Bridging the Capacity Building Gap for Antimicrobial Stewardship Implementation: Evidence from Virtual Communities of Practice in Kenya, Ghana, and Malawi
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Setting
2.2. Study Design
2.3. Study Analysis
3. Results
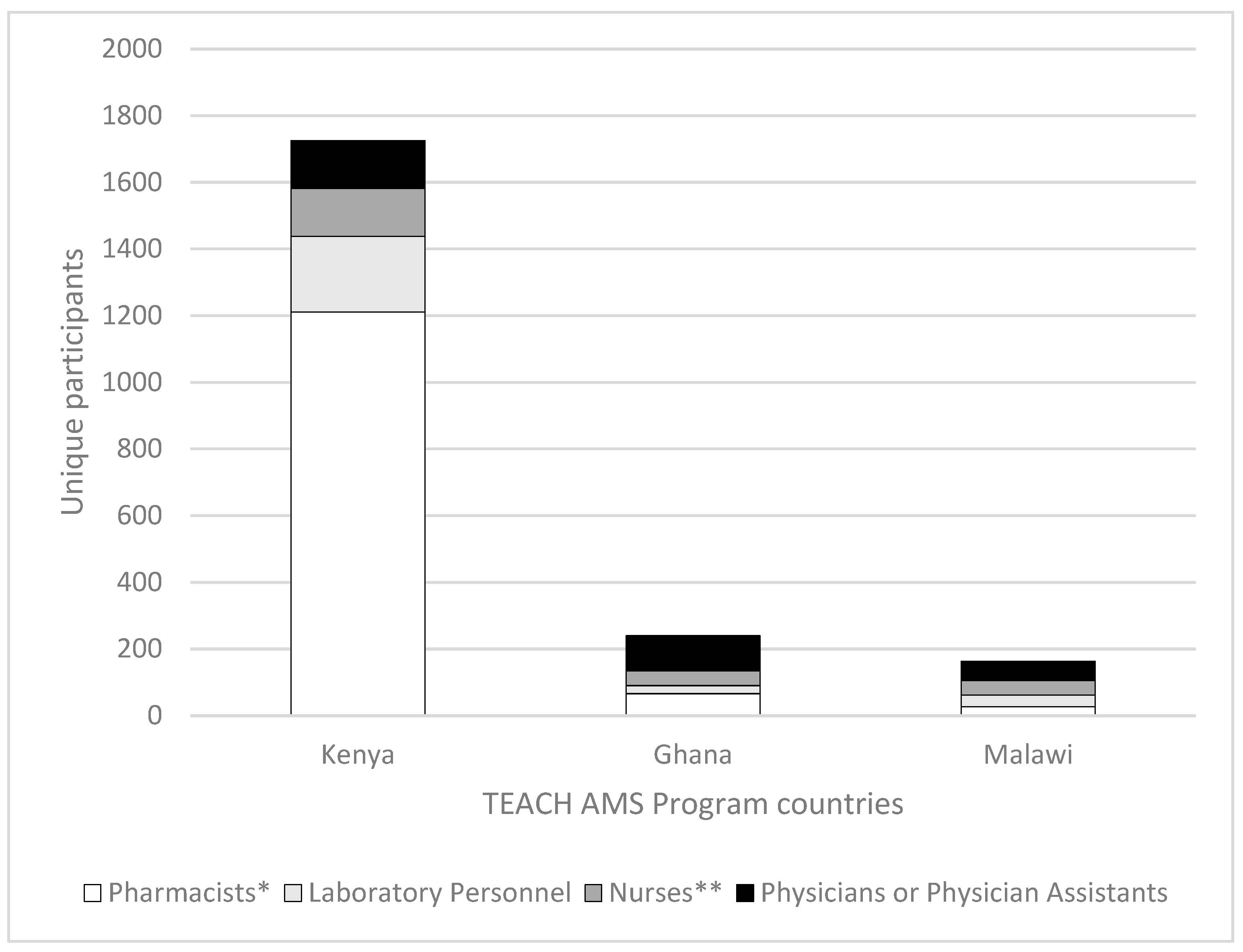
3.1. Attendance Reach
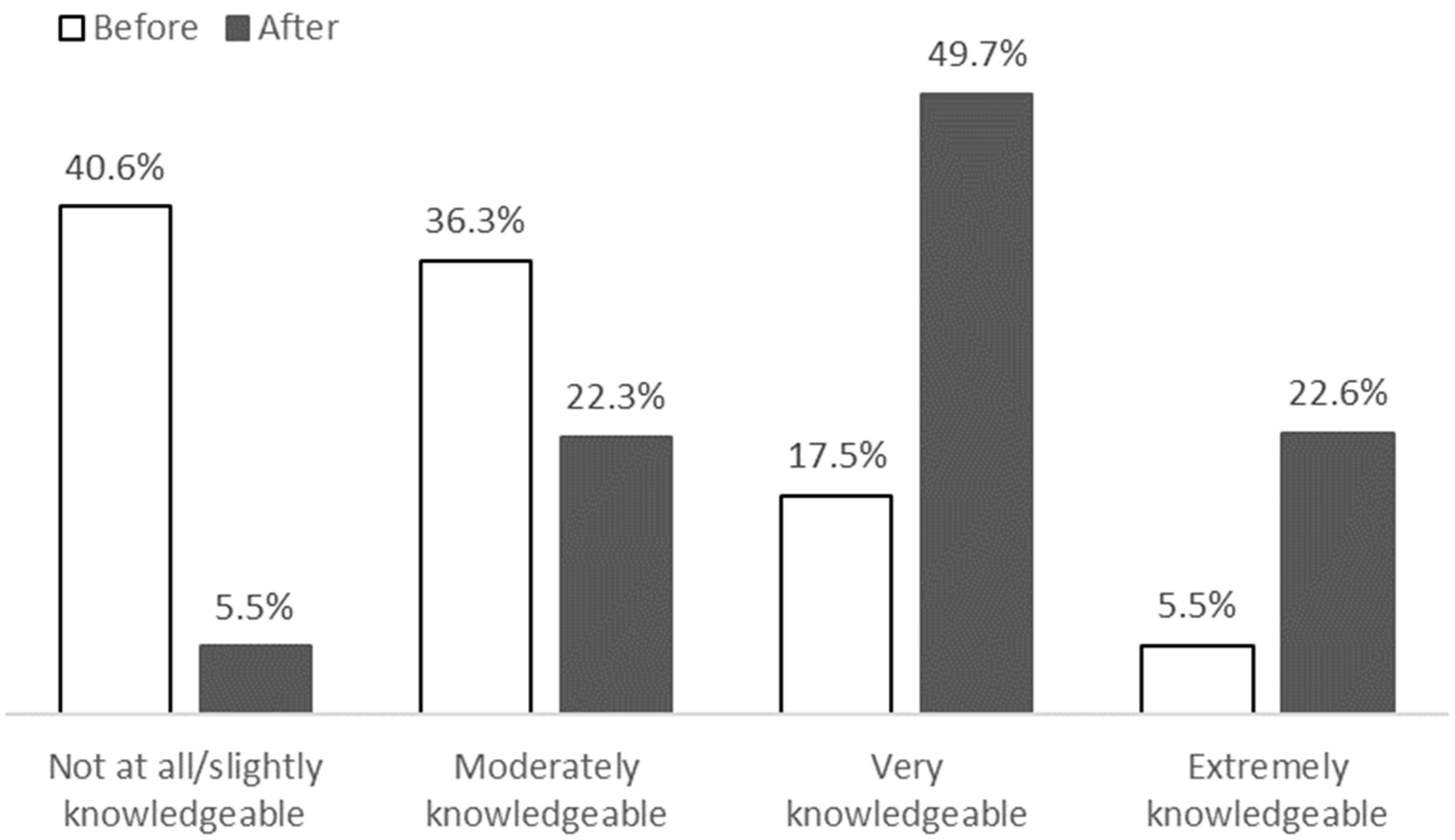
3.2. Session Satisfaction and Knowledge Gain
3.3. Implementation of AMS Healthcare Facility Core Elements
3.4. Practice and Systems Changes
3.4.1. Prescription Practice, AMS Interventions, and Use of Microbiology Laboratory Themes
“[During a TEACH AMS case presentation] the subject matter experts made us aware that [antimicrobial A] is useful for cystitis but not for pyelonephritis. And interestingly enough, about a week or two later, I had a patient with a similar presentation and (…) due to the information gathered from the session, there was enough confidence to immediately decide to go with [antimicrobial B].”(participant G03)
“We were able to do baseline assessments and specific tasks during the implementation. We were able to bring aspects of surveillance and diagnostic stewardship and dissemination of the findings through the AMS team and the health management team for actual policy change.”(participant K04)
“The TEACH AMS platform has really impacted our practice in terms of antimicrobial prescribing in the hospital. (…) Any time a prescriber wants to prescribe a Watch or a Reserve antibiotic, a pharmacist has to be consulted before such an antibiotic is given. And per the policy we drafted from sessions from the TEACH AMS, cultures have to be requested (…) before such antibiotics are started.”(participant G07)
“So one of the things that I have learned through these ECHO sessions is to draw samples first. (…) So with the prompt action on [maternal sepsis] to investigate and to take pus swabs for culture, and to take blood cultures at an earliest time, we have reduced some other cases that we usually had to refer to tertiary hospitals”.(participant M06)
3.4.2. Other Qualitative Analysis Themes
“We noted that different clinicians were not conversant with the different standard turnaround times for results (…). [Therefore] we had sensitization meetings from the lab, microbiology department in the different facilities. And clinicians were able to realize that the turnaround time for blood cultures was not the same as the turnaround for [other cultures]. (…) Most of the staff who joined the TEACH program from the facilities (…) [realized] there is need for us to dig deeper and understand patient care in collaboration with the other colleagues”.(participant K05)
“(…) if a child is diagnosed with UTI, for instance, the mother is taught to constantly change the diapers, wash their hand (…). We educate [parents] very well as to some of the things that they are supposed to do so that the hospital stay will be reduced”(participant G06)
“Following these TEACH AMS sessions, we were able to regroup and refocus [in the Antimicrobial Stewardship Committee]. (…) We were also able to bring in the administration and have a budget allocated for AMS activities”(participant K04)
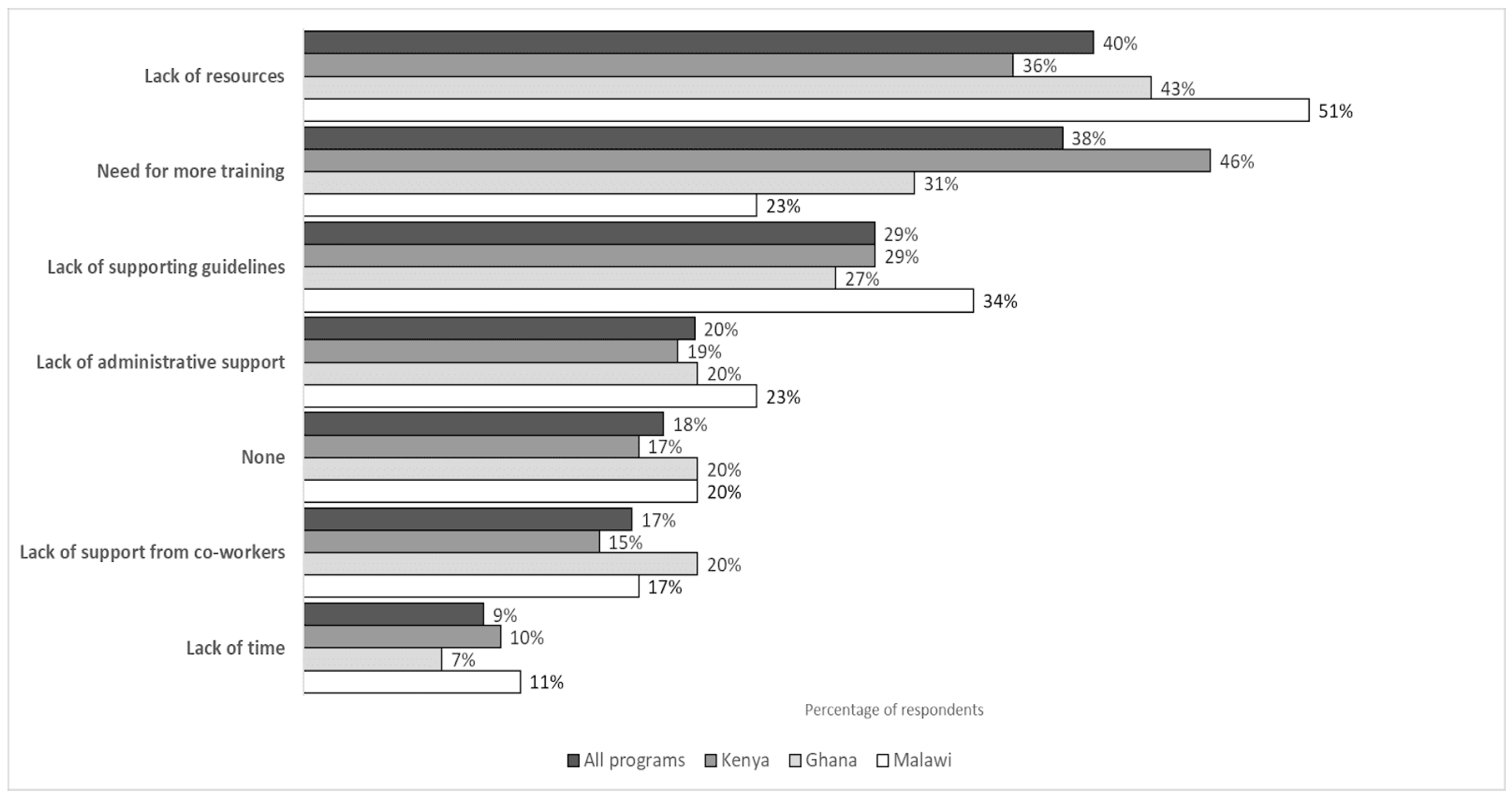
3.4.3. Barriers to Knowledge Application
“(…) Most of the time, the antibiotics that matter, that we need to dispense to the patients, are not available. (…) even if you encourage the patients to buy, they will tell you, ‘I don’t have funds to buy such a drug’”.(participant M06)
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMR | Antimicrobial Resistance |
| AMS | Antimicrobial Stewardship |
| ECHO | Extension for Community Health Outcomes |
| FGD | Focus Group Discussion |
| IPC | Infection Prevention and Control |
| MoH | Ministry of Health |
| SME | Subject Matter Expert |
| ToR | Terms of Reference |
Appendix A
| Number of Sessions Attended | Number of Attendees | Percentage of Attendees |
|---|---|---|
| 1 | 919 | 37.6% |
| 2 | 398 | 16.3% |
| 3 | 251 | 10.3% |
| 4 | 206 | 8.4% |
| 5 | 139 | 5.7% |
| 6 | 112 | 4.6% |
| 7 | 83 | 3.4% |
| 8 | 73 | 3% |
| 9 | 50 | 2% |
| 10 or more | 214 | 8.8% |
References
- World Health Organization. Global Action Plan on Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2015; p. 28. ISBN 9789241509763. [Google Scholar]
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Tattevin, P.; Hara, G.L.; Toumi, A.; Enani, M.; Coombs, G.; Voss, A.; Wertheim, H.; Poda, A.; Daoud, Z.; Laxminarayan, R.; et al. Advocacy for Increased International Efforts for Antimicrobial Stewardship Actions in Low-and Middle-Income Countries on Behalf of Alliance for the Prudent Use of Antimicrobials (APUA), Under the Auspices of the International Society of Antimicrobial Chemotherapy (ISAC). Front. Med. 2020, 7, 503. [Google Scholar] [CrossRef]
- Godman, B.; Egwuenu, A.; Wesangula, E.; Schellack, N.; Kalungia, A.C.; Tiroyakgosi, C.; Kgatlwane, J.; Mwita, J.C.; Patrick, O.; Niba, L.L.; et al. Tackling antimicrobial resistance across sub-Saharan Africa: Current challenges and implications for the future. Expert Opin. Drug Saf. 2022, 21, 1089–1111. [Google Scholar] [CrossRef]
- Harun, G.D.; Sumon, S.A.; Hasan, I.; Akther, F.M.; Islam, S.; Anwar, M.U. Barriers, facilitators, perceptions and impact of interventions in implementing antimicrobial stewardship programs in hospitals of low-middle and middle countries: A scoping review. Antimicrob. Resist. Infect. Control. 2024, 13, 8. [Google Scholar] [CrossRef]
- Charani, E.; Mendelson, M.; Pallett, S.J.C.; Ahmad, R.; Mpundu, M.; Mbamalu, O.; Bonaconsa, C.; Nampoothiri, V.; Singh, S.; Peiffer-Smadja, N.; et al. An analysis of existing national action plans for antimicrobial resistance—Gaps and opportunities in strategies optimising antibiotic use in human populations. Lancet Glob. Health 2023, 11, e466–e474. [Google Scholar] [CrossRef]
- World Health Organization. Antimicrobial Stewardship Programmes in Health-Care Facilities in Low- and Middle-Income Countries: A WHO Practical Toolkit; World Health Organization: Geneva, Switzerland, 2019; ISBN 978-92-4-151548-1. Available online: https://www.who.int/publications/i/item/9789241515481 (accessed on 9 May 2025).
- Minja, C. Antimicrobial Stewardship Guidance Document–ECSA-Health Community. Available online: https://ecsahc.org/document/antimicrobial-stewardship-guidance-document/ (accessed on 27 May 2025).
- Gitonga, N.; Okumu, M.; Agoro, O.; Kusu, N.; Mukoko, J.; Wangai, H.; Hafner, T.; Joshi, M.P. Assessment of the implementation of antimicrobial stewardship programs in selected healthcare facilities in Kenya. Front. Trop. Dis. 2025, 5, 1497220. [Google Scholar] [CrossRef]
- Sefah, I.A.; Chetty, S.; Yamoah, P.; Godman, B.; Bangalee, V. An Assessment of the Current Level of Implementation of the Core Elements of Antimicrobial Stewardship Programs in Public Hospitals in Ghana. Hosp. Pharm. 2024, 59, 367–377. [Google Scholar] [CrossRef]
- Chitatanga, R.; Yiwombe, C.; Divala, O.; Msokera, M.P.; Banda, E.; Chadwala, H.; Gilmon, M.W.; Kaminyoghe, W.; Chibwe, I.; Milala, H.; et al. A baseline assessment of antimicrobial stewardship core element implementation in selected public hospitals in Malawi: Findings from the 2023 National Program Audit. Front. Public Health 2025, 13, 1588778. [Google Scholar] [CrossRef]
- Ashiru-Oredope, D.; Langford, B.J.; Bonaconsa, C.; Nampoothiri, V.; Charani, E.; Goff, D.A. Global collaborations in antimicrobial stewardship: All hands on deck. Antimicrob. Steward. Healthc. Epidemiol. 2023, 3, e66. [Google Scholar] [CrossRef]
- Sneddon, J.; Guise, T.; Jenkins, D.; Mpundu, M.; Van Dongen, M.; Schouten, J.; Xiao, J.; Cordoba, G.; Nathwani, D. Introducing the global antimicrobial stewardship partnership hub (GASPH): Creating conditions for successful global partnership collaboration. JAC-Antimicrob. Resist. 2022, 4, dlac115. [Google Scholar] [CrossRef]
- US Centers for Disease Control and Prevention. Antibiotic Stewardship Trainings. Available online: https://www.cdc.gov/antibiotic-use/hcp/training/index.html (accessed on 9 May 2025).
- Shamas, N.; Stokle, E.; Ashiru-Oredope, D.; Wesangula, E. Challenges of implementing antimicrobial stewardship tools in Low to Middle Income Countries (LMICs). Infect. Prev. Pract. 2023, 5, 100315. [Google Scholar] [CrossRef]
- Chizimu, J.Y.; Mudenda, S.; Yamba, K.; Lukwesa, C.; Chanda, R.; Nakazwe, R.; Simunyola, B.; Shawa, M.; Kalungia, A.C.; Chanda, D.; et al. Antimicrobial stewardship situation analysis in selected hospitals in Zambia: Findings and implications from a national survey. Front. Public Health 2024, 12, 1367703. [Google Scholar] [CrossRef]
- Fuller, W.; Kapona, O.; Aboderin, A.O.; Adeyemo, A.T.; Olatunbosun, O.I.; Gahimbare, L.; Ahmed, Y.A. Education and Awareness on Antimicrobial Resistance in the WHO African Region: A Systematic Review. Antibiotics 2023, 12, 1613. [Google Scholar] [CrossRef]
- WHO Learn to Build a Healthier World | WHO Academy. Available online: https://whoacademy.org (accessed on 23 May 2025).
- Catanzaro, M.T. Antibiotic stewardship for nurses: Using e-learning modules to bridge the education gap. Antimicrob. Steward. Heal. Epidemiol. 2022, 2, e7. [Google Scholar] [CrossRef]
- Polisetty, R.S.; Borkowski, J.; Georges, D.; Mowers, S.; Bolch, C.; Quiñones-Boex, A.; Murray, M. Antibiotic Stewardship Attitudes and Beliefs Among Frontline Staff Nurses: Impact of Virtual Education. EMJ Microbiol. Infect. Dis. 2022. [Google Scholar] [CrossRef]
- Laks, M.; Guerra, C.M.; Miraglia, J.L.; Medeiros, E.A. Distance learning in antimicrobial stewardship: Innovation in medical education. BMC Med Educ. 2019, 19, 191. [Google Scholar] [CrossRef]
- Freire, P.I.A.; Gaspar, G.G.; Zurita, J.; Salazar, G.; Velez, J.W.; Bollela, V.R. E-Learning versus Face-to-Face Methodology for Learning Antimicrobial Resistance and Prescription Practice in a Tertiary Hospital of a Middle-Income Country. Antibiotics 2022, 11, 1829. [Google Scholar] [CrossRef]
- Sneddon, J.; Barlow, G.; Bradley, S.; Brink, A.; Chandy, S.J.; Nathwani, D. Development and impact of a massive open online course (MOOC) for antimicrobial stewardship. J. Antimicrob. Chemother. 2018, 73, 1091–1097. [Google Scholar] [CrossRef]
- Hartsough, K.; Safstrom, J.; Evans, M.; Pierre, M.; Schroder, E.; Herzig, C.; Da Costa, A.; Githii, S.; Abubeker, R.; Alebachew, G.; et al. Lessons from an evaluation of an antimicrobial resistance laboratory capacity telementoring program in Ethiopia and Kenya. Antimicrob. Steward. Heal. Epidemiol. 2023, 3, s123. [Google Scholar] [CrossRef]
- Wilcha, R.-J. Effectiveness of Virtual Medical Teaching During the COVID-19 Crisis: Systematic Review. JMIR Med. Educ. 2020, 6, e20963. [Google Scholar] [CrossRef]
- Goldin, S.; Hood, N.; Pascutto, A.; Bennett, C.; de Lima, A.C.B.; Devereaux, N.; Caric, A.; Rai, K.; Desai, S.; Lindstrand, A.; et al. Building global capacity for COVID-19 vaccination through interactive virtual learning. Hum. Resour. Health 2022, 20, 16. [Google Scholar] [CrossRef]
- Bonkoungou, B.; Mosha, F.; Abianuru, A.; Okeibunor, J.; Utunen, H.; Balaciano, G.; de Lima, A.C.B.; Burke, L.; McKenna, S.; Nag, S.; et al. The evolutionary journey to a new normal for learning and capacity building of healthcare workers to prepare and respond to health emergencies across Africa. Front. Public Health 2025, 12, 1455444. [Google Scholar] [CrossRef]
- Figueras, A. A Global Call to Action on Antimicrobial Resistance at the UN General Assembly. Antibiotics 2024, 13, 915. [Google Scholar] [CrossRef]
- Charani, E.; Holmes, A. Antibiotic Stewardship—Twenty Years in the Making. Antibiotics 2019, 8, 7. [Google Scholar] [CrossRef]
- Mendelson, M.; Laxminarayan, R.; Limmathurotsakul, D.; Kariuki, S.; Gyansa-Lutterodt, M.; Charani, E.; Singh, S.; Walia, K.; Gales, A.C.; Mpundu, M. Antimicrobial resistance and the great divide: Inequity in priorities and agendas between the Global North and the Global South threatens global mitigation of antimicrobial resistance. Lancet Glob. Health 2024, 12, e516–e521. [Google Scholar] [CrossRef]
- Freire, P.; Macedo, D.P. Pedagogy of the Oppressed: 30th Anniversary Edition; Bloomsbury Publishing: New York, NY, USA, 2014; ISBN 978-0-8264-1276-8. [Google Scholar]
- Arora, S.; Thornton, K.; Murata, G.; Deming, P.; Kalishman, S.; Dion, D.; Parish, B.; Burke, T.; Pak, W.; Dunkelberg, J.; et al. Outcomes of Treatment for Hepatitis C Virus Infection by Primary Care Providers. N. Engl. J. Med. 2011, 364, 2199–2207. [Google Scholar] [CrossRef]
- Struminger, B.; Arora, S.; Zalud-Cerrato, S.; Lowrance, D.; Ellerbrock, T. Building virtual communities of practice for health. Lancet 2017, 390, 632–634. [Google Scholar] [CrossRef]
- Bikinesi, L.; O’bRyan, G.; Roscoe, C.; Mekonen, T.; Shoopala, N.; Mengistu, A.T.; Sawadogo, S.; Agolory, S.; Mutandi, G.; Garises, V.; et al. Implementation and evaluation of a Project ECHO telementoring program for the Namibian HIV workforce. Hum. Resour. Health 2020, 18, 61. [Google Scholar] [CrossRef]
- Njukeng, P.A.; Njumkeng, C.; Ntongowa, C.; Abdulaziz, M. Strengthening laboratory networks in the Central Africa region: A milestone for epidemic preparedness and response. Afr. J. Lab. Med. 2022, 11, 5. [Google Scholar] [CrossRef]
- Talisuna, A.O.; Bonkoungou, B.; Mosha, F.S.; Struminger, B.B.; Lehmer, J.; Arora, S.; Conteh, I.N.; Appiah, J.A.; Nel, J.; Mehtar, S.; et al. The COVID-19 pandemic: Broad partnerships for the rapid scale up of innovative virtual approaches for capacity building and credible information dissemination in Africa. Pan Afr. Med. J. 2020, 37. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention Framework for Program Evaluation in Public Health. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr4811a1.htm (accessed on 2 May 2025).
- Drennan, J.; Hyde, A. Controlling response shift bias: The use of the retrospective pre-test design in the evaluation of a master’s programme. Assess. Eval. High. Educ. 2008, 33, 699–709. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2022; Available online: https://www.R-project.org (accessed on 2 May 2025).
- Posit Team. RStudio: Integrated Development Environment for R; Posit Team: Boston, MA, USA, 2022; Available online: http://www.posit.co (accessed on 2 May 2025).
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S. In Statistics and Computing, 4th ed.; Springer: New York, NY, USA, 2002; ISBN 978-0-387-95457-8. [Google Scholar]
- Hothorn, T.; Hornik, K.; van de Wiel, M.A.; Zeileis, A. Implementing a Class of Permutation Tests: ThecoinPackage. J. Stat. Softw. 2008, 28, 418. [Google Scholar] [CrossRef]
- Krippendorff, K. Content Analysis: An Introduction to Its Methodology; Sage Publications: New York, NY, USA, 2018. [Google Scholar]
- Lumivero NVivo 14. Denver, USA. 2023. Available online: www.lumivero.com (accessed on 2 May 2025).
- Wilkinson, A.; Ebata, A.; MacGregor, H. Interventions to Reduce Antibiotic Prescribing in LMICs: A Scoping Review of Evidence from Human and Animal Health Systems. Antibiotics 2018, 8, 2. [Google Scholar] [CrossRef]
- Cuevas, C.; Batura, N.; Wulandari, L.P.L.; Khan, M.; Wiseman, V. Improving antibiotic use through behaviour change: A systematic review of interventions evaluated in low- and middle-income countries. Health Policy Plan. 2021, 36, 754–773. [Google Scholar] [CrossRef]
- Van Dijck, C.; Vlieghe, E.; Cox, J.A. Antibiotic stewardship interventions in hospitals in low-and middle-income countries: A systematic review. Bull. World Health Organ. 2018, 96, 266–280. [Google Scholar] [CrossRef]
| Core Primary AMS Components | Z | p-Value | Increased | Unchanged | Decreased |
| AMS prioritized with management action plan | 1.9 | 0.06 | 32% | 60% | 8% |
| Accessible laboratory and imaging services | 1.7 | 0.08 | 12% | 88% | 0% |
| Antibiogram informed by antimicrobial use and resistance data | 1.6 | 0.11 | 36% | 52% | 12% |
| Dedicated AMS leader/champion identified | 1.4 | 0.17 | 16% | 80% | 4% |
| Multidisciplinary AMS leadership committee with clear ToR | 1.3 | 0.18 | 16% | 80% | 4% |
| Standardized facility prescription charts and medical records | 1.4 | 0.2 | 16% | 80% | 4% |
| Antimicrobial use regularly evaluated and shared | −1.1 | 0.3 | 8% | 72% | 20% |
| Basic training in optimal antimicrobial use | 0.9 | 0.4 | 32% | 48% | 20% |
| Resistance rates regularly evaluated and shared | 0.9 | 0.4 | 32% | 48% | 20% |
| Up-to-date standard treatment guidelines | 0.7 | 0.5 | 20% | 68% | 12% |
| Accessible IT services to support AMS activities | 0.6 | 0.5 | 24% | 60% | 16% |
| Approved antimicrobials list | 0.5 | 0.6 | 24% | 56% | 20% |
| Policy for documenting prescribed medicines | 0.5 | 0.6 | 20% | 68% | 12% |
| Restricted antimicrobial list and implementation guidelines | 0.4 | 0.7 | 24% | 60% | 16% |
| Core Secondary AMS components | Z | p-value | Increased | Unchanged | Decreased |
| Dedicated financial support for AMS action plan | 2 | 0.05 | 48% | 36% | 16% |
| Multidisciplinary AMS team with ToR | 1.2 | 0.22 | 44% | 44% | 12% |
| AMS action plan endorsed with progress and accountability measures | 1.2 | 0.23 | 44% | 40% | 16% |
| Continued training in optimal antimicrobial use | 0.82 | 0.41 | 40% | 40% | 20% |
| Core Tertiary AMS components | Z | p-value | Increased | Unchanged | Decreased |
| Regular ward rounds and other interventions by AMS team in select departments | 2.0 | 0.04 (*) | 48% | 36% | 16% |
| Monitoring antimicrobial susceptibility and resistance rates for a key indicator bacteria | 2.1 | 0.04 (*) | 44% | 44% | 12% |
| Initial and regular training of the AMS team in infection management | 1.8 | 0.06 | 44% | 40% | 16% |
| Monitoring of compliance of AMS interventions by AMS committee | 1.4 | 0.16 | 40% | 40% | 20% |
| Monitoring of quantity and types of antimicrobial use (purchased/prescribed/dispensed) | 1.4 | 0.17 | 32% | 56% | 12% |
| Defined collaboration between the AMS and IPC | 1.3 | 0.18 | 40% | 36% | 24% |
| Regular (descriptive) activity reports on AMS implementation | 1.1 | 0.25 | 36% | 44% | 20% |
| Regular activity reports (status and outcomes) on AMS implementation | 1.1 | 0.25 | 36% | 44% | 20% |
| Regular AMS team review/audit (antimicrobial therapy or clinical conditions) | 1.1 | 0.27 | 40% | 36% | 24% |
| Audits or PPSs monitoring for appropriate antimicrobial use | 1.0 | 0.31 | 36% | 44% | 20% |
| AMS team feedback easily available to all prescribers | 1.0 | 0.33 | 40% | 36% | 24% |
| Other health professionals identified and involved in AMS activities | 0 | 1 | 12% | 64% | 24% |
| Themes | Inclusion Criteria/Definition |
|---|---|
| Prescription Practice Improvements | Quotes from focus group participants or examples shared in follow-up surveys open questions in which respondents mentioned or wrote about improved antimicrobial prescription at the individual level, including use of culture results for prescribing, prescribing antimicrobials supported by laboratory results, use of target therapy, ensuring right frequency and dose is prescribed, and others, after participating in the TEACH AMS program. |
| AMS Interventions Application | Quotes from focus group participants or examples shared in follow-up surveys open questions in which respondents mentioned or wrote about implementing AMS activities, including use of the AWaRe classification (Access, Watch, reserve WHO system), conducting prescription audits, conducting ward rounds and panels, implementing point-prevalence survey, antibiotic inventory management, review of antibiotics after 48 h, creating or using antibiotics guidelines, SOPs, and workplans, creating restricted antimicrobials policy, cost implications, diagnostic stewardship, policies regarding any of the previously listed AMS interventions, system levels improvements of prescriptions, and others, after participating in the TEACH AMS program. |
| Improved Use of Microbiology Laboratory | Quotes from focus group participants or examples shared in follow-up surveys open questions in which respondents mentioned or wrote about improved sample collection and processing; improvements in test quality, safety, and efficiency; regular control testing for facility antibiotics for AST; confirming isolates; and having cultures done before prescribing antimicrobials, after participating in the TEACH AMS program. |
| Education or Training of Health Care Staff | Quotes from focus group participants or examples shared in follow-up surveys open questions in which respondents mentioned or wrote about educating other health care workers (e.g., discouraging the use of unnecessary antimicrobials, including specific learnings in facility trainings, presentations at the facility, advocating for proper and correct use of antimicrobials in facilities, advocate for forming AMS committee, advising prescribers) about AMS topics after participating in the TEACH AMS program. |
| IPC measures | Quotes from focus group participants or examples shared in follow-up surveys open questions in which respondents mentioned or wrote about infection, prevention, and control or facility waste management, often accompanied by examples such as equipment decontamination, hand washing, restricted entry to possibly contaminated areas, WASH, and others after participating in the TEACH AMS program. |
| Communication Across Diverse Health Professionals | Quotes from focus group participants or examples shared in follow-up surveys open questions in which respondents mentioned or wrote about applying a multidisciplinary approach, membership, or similar in their AMS-related activities in the workplace, communicating across diverse professional staff, after participating in the TEACH AMS program. |
| Communication with Patients and/or Community | Quotes from focus group participants or examples shared in follow-up surveys open questions in which respondents mentioned or wrote about educating patients, guardians, and/or the community after participating in the TEACH AMS program. |
| Advances in the Facility AMS Committee | Quotes from focus group participants or examples shared in follow-up surveys open questions in which respondents specifically mentioned or wrote about AMS committee improvements, including reactivating or participating in the committee, after participating in the TEACH AMS program. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbosa de Lima, A.C.; Buabeng, K.O.; Sakyi, M.; Chadwala, H.M.; Devereaux, N.; Mitambo, C.; Mugo-Sitati, C.; Njuhigu, J.; Revathi, G.; Tanui, E.; et al. Bridging the Capacity Building Gap for Antimicrobial Stewardship Implementation: Evidence from Virtual Communities of Practice in Kenya, Ghana, and Malawi. Antibiotics 2025, 14, 794. https://doi.org/10.3390/antibiotics14080794
Barbosa de Lima AC, Buabeng KO, Sakyi M, Chadwala HM, Devereaux N, Mitambo C, Mugo-Sitati C, Njuhigu J, Revathi G, Tanui E, et al. Bridging the Capacity Building Gap for Antimicrobial Stewardship Implementation: Evidence from Virtual Communities of Practice in Kenya, Ghana, and Malawi. Antibiotics. 2025; 14(8):794. https://doi.org/10.3390/antibiotics14080794
Chicago/Turabian StyleBarbosa de Lima, Ana C., Kwame Ohene Buabeng, Mavis Sakyi, Hope Michael Chadwala, Nicole Devereaux, Collins Mitambo, Christine Mugo-Sitati, Jennifer Njuhigu, Gunturu Revathi, Emmanuel Tanui, and et al. 2025. "Bridging the Capacity Building Gap for Antimicrobial Stewardship Implementation: Evidence from Virtual Communities of Practice in Kenya, Ghana, and Malawi" Antibiotics 14, no. 8: 794. https://doi.org/10.3390/antibiotics14080794
APA StyleBarbosa de Lima, A. C., Buabeng, K. O., Sakyi, M., Chadwala, H. M., Devereaux, N., Mitambo, C., Mugo-Sitati, C., Njuhigu, J., Revathi, G., Tanui, E., Lehmer, J., Mera, J., & Groom, A. V. (2025). Bridging the Capacity Building Gap for Antimicrobial Stewardship Implementation: Evidence from Virtual Communities of Practice in Kenya, Ghana, and Malawi. Antibiotics, 14(8), 794. https://doi.org/10.3390/antibiotics14080794









