CrossRef]
- Maruo, N.; Morita, I.; Shirao, M.; Murota, S. IL-6 increases endothelial permeability in vitro. Endocrinology 1992, 131, 710–714. [Google Scholar]
- Flynn, K.M.; Michaud, M.; Madri, J.A. CD44 deficiency contributes to enhanced experimental autoimmune encephalomyelitis: A role in immune cells and vascular cells of the blood-brain barrier. Am. J. Pathol. 2013, 182, 1322–1336. [Google Scholar] [CrossRef] [PubMed]
- Phoenix, K.N.; Yue, Z.; Yue, L.; Cronin, C.G.; Liang, B.T.; Hoeppner, L.H.; Claffey, K.P. PLCβ2 promotes VEGF-induced vascular permeability. Arterioscler. Thromb. Vasc. Biol. 2022, 42, 1229–1241. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Lin, Q.; Qin, X.; Ruan, Z.; Zhou, J.; Lin, Z.; Su, Y.; Zheng, J.; Liu, Z. ACE2 antagonizes VEGFa to reduce vascular permeability during acute lung injury. Cell Physiol. Biochem. 2016, 38, 1055–1062. [Google Scholar] [CrossRef]
- Zhang, Q.; Zheng, M.; Betancourt, C.E.; Liu, L.; Sitikov, A.; Sladojevic, N.; Zhao, Q.; Zhang, J.H.; Liao, J.K.; Wu, R. Increase in blood-brain barrier (BBB) permeability is regulated by MMP3 via the ERK signaling pathway. Oxid. Med. Cell Longev. 2021, 2021, 6655122. [Google Scholar] [CrossRef]
- Cui, Y.L.; Zhang, S.; Tian, Z.T.; Lin, Z.F.; Chen, D.C. Rhubarb antagonizes matrix metalloproteinase-9-induced vascular endothelial permeability. Chin. Med. J. (Engl.) 2016, 129, 1737–1743. [Google Scholar] [CrossRef]
- Kumar, M.; Sandhir, R. Hydrogen sulfide attenuates hyperhomocysteinemia-induced blood-brain barrier permeability by inhibiting MMP-9. Int. J. Neurosci. 2022, 132, 1061–1071. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; He, Q. Inhibition of c-Src protects paraquat induced microvascular endothelial injury by modulating caveolin-1 phosphorylation and caveolae mediated transcellular permeability. Environ. Toxicol. Pharmacol. 2017, 52, 62–68. [Google Scholar] [CrossRef]
- Ninchoji, T.; Love, D.T.; Smith, R.O.; Hedlund, M.; Vestweber, D.; Sessa, W.C.; Claesson-Welsh, L. eNOS-induced vascular barrier disruption in retinopathy by c-Src activation and tyrosine phosphorylation of VE-cadherin. Elife 2021, 10, e64944. [Google Scholar] [CrossRef]

| Gene | Primer | Primer Sequence (5′→3′) |
|---|---|---|
| GAPDH | Forward | TCGGAGTGAACGGATTTGGC |
| Reverse | TGCCGTGGGTGGAATCATAC | |
| TNF | Forward | CGCTCTTCTGCCTACTGCACTTC |
| Reverse | CTGTCCCTCGGCTTTGACATT | |
| IL-6 | Forward | TGTCGAGGCTGTGCAGATTAGT |
| Reverse | ATCCACTCGTTCTGTGACTGC | |
| IL-8 | Forward | ACAGCAGTAACAACAACAAG |
| Reverse | GACCAGCACAGGAATGAG | |
| IL-1β | Forward | GCTGGAGGATATAGACCCC |
| Reverse | GTTGGGGTACAGGGCAGAC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, P.; Liu, X.; Li, X.; Ihsan, A.; Wu, Z.; Fu, S.; Ye, C.; Qiu, Y.; Wang, X.; Lu, Q.; et al. Protective Effects of Luteolin on Glaesserella parasuis-Induced Injury: An In Vitro Study with Porcine Vascular Endothelial Cells. Antibiotics 2025, 14, 824. https://doi.org/10.3390/antibiotics14080824
Guo P, Liu X, Li X, Ihsan A, Wu Z, Fu S, Ye C, Qiu Y, Wang X, Lu Q, et al. Protective Effects of Luteolin on Glaesserella parasuis-Induced Injury: An In Vitro Study with Porcine Vascular Endothelial Cells. Antibiotics. 2025; 14(8):824. https://doi.org/10.3390/antibiotics14080824
Chicago/Turabian StyleGuo, Pu, Xuwen Liu, Xiaoyi Li, Awais Ihsan, Zhongyuan Wu, Shulin Fu, Chun Ye, Yinsheng Qiu, Xu Wang, Qirong Lu, and et al. 2025. "Protective Effects of Luteolin on Glaesserella parasuis-Induced Injury: An In Vitro Study with Porcine Vascular Endothelial Cells" Antibiotics 14, no. 8: 824. https://doi.org/10.3390/antibiotics14080824
APA StyleGuo, P., Liu, X., Li, X., Ihsan, A., Wu, Z., Fu, S., Ye, C., Qiu, Y., Wang, X., Lu, Q., & Liu, Y. (2025). Protective Effects of Luteolin on Glaesserella parasuis-Induced Injury: An In Vitro Study with Porcine Vascular Endothelial Cells. Antibiotics, 14(8), 824. https://doi.org/10.3390/antibiotics14080824







