Biosynthesized Gold Nanoparticles from Eruca sativa Mill. Leaf Extract Exhibit In Vivo Biocompatibility, Antimicrobial, and Antioxidant Activities
Abstract
1. Introduction
2. Results and Discussion
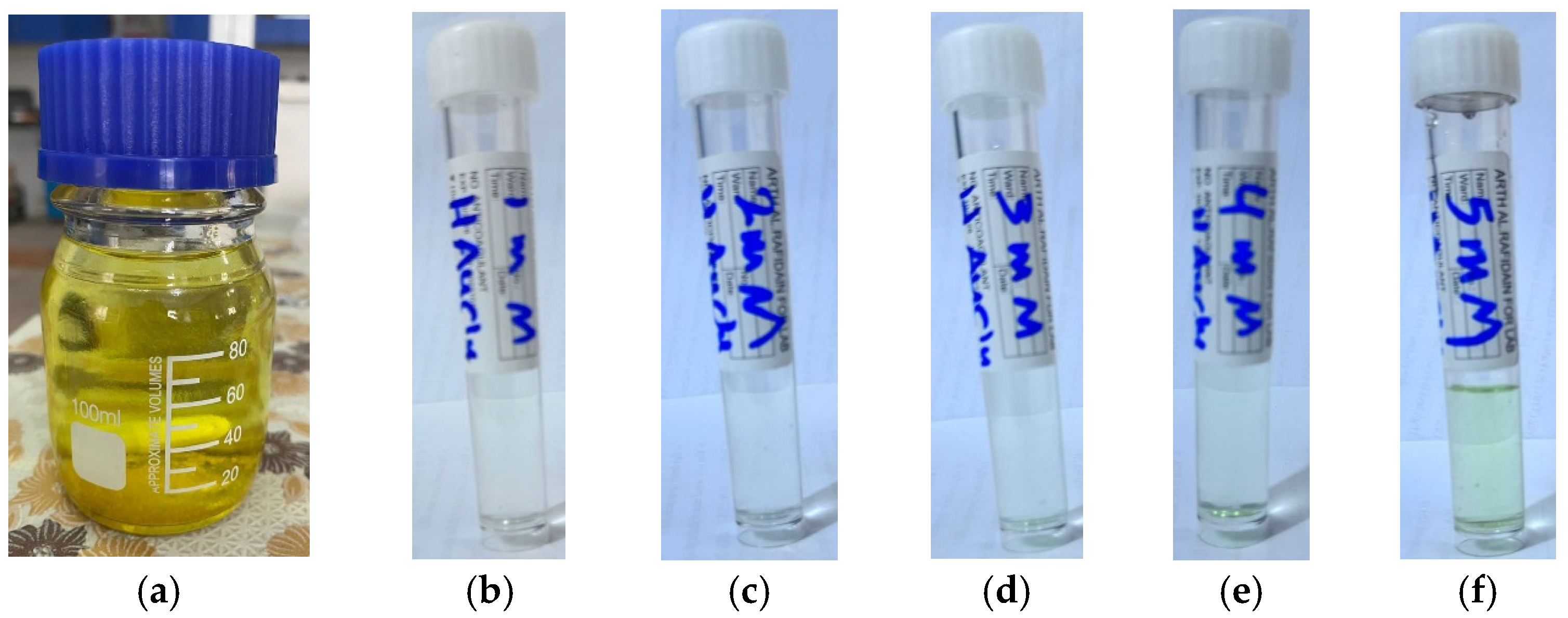
2.1. Preparation of the Eruca sativa Leaves Extract
2.2. Preparation of the Gold Stock Solution and Its Dilution
2.3. Green Synthesis of the AuNPs Using Eruca sativa Leaves Extract
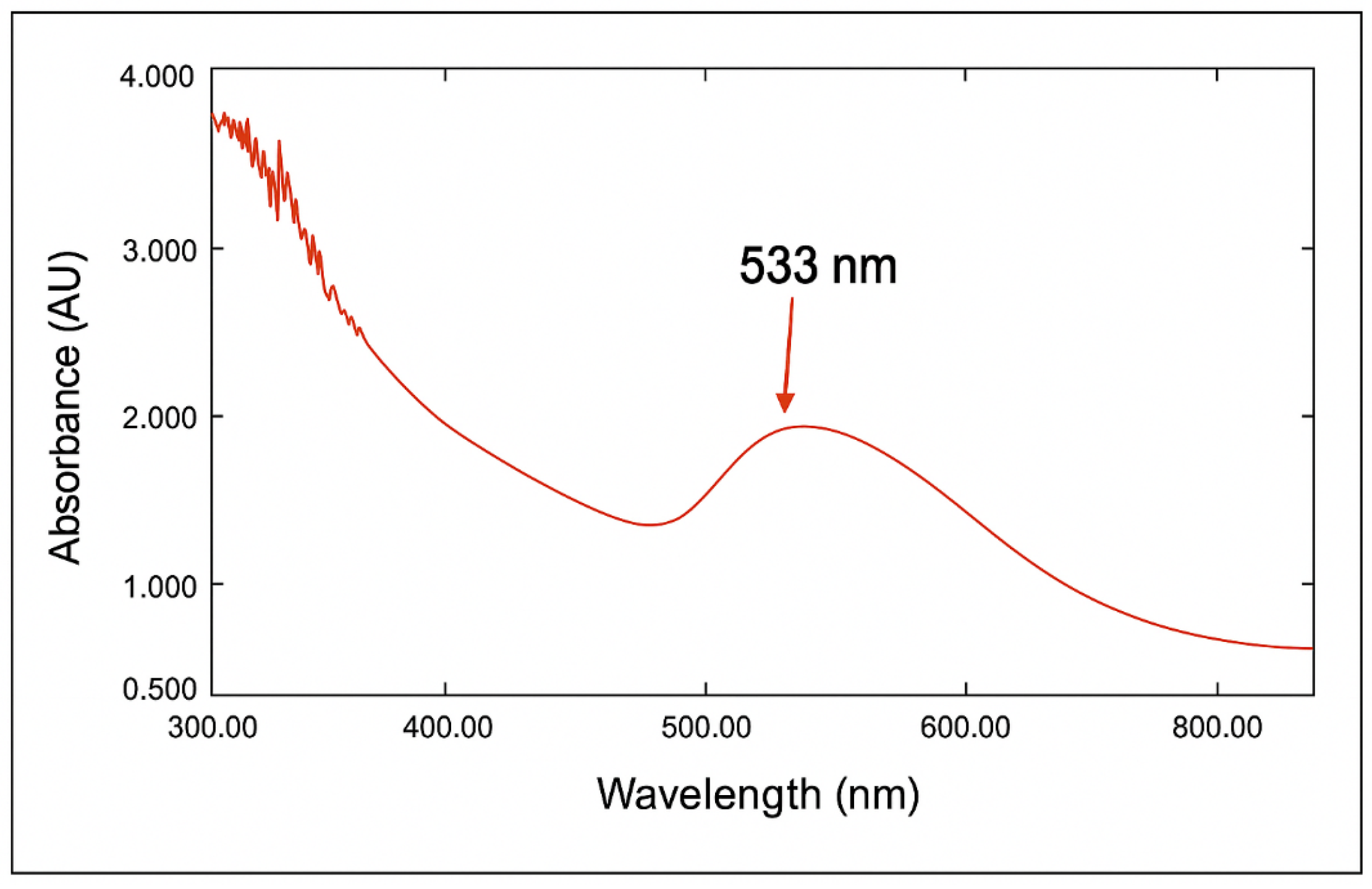
2.4. UV-Vis Spectroscopy Study
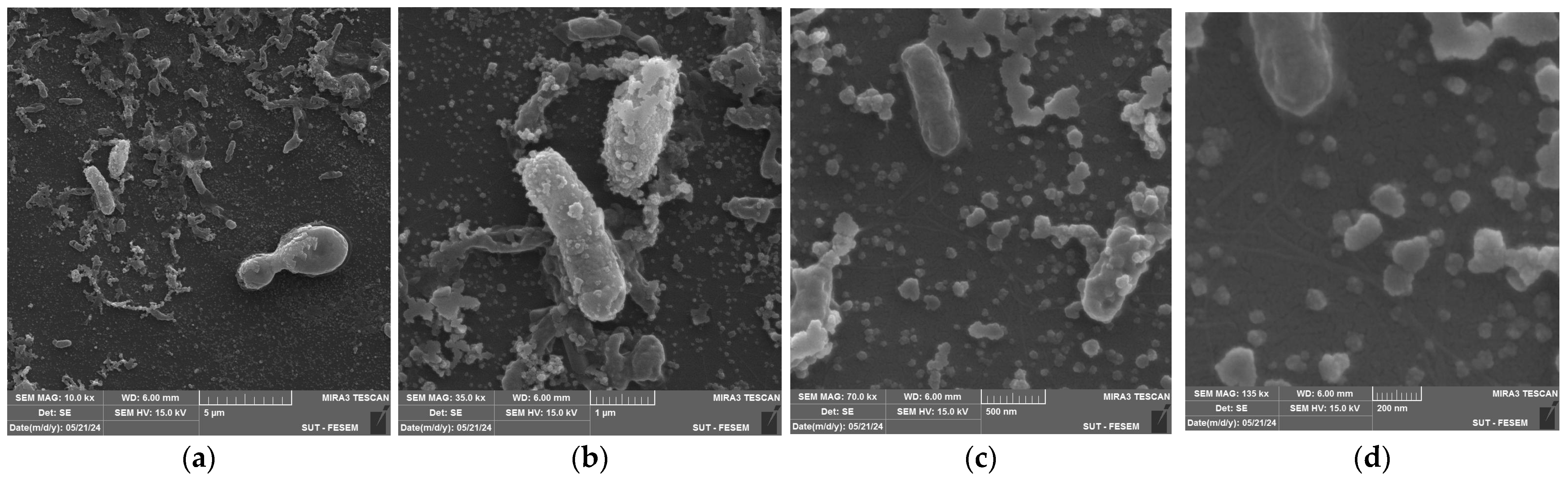
2.5. Field Emission Scanning Electron Microscopy (FESEM) Analysis
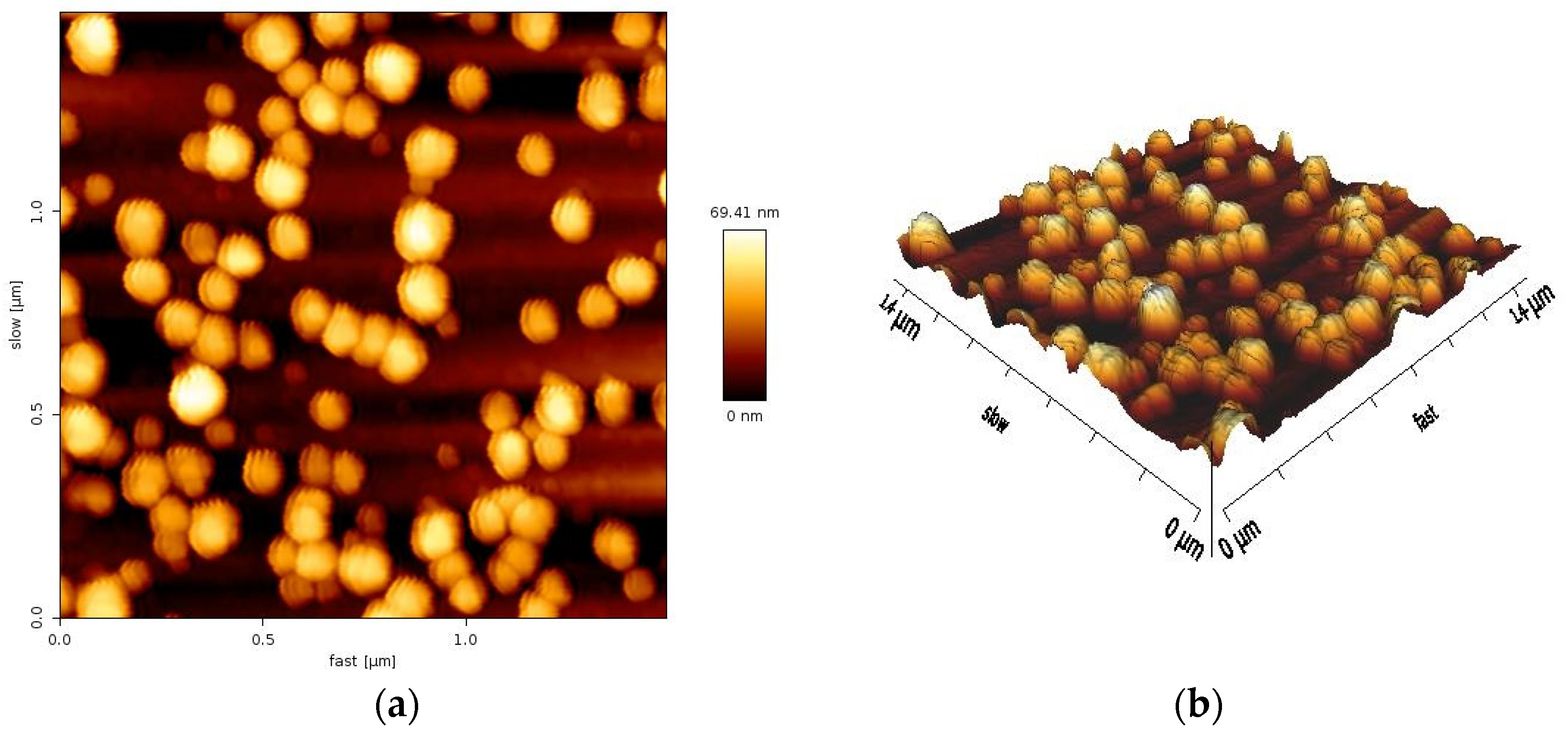
2.6. Atomic Force Microscopy (AFM) Analysis
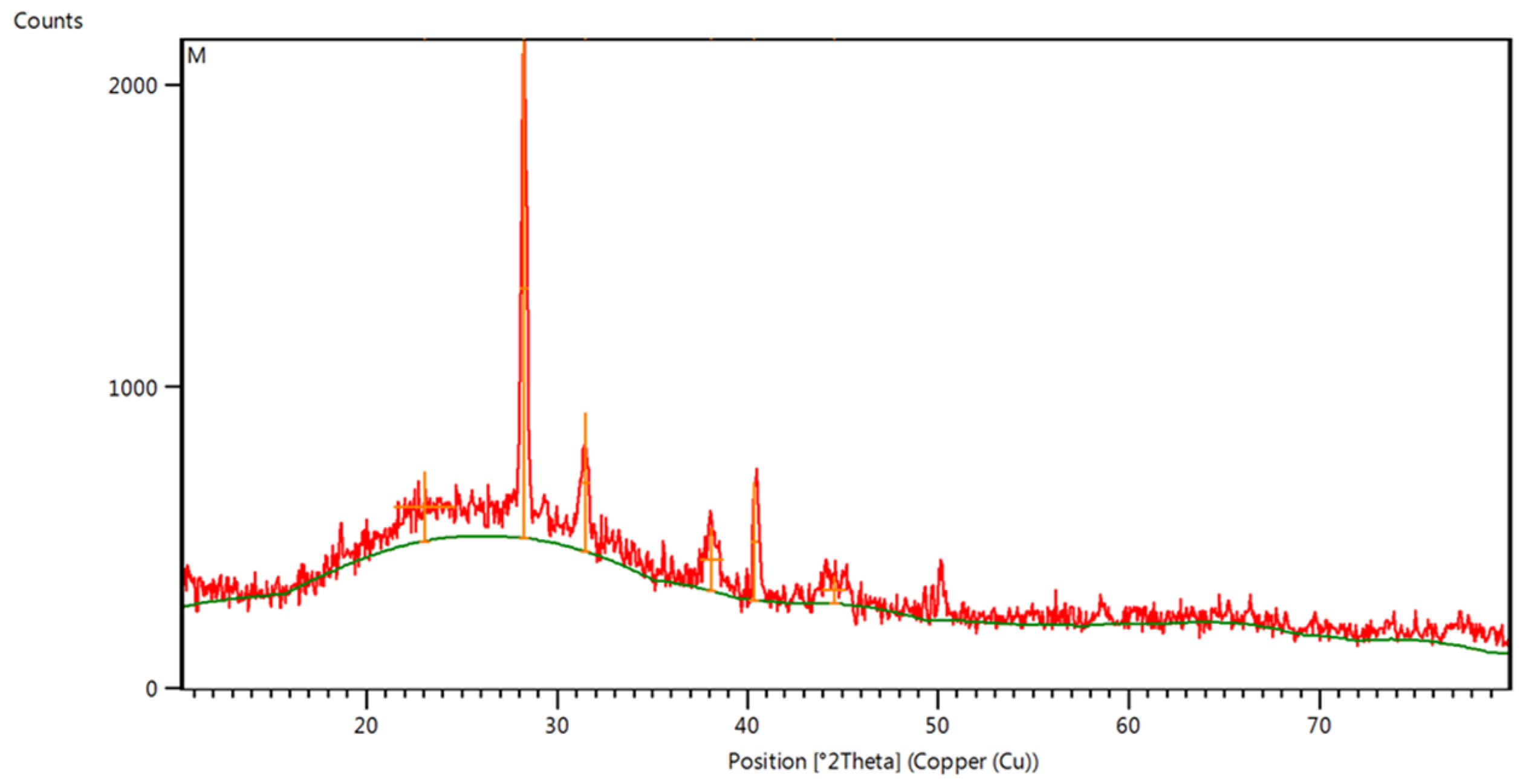
2.7. X-Ray Diffraction (XRD) Analysis
2.8. Zeta Potential Analysis
2.9. Inductively Coupled Plasma–Mass Spectrometry (ICP-MS) Analysis
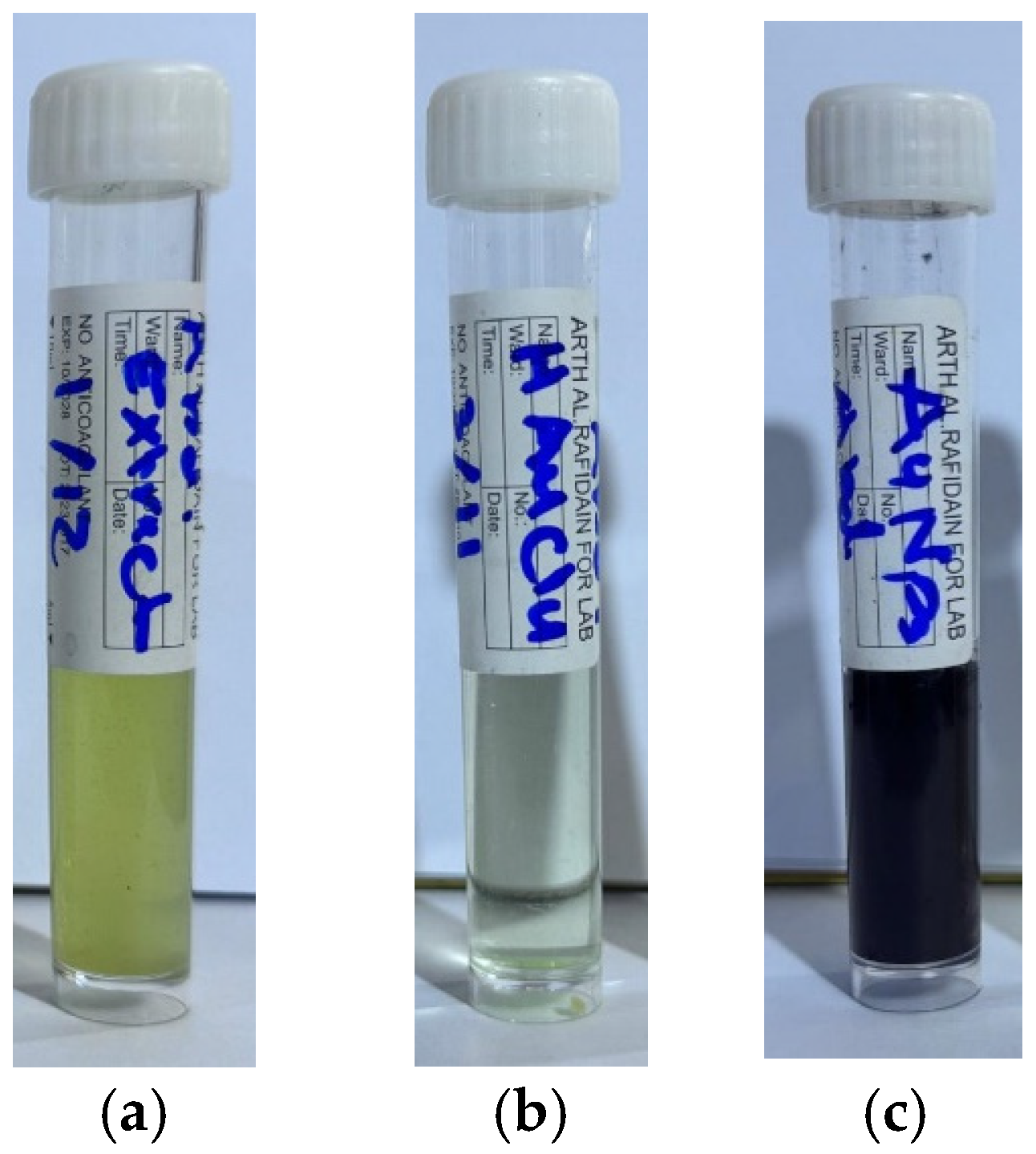
2.10. Hemolysis Assay
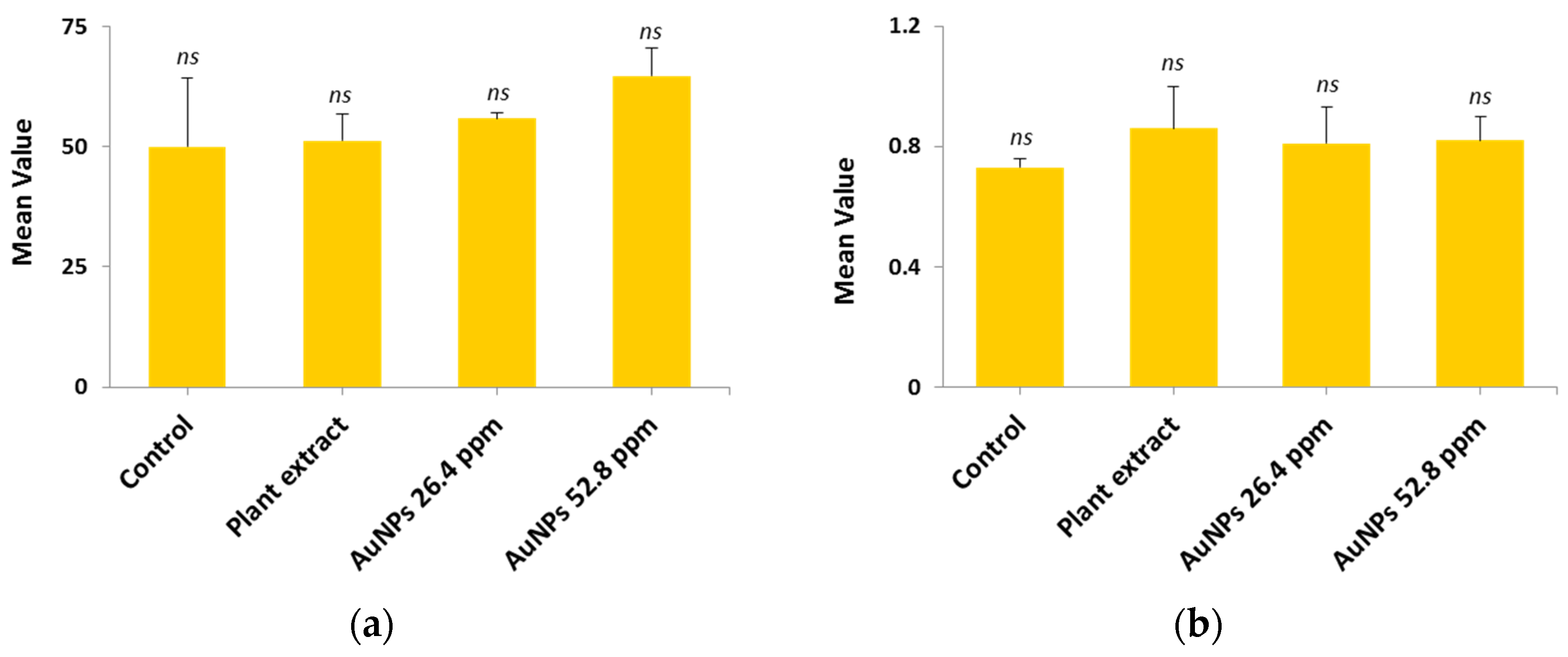
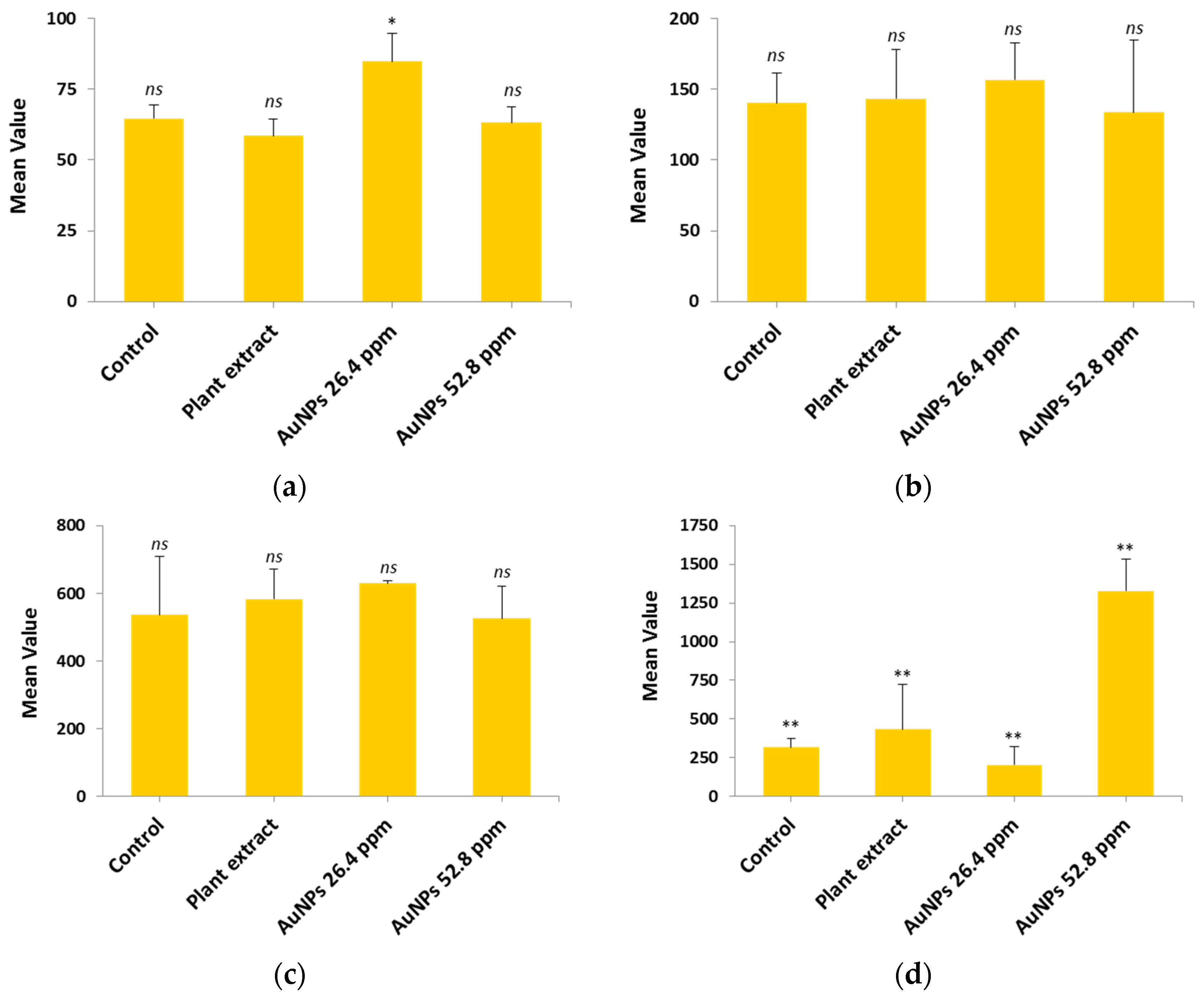
2.11. Hepatic and Renal Functions
2.11.1. Hepatic Function
2.11.2. Renal Function
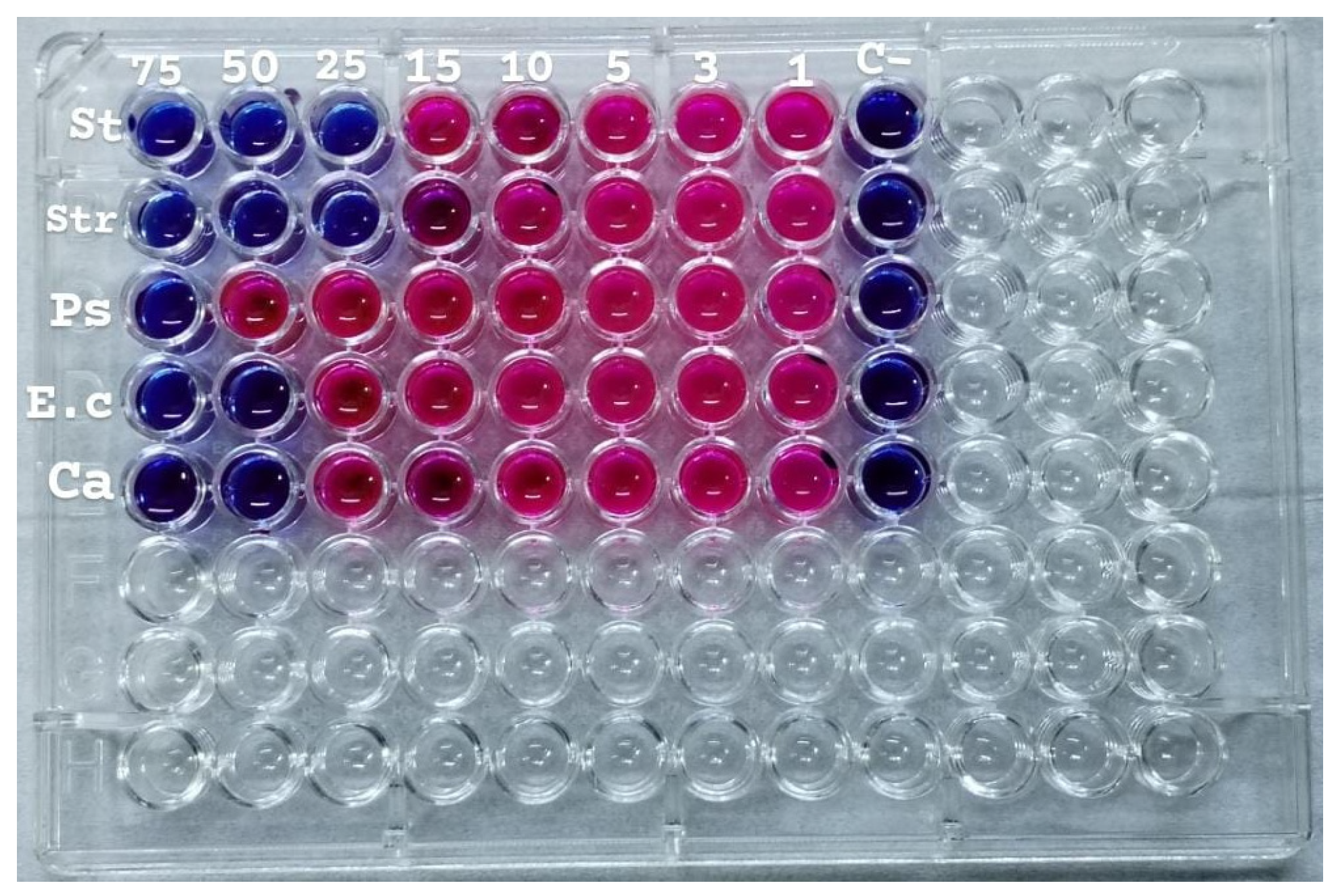
2.12. Minimum Inhibitory Concentration (MIC)
2.13. Antimicrobial Activity
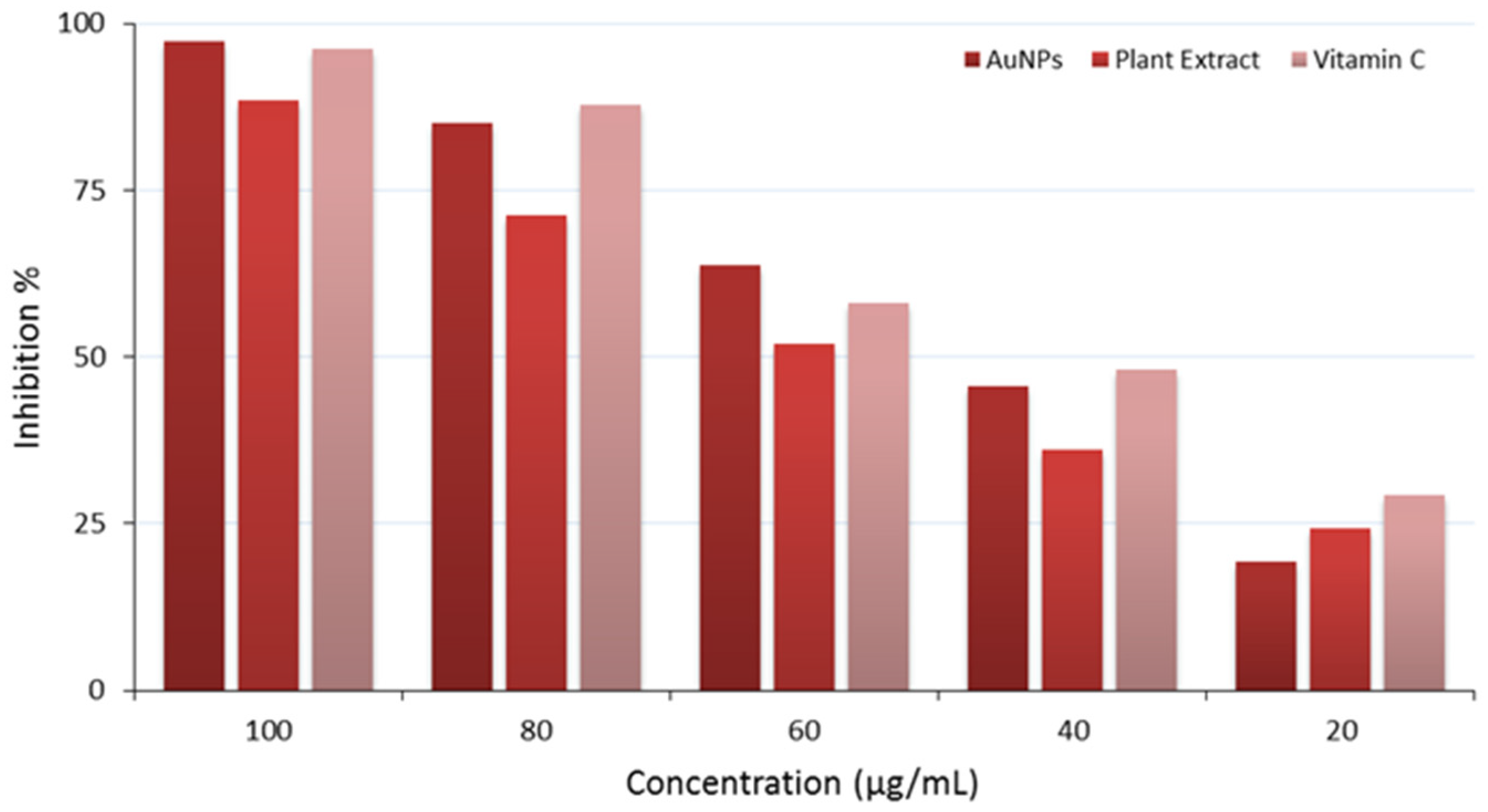
2.14. DPPH Free Radical Scavenging Activity
3. Materials and Methods
3.1. General
3.2. Preparation of the Eruca sativa Mill. Leaves Extract
3.3. Preparation of the Gold Stock Solution and Its Dilution
3.4. Green Synthesis of AuNPs
3.5. Characterization of the Biosynthesized Gold Nanoparticles (AuNPs)
3.5.1. Ultraviolet–Visible (UV–Vis) Spectroscopy
3.5.2. The Field Emission Scanning Electron Microscopy (FE-SEM) Analysis
3.5.3. The Atomic Force Microscopy (AFM) Analysis
3.5.4. X-Ray Diffraction (XRD) Analysis
3.5.5. Zeta Potential Analysis
3.5.6. Inductively Coupled Plasma–Mass Spectrometry (ICP-MS) Analysis
3.6. Ethical Approval
3.7. Hemolytic Activity
3.8. Animals and Experimental Design
3.9. Hepatic and Renal Function Analysis
3.9.1. Blood Samples Collection
3.9.2. Serum Analysis of Hepatic and Renal Function
3.10. Antimicrobial Activity
3.11. Minimum Inhibitory Concentration (MIC)
3.12. DPPH Free Radical Scavenging Activity
3.13. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A0 | Absorbance of the control |
| A1 | Absorbance of the sample |
| AFM | Atomic Force Microscopy |
| ALP | Alkaline phosphatase |
| ALT | Alanine transaminase |
| AMR | Antimicrobial resistance |
| AST | Aspartate aminotransferase |
| AU | Arbitrary unit |
| AuNPs | gold nanoparticles |
| dl | Deciliter |
| DPPH | 2,2-DiPhenyl-1-Picrylhydrazyl |
| FESEM | Field Emission Scanning Electron Microscope |
| FTIR | Fourier Transform Infrared Spectroscopy |
| HC | Hemolytic Activity |
| ICDD | International Centre for Diffraction Data |
| ICP-MS | Inductively coupled plasma-mass spectrometry |
| IU | International Unit |
| kx | Thousands of times magnification |
| LDH | Lactate dehydrogenase |
| MIC | Minimum inhibitory concentration |
| NC | Negative control |
| O | Optical density |
| PC | Positive control |
| p-value | Probability value |
| ROS | Reactive Oxygen Species |
| SPR | Surface Plasmon Resonance |
| U/L | Unit/Liter |
| UV-Vis | Ultraviolet–visible |
| XRD | X-Ray Diffraction |
References
- Ho, C.S.; Wong, C.T.H.; Aung, T.T.; Lakshminarayanan, R.; Mehta, J.S.; Rauz, S.; McNally, A.; Kintses, B.; Peacock, S.J.; de la Fuente-Nunez, C.; et al. Antimicrobial Resistance: A Concise Update. Lancet Microbe 2025, 6, 100947. [Google Scholar] [CrossRef]
- Mahdi, I.S.; Abdula, A.M.; Jassim, A.M.N.; Baqi, Y. Design, Synthesis, Antimicrobial Properties, and Molecular Docking of Novel Furan-Derived Chalcones and Their 3,5-Diaryl-Δ2-pyrazoline Derivatives. Antibiotics 2023, 13, 21. [Google Scholar] [CrossRef] [PubMed]
- Chandimali, N.; Bak, S.G.; Park, E.H.; Lim, H.J.; Won, Y.S.; Kim, E.K.; Park, S.I.; Lee, S.J. Free Radicals and Their Impact on Health and Antioxidant Defenses: A Review. Cell Death Discov. 2025, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Joudeh, N.; Linke, D. Nanoparticle Classification, Physicochemical Properties, Characterization, and Applications: A Comprehensive Review for Biologists. J. Nanobiotechnol. 2022, 20, 262. [Google Scholar] [CrossRef] [PubMed]
- Khushnood, M. Nanomaterials: Surface Area to Volume Ratio. J. Mater. Sci. Nanomater. 2024, 8, 159. [Google Scholar] [CrossRef]
- Pomerantseva, E.; Bonaccorso, F.; Feng, X.; Cui, Y.; Gogotsi, Y. Energy Storage: The Future Enabled by Nanomaterials. Science 2019, 366, eaan8285. [Google Scholar] [CrossRef]
- Cao, H.; Yang, E.; Kim, Y.; Zhao, Y.; Ma, W. Biomimetic Chiral Nanomaterials with Selective Catalysis Activity. Adv. Sci. 2024, 11, e2306979. [Google Scholar] [CrossRef]
- Illath, K.; Kar, S.; Gupta, P.; Shinde, A.; Wankhar, S.; Tseng, F.G.; Lim, K.T.; Nagai, M.; Santra, T.S. Microfluidic Nanomaterials: From Synthesis to Biomedical Applications. Biomaterials 2022, 280, 121247. [Google Scholar] [CrossRef] [PubMed]
- Awadelkareem, A.M.; Al-Shammari, E.; Elkhalifa, A.O.; Adnan, M.; Siddiqui, A.J.; Patel, M.; Khan, M.I.; Mehmood, K.; Ashfaq, F.; Badraoui, R.; et al. Biosynthesized Silver Nanoparticles from Eruca sativa Miller Leaf Extract Exhibits Antibacterial, Antioxidant, Anti-Quorum-Sensing, Antibiofilm, and Anti-Metastatic Activities. Antibiotics 2022, 11, 853. [Google Scholar] [CrossRef] [PubMed]
- Omar, N.; McCann, T.; Al-Juboury, A.I.; Ustinova, M.A.; Sharezwri, A.O. Early Jurassic–Early Cretaceous Calcareous Nannofossil Biostratigraphy and Geochemistry, Northeastern Iraqi Kurdistan: Implications for Paleoclimate and Paleoecological Conditions. Geosciences 2022, 12, 94. [Google Scholar] [CrossRef]
- Georgeous, J.; AlSawaftah, N.; Abuwatfa, W.H.; Husseini, G.A. Review of Gold Nanoparticles: Synthesis, Properties, Shapes, Cellular Uptake, Targeting, Release Mechanisms and Applications in Drug Delivery and Therapy. Pharmaceutics 2024, 16, 1332. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Crawford, B.M.; Vo-Dinh, T. Gold nanoparticles-mediated photothermal therapy and immunotherapy. Immunotherapy 2018, 10, 1175–1188. [Google Scholar] [CrossRef] [PubMed]
- Bloise, N.; Strada, S.; Dacarro, G.; Visai, L. Gold Nanoparticles Contact with Cancer Cell: A Brief Update. Int. J. Mol. Sci. 2022, 23, 7683. [Google Scholar] [CrossRef] [PubMed]
- Turkmen Koc, S.N.; Rezaei Benam, S.; Aral, I.P.; Shahbazi, R.; Ulubayram, K. Gold Nanoparticles-Mediated Photothermal and Photodynamic Therapies for Cancer. Int. J. Pharm. 2024, 655, 124057. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, P.; Ma, R.; Sang, L.; Fatima, M.; Sheikh, A.; Abourehab, M.A.S.; Gupta, N.; Chen, Z.S.; Zhou, Y. Gold Nanoparticles and Gold Nanorods in the Landscape of Cancer Therapy. Mol. Cancer 2023, 22, 98. [Google Scholar] [CrossRef]
- Sani, A.; Cao, C.; Cui, D. Toxicity of Gold Nanoparticles (AuNPs): A Review. Biochem. Biophys. Rep. 2021, 26, 100991. [Google Scholar] [CrossRef]
- Alaabedin, A.A.Z.; Hamza, B.H.; Abdual-Majeed, A.M.A.-M.; Bamsaoud, S.F. Green Synthesis of Zinc Oxide Nanoparticles to Study Its Effect on the Skin Using IR Thermography. Al-Mustansiriyah J. Sci. 2023, 34, 115–123. [Google Scholar] [CrossRef]
- Lazim, K.A.; Moghaddam, H.M. Green Synthesis of Nickel Nanoparticles Using Lawsonia inermis Extract and Evaluation of Their Effectiveness against Staphylococcus aureus. Al-Mustansiriyah J. Sci. 2025, 36, 84–91. [Google Scholar] [CrossRef]
- Mikhailova, E.O. Gold Nanoparticles: Biosynthesis and Potential of Biomedical Application. J. Funct. Biomater. 2021, 12, 70. [Google Scholar] [CrossRef]
- Bashir, S.; Al-Habib, A.A.S. Evaluation of the Activity of Petroselinum crispum Aqueous Extract as a Promoter for Rooting of Stem Cuttings of Some Plants. Al-Mustansiriyah J. Sci. 2020, 31, 22–30. [Google Scholar] [CrossRef][Green Version]
- Al-Alawi, R.A.; Al-Mashiqri, J.H.; Al-Nadabi, J.S.M.; Al-Shihi, B.I.; Baqi, Y. Date Palm Tree (Phoenix dactylifera L.): Natural Products and Therapeutic Options. Front. Plant Sci. 2017, 8, 845. [Google Scholar] [CrossRef] [PubMed]
- Al Alawi, R.; Alhamdani, M.S.S.; Hoheisel, J.D.; Baqi, Y. Antifibrotic and Tumor Microenvironment Modulating Effect of Date Palm Fruit (Phoenix dactylifera L.) Extracts in Pancreatic Cancer. Biomed. Pharmacother. 2020, 121, 109522. [Google Scholar] [CrossRef] [PubMed]
- Al Alawi, R.A.; Hoheisel, J.D.; Alhamdani, M.S.S.; Baqi, Y. Phoenix dactylifera L. (date palm) fruit extracts and fractions exhibit anti-proliferative activity against human pancreatic cancer cell lines. Heliyon 2025, 11, e42274. [Google Scholar] [CrossRef] [PubMed]
- Awla, N.J.; Naqishbandi, A.M.; Baqi, Y. Preventive and Therapeutic Effects of Silybum marianum Seed Extract Rich in Silydianin and Silychristin in a Rat Model of Metabolic Syndrome. ACS Pharmacol. Transl. Sci. 2023, 6, 1715–1723. [Google Scholar] [CrossRef]
- Azimova, S.S.; Glushenkova, A.I. Eruca sativa Mill. In Lipids, Lipophilic Components and Essential Oils from Plant Sources; Azimova, S.S., Glushenkova, A.I., Eds.; Springer: London, UK, 2012. [Google Scholar] [CrossRef]
- Testai, L.; Pagnotta, E.; Piragine, E.; Flori, L.; Citi, V.; Martelli, A.; Mannelli, L.D.C.; Ghelardini, C.; Matteo, R.; Suriano, S.; et al. Cardiovascular Benefits of Eruca sativa Mill. Defatted Seed Meal Extract: Potential Role of Hydrogen Sulfide. Phytother. Res. 2022, 36, 2616–2627. [Google Scholar] [CrossRef]
- Grami, D.; Selmi, S.; Rtibi, K.; Sebai, H.; De Toni, L. Emerging Role of Eruca sativa Mill. in Male Reproductive Health. Nutrients 2024, 16, 253. [Google Scholar] [CrossRef]
- Barillari, J.; Canistro, D.; Paolini, M.; Ferroni, F.; Pedulli, G.F.; Iori, R.; Valgimigli, L. Direct Antioxidant Activity of Purified Glucoerucin, the Dietary Secondary Metabolite Contained in Rocket (Eruca sativa Mill.) Seeds and Sprouts. J. Agric. Food Chem. 2005, 53, 2475–2482. [Google Scholar] [CrossRef]
- Amiripour, H.; Iranbakhsh, A.; Saadatmand, S.; Mousavi, F.; Oraghi Ardebili, Z. Exogenous Application of Melatonin and Chitosan Mitigate Simulated Microgravity Stress in the Rocket (Eruca sativa L.) Plant. Plant Physiol. Biochem. 2025, 218, 109294. [Google Scholar] [CrossRef]
- Ganesh, K.M.; Bhaskar, S.; Cheerala, V.S.K.; Battampara, P.; Reddy, R.; Neelakantan, S.C.; Reddy, N.; Ramamurthy, S.S. Review of Gold Nanoparticles in Surface Plasmon-Coupled Emission Technology: Effect of Shape, Hollow Nanostructures, Nano-Assembly, Metal–Dielectric and Heterometallic Nanohybrids. Nanomaterials 2024, 14, 111. [Google Scholar] [CrossRef]
- Verma, K.; Kathuria, D.; Ram, A.; Verma, K.; Sharma, S.; Tohra, S.K.; Sharma, A. Evaluation of Cytotoxic and Antioxidant Potential of Green-Synthesized Silver and Gold Nanoparticles from Nepeta leucophylla Benth. Chem. Biodivers. 2025, 22, e202402679. [Google Scholar] [CrossRef]
- Zhu, W.; Hu, C.; Ren, Y.; Lu, Y.; Song, Y.; Ji, Y.; He, J. Green Synthesis of Zinc Oxide Nanoparticles Using Cinnamomum camphora (L.) Presl Leaf Extracts and Its Antifungal Activity. J. Environ. Chem. Eng. 2021, 9, 106659. [Google Scholar] [CrossRef]
- Alharbi, F.N.; Abaker, Z.M.; Makawi, S.Z.A. Phytochemical Substances—Mediated Synthesis of Zinc Oxide Nanoparticles (ZnO NPs). Inorganics 2023, 11, 328. [Google Scholar] [CrossRef]
- Hu, X.; Qiu, D.; Jiang, Q.; Xu, Q.; Li, J. Cu2+-Doped Zeolitic Imidazolate Frameworks and Gold Nanoparticle (AuNPs@ZIF-8/Cu) Nanocomposites Enable Label-Free and Highly Sensitive Electrochemical Detection of Oral Cancer-Related Biomarkers. Anal. Methods 2024, 16, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.X.; Shameli, K.; Miyake, M.; Kuwano, N.; Ahmad Khairudin, N.B.; Mohamad, S.E.; Yew, Y.P. Green Synthesis of Gold Nanoparticles Using Aqueous Extract of Garcinia mangostana Fruit Peels. J. Nanomater. 2016, 2016, 8489094. [Google Scholar] [CrossRef]
- Oliveira, A.E.F.; Pereira, A.C.; Resende, M.A.C.; Ferreira, L.F. Gold Nanoparticles: A Didactic Step-by-Step of the Synthesis Using the Turkevich Method, Mechanisms, and Characterizations. Analytica 2023, 4, 250–263. [Google Scholar] [CrossRef]
- Francis, S.; Joseph, S.; Koshy, E.P.; Mathew, B. Green Synthesis and Characterization of Gold and Silver Nanoparticles Using Mussaenda glabrata Leaf Extract and Their Environmental Applications to Dye Degradation. Environ. Sci. Pollut. Res. 2017, 24, 17347–17357. [Google Scholar] [CrossRef]
- Sæbø, I.P.; Bjørås, M.; Franzyk, H.; Helgesen, E.; Booth, J.A. Optimization of the Hemolysis Assay for the Assessment of Cytotoxicity. Int. J. Mol. Sci. 2023, 24, 2914. [Google Scholar] [CrossRef]
- Duncan, D.B. Multiple Range and Multiple F Tests. Biometrics 1955, 11, 1–42. [Google Scholar] [CrossRef]
- Alkilany, A.M.; Murphy, C.J. Toxicity and cellular uptake of gold nanoparticles: What we have learned so far? Adv. Drug Deliv. Rev. 2012, 64, 190–199. [Google Scholar] [CrossRef]
- Gómez-Lechón, M.J.; Tolosa, L.; Conde, I.; Donato, M.T. Competency of different cell models to predict human hepatotoxic drugs. Expert Opin. Drug Metab. Toxicol. 2014, 10, 1553–1568. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Burmistrov, D.E.; Fomina, P.A.; Validov, S.Z.; Kozlov, V.A. Antibacterial Properties of Copper Oxide Nanoparticles. Int. J. Mol. Sci. 2024, 25, 11563. [Google Scholar] [CrossRef]
- Mikhailova, E.O. Green Silver Nanoparticles: An Antibacterial Mechanism. Antibiotics 2024, 14, 5. [Google Scholar] [CrossRef]
- Jha, D.; Kumar, P.; Gautam, H.K. Citrus maxima Extract-Coated Versatile Gold Nanoparticles Display ROS-Mediated Inhibition of MDR-Pseudomonas aeruginosa and Cancer Cells. Bioorg. Chem. 2025, 155, 108152. [Google Scholar] [CrossRef]
- Rmaidh, A.Y.; Al-Mahdawi, A.S.; Al-Obaidi, N.S.; Al-Obaidi, M.A. Organic Ligands as Adsorbent Surface of Heavy Metals and Evaluating Antibacterial Activity of Synthesized Complexes. Inorg. Chem. Commun. 2023, 148, 111148. [Google Scholar] [CrossRef]
- Al-Radadi, N.S.; Al-Bishri, W.M.; Salem, N.A.; ElShebiney, S.A. Plant-Mediated Green Synthesis of Gold Nanoparticles Using an Aqueous Extract of Passiflora ligularis, Optimization, Characterizations, and Their Neuroprotective Effect on Propionic Acid-Induced Autism in Wistar Rats. Saudi Pharm. J. 2024, 32, 101921. [Google Scholar] [CrossRef] [PubMed]
- Galúcio, J.M.P.; de Souza, S.G.B.; Vasconcelos, A.A.; Lima, A.K.O.; da Costa, K.S.; de Campos Braga, H.; Taube, P.S. Synthesis, Characterization, Applications, and Toxicity of Green Synthesized Nanoparticles. Curr. Pharm. Biotechnol. 2022, 23, 420–443. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Dasgupta, S.C.; Dasgupta, A.K.; Gomes, A.; Gomes, A. Gold Nanoparticles (AuNPs) Conjugated with Andrographolide Ameliorated Viper (Daboia russellii russellii) Venom-Induced Toxicities in Animal Model. J. Nanosci. Nanotechnol. 2020, 20, 3404–3414. [Google Scholar] [CrossRef] [PubMed]
- Święch, D.; Kollbek, K.; Jabłoński, P.; Gajewska, M.; Palumbo, G.; Oćwieja, M.; Piergies, N. Exploring the Nanoscale: AFM-IR Visualization of Cysteine Adsorption on Gold Nanoparticles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2024, 318, 124433. [Google Scholar] [CrossRef]
- Ghramh, H.A.; Khan, K.A.; Ibrahim, E.H.; Setzer, W.N. Synthesis of Gold Nanoparticles (AuNPs) Using Ricinus communis Leaf Ethanol Extract, Their Characterization, and Biological Applications. Nanomaterials 2019, 9, 765. [Google Scholar] [CrossRef]
- Korolev, D.; Shumilo, M.; Shulmeyster, G.; Krutikov, A.; Golovkin, A.; Mishanin, A.; Gorshkov, A.; Spiridonova, A.; Domorad, A.; Krasichkov, A.; et al. Hemolytic Activity, Cytotoxicity, and Antimicrobial Effects of Human Albumin- and Polysorbate-80-Coated Silver Nanoparticles. Nanomaterials 2021, 11, 1484. [Google Scholar] [CrossRef]
- Moatamed, E.R.; Hussein, A.A.; El-Desoky, M.M.; Khayat, Z.E. Comparative Study of Zinc Oxide Nanoparticles and Its Bulk Form on Liver Function of Wistar Rat. Toxicol. Ind. Health 2019, 35, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.E.; Hassan, M.A.; El-Nekeety, A.A.; Abdel-Aziem, S.H.; Bakeer, R.M.; Abdel-Wahhab, M.A. Zinc-Loaded Whey Protein Nanoparticles Alleviate the Oxidative Damage and Enhance the Gene Expression of Inflammatory Mediators in Rats. J. Trace Elem. Med. Biol. 2022, 73, 127030. [Google Scholar] [CrossRef]
- Holder, I.A.; Boyce, S.T. Agar Well Diffusion Assay Testing of Bacterial Susceptibility to Various Antimicrobials in Concentrations Non-Toxic for Human Cells in Culture. Burns 1994, 20, 426–429. [Google Scholar] [CrossRef]
- Elshikh, M.; Ahmed, S.; Funston, S.; Dunlop, P.; McGaw, M.; Marchant, R.; Banat, I.M. Resazurin-Based 96-Well Plate Microdilution Method for the Determination of Minimum Inhibitory Concentration of Biosurfactants. Biotechnol. Lett. 2016, 38, 1015–1019. [Google Scholar] [CrossRef] [PubMed]
- Parejo, I.; Codina, C.; Petrakis, C.; Kefalas, P. Evaluation of Scavenging Activity Assessed by Co(II)/EDTA-Induced Luminol Chemiluminescence and DPPH* (2,2-Diphenyl-1-picrylhydrazyl) Free Radical Assay. J. Pharmacol. Toxicol. Methods 2000, 44, 507–512. [Google Scholar] [CrossRef] [PubMed]

| Sample | Nanoparticles and Controls | Absorbance (λ = 415 nm) AU 1 |
|---|---|---|
| AuNPs | Gold nanoparticles | 0.095 |
| Saline | Negative control | 0.087 |
| Deionized water | Positive control | 0.991 |
| Parameters | Group | Mean | SD | p-Value |
|---|---|---|---|---|
| ALT (U/L) | Control | 64.63 ns | 4.66 | p > 0.05 |
| Plant extract | 58.60 ns | 5.92 | ||
| AuNPs 26.4 µg/mL | 84.80 * | 10.05 | ||
| AuNPs 52.8 µg/mL | 63.17 ns | 5.50 | ||
| AST (U/L) | Control | 140.30 ns | 21.34 | p > 0.05 |
| Plant extract | 143.43 ns | 34.84 | ||
| AuNPs 26.4 µg/mL | 156.53 ns | 26.17 | ||
| AuNPs 52.8 µg/mL | 133.60 ns | 51.21 | ||
| ALP (U/L) | Control | 536.00 ns | 173.22 | p > 0.05 |
| Plant extract | 583.33 ns | 89.18 | ||
| AuNPs 26.4 µg/mL | 629.00 ns | 8.72 | ||
| AuNPs 52.8 µg/mL | 525.67 ns | 95.13 | ||
| LDH (U/L) | Control | 317.33 ** | 56.58 | p < 0.001 |
| Plant extract | 434.33 ** | 289.83 | ||
| AuNPs 26.4 µg/mL | 203.00 ** | 116.05 | ||
| AuNPs 52.8 µg/mL | 1326.67 ** | 206.40 |
| Parameters | Group | Mean | SD | p-Value |
|---|---|---|---|---|
| Urea | Control | 50.00 ns | 14.36 | p > 0.05 |
| (mg/dL) | Plant extract | 51.23 ns | 5.55 | |
| AuNPs 26.4 µg/mL | 55.87 ns | 1.08 | ||
| AuNPs 52.8 µg/mL | 64.70 ns | 5.72 | ||
| Creatinine | Control | 0.73 ns | 0.03 | p > 0.05 |
| (mg/dL) | Plant extract | 0.86 ns | 0.14 | |
| AuNPs 26.4 µg/mL | 0.81 ns | 0.12 | ||
| AuNPs 52.8 µg/mL | 0.82 ns | 0.08 |
| Microorganism | S. aureus | S. pneumoniae | P. aeruginosa | E. coli | C. albicans |
|---|---|---|---|---|---|
| Inhibition zone (mm) |  |  |  |  |  |
| AuNPs | 40 | 40 | 35 | 36 | 40 |
| Plant Extract | 37 | 35 | 35 | 34 | 35 |
| HAuCl4 | 30 | 29 | 30 | 28 | 30 |
| Concentration (µg/mL) | AuNPs 1 | Plant Extract 1 | Vitamin C 1 | p-Value * |
|---|---|---|---|---|
| 100 | 97.22 | 88.56 | 96.29 | p < 0.05 |
| 80 | 84.98 | 71.21 | 87.90 | p < 0.05 |
| 60 | 63.76 | 51.90 | 58.09 | p < 0.05 |
| 40 | 45.65 | 36.20 | 48.04 | p < 0.05 |
| 20 | 19.27 | 24.31 | 29.21 | p < 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hazbar, A.M.; Jassim, A.M.N.; Mohammed, M.T.; Baqi, Y. Biosynthesized Gold Nanoparticles from Eruca sativa Mill. Leaf Extract Exhibit In Vivo Biocompatibility, Antimicrobial, and Antioxidant Activities. Antibiotics 2025, 14, 776. https://doi.org/10.3390/antibiotics14080776
Hazbar AM, Jassim AMN, Mohammed MT, Baqi Y. Biosynthesized Gold Nanoparticles from Eruca sativa Mill. Leaf Extract Exhibit In Vivo Biocompatibility, Antimicrobial, and Antioxidant Activities. Antibiotics. 2025; 14(8):776. https://doi.org/10.3390/antibiotics14080776
Chicago/Turabian StyleHazbar, Abdullah Muhsin, Abdulkadir Mohammed Noori Jassim, Mustafa Taha Mohammed, and Younis Baqi. 2025. "Biosynthesized Gold Nanoparticles from Eruca sativa Mill. Leaf Extract Exhibit In Vivo Biocompatibility, Antimicrobial, and Antioxidant Activities" Antibiotics 14, no. 8: 776. https://doi.org/10.3390/antibiotics14080776
APA StyleHazbar, A. M., Jassim, A. M. N., Mohammed, M. T., & Baqi, Y. (2025). Biosynthesized Gold Nanoparticles from Eruca sativa Mill. Leaf Extract Exhibit In Vivo Biocompatibility, Antimicrobial, and Antioxidant Activities. Antibiotics, 14(8), 776. https://doi.org/10.3390/antibiotics14080776







