TDM-Based Approach for Properly Managing Intravenous Isavuconazole Treatment in a Complex Case Mix of Critically Ill Patients
Abstract
1. Introduction
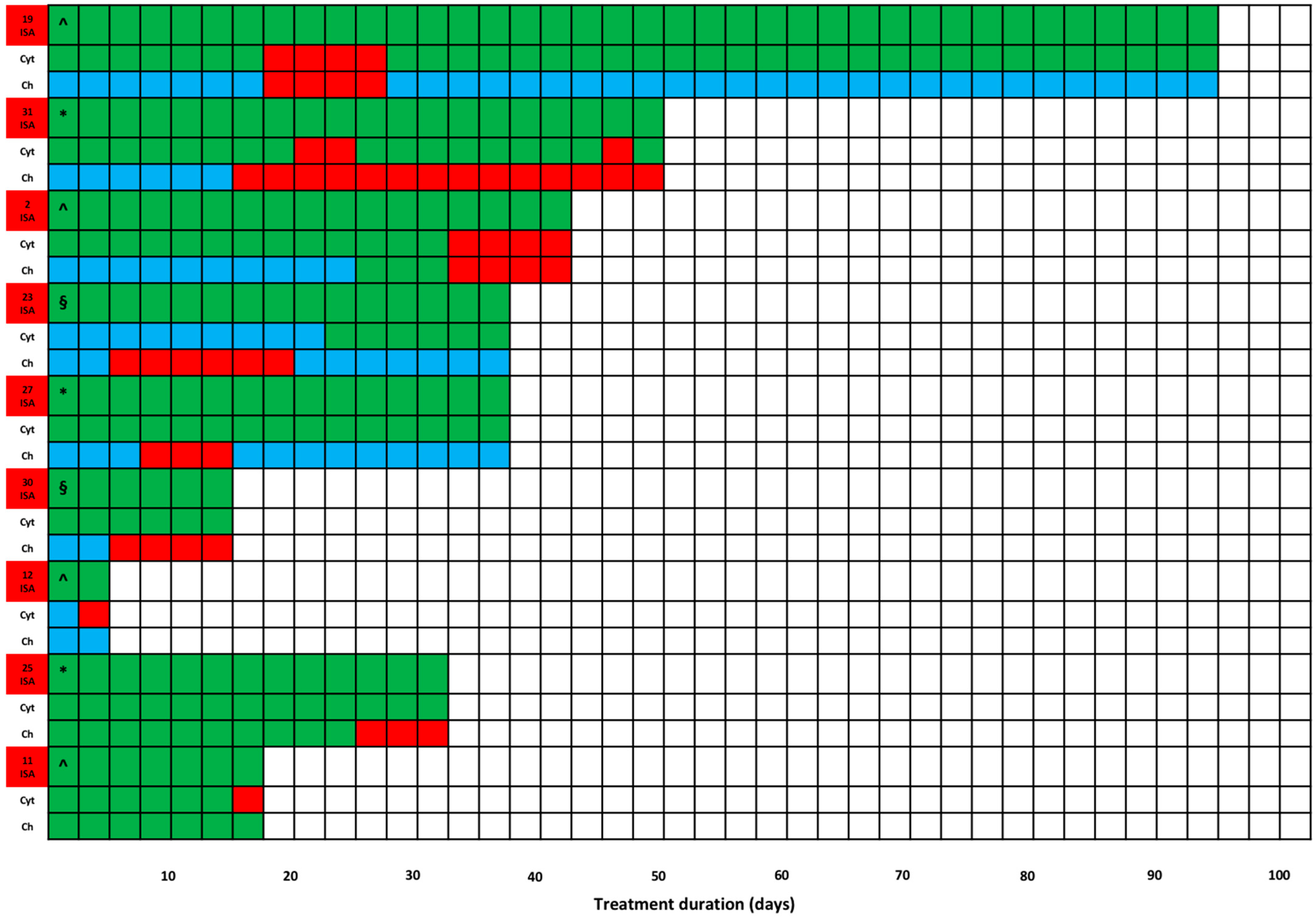
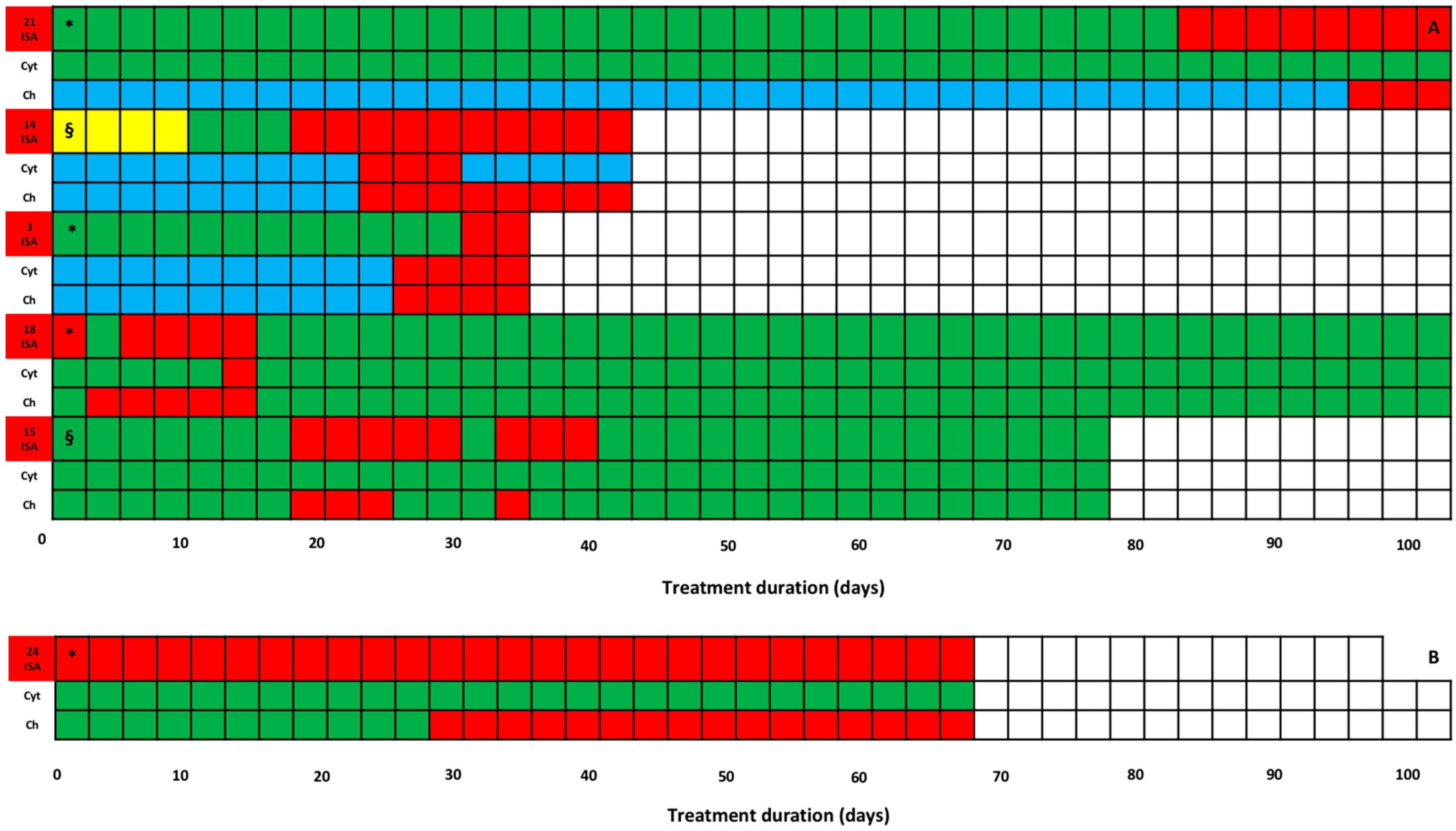
2. Results
3. Discussion
4. Materials and Methods
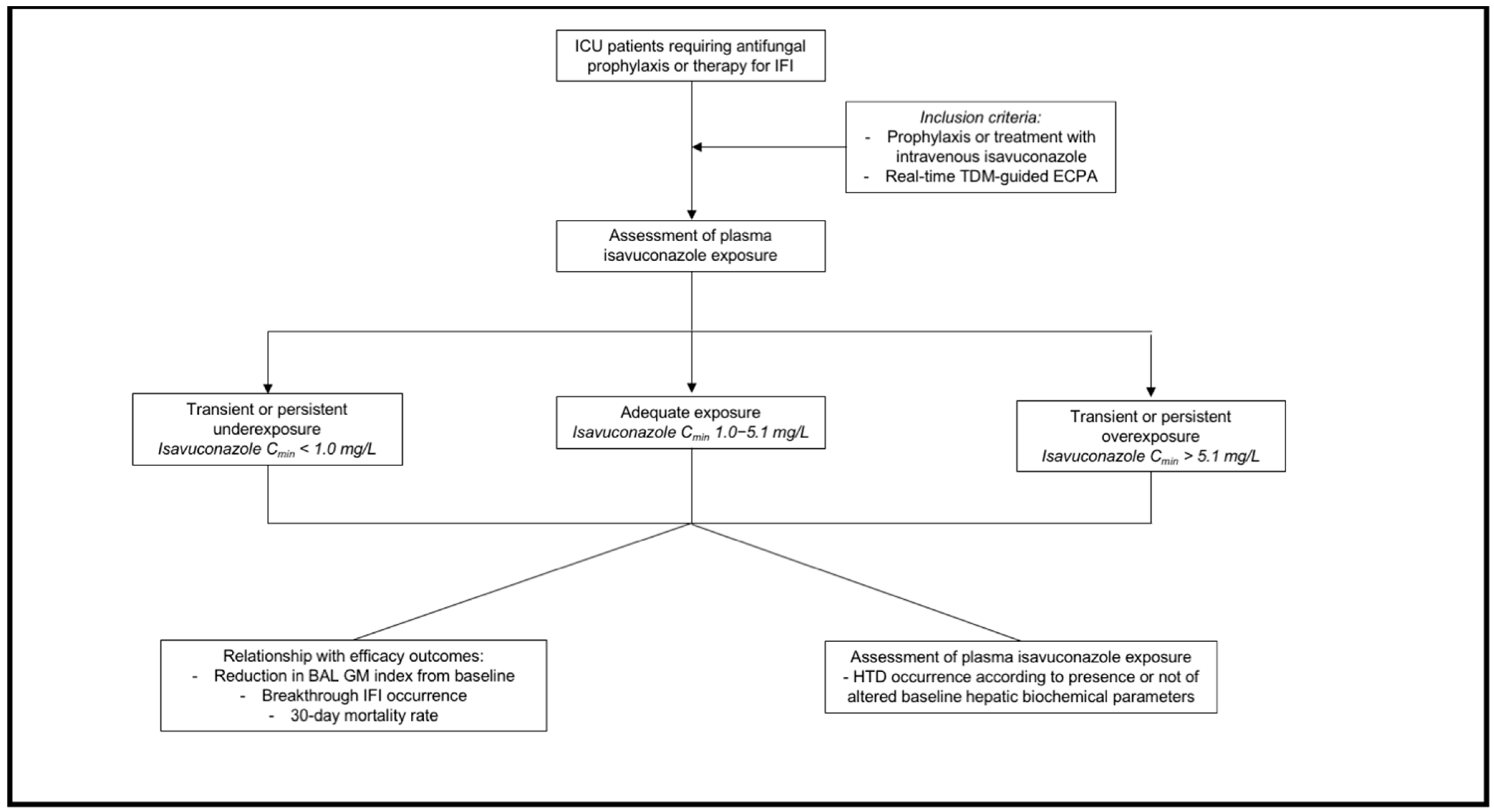
4.1. Study Design
4.2. Data Collection
4.3. Outcome Definition
4.4. Isavuconazole Dosing Regimens, Sampling Procedure, and Definition of Optimal Exposure
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blot, S.; Rello, J.; Koulenti, D. Diagnosing Invasive Pulmonary Aspergillosis in ICU Patients: Putting the Puzzle Together. Curr. Opin. Crit. Care 2019, 25, 430–437. [Google Scholar] [CrossRef]
- Bassetti, M.; Bouza, E. Invasive Mould Infections in the ICU Setting: Complexities and Solutions. J. Antimicrob. Chemother. 2017, 72, i39–i47. [Google Scholar] [CrossRef]
- Jenks, J.D.; Nam, H.H.; Hoenigl, M. Invasive Aspergillosis in Critically Ill Patients: Review of Definitions and Diagnostic Approaches. Mycoses 2021, 64, 1002–1014. [Google Scholar] [CrossRef]
- Cornely, O.A.; Alastruey-Izquierdo, A.; Arenz, D.; Chen, S.C.A.; Dannaoui, E.; Hochhegger, B.; Hoenigl, M.; Jensen, H.E.; Lagrou, K.; Lewis, R.E.; et al. Global Guideline for the Diagnosis and Management of Mucormycosis: An Initiative of the European Confederation of Medical Mycology in Cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect. Dis. 2019, 19, e405–e421. [Google Scholar] [CrossRef]
- Ullmann, A.J.; Aguado, J.M.; Arikan-Akdagli, S.; Denning, D.W.; Groll, A.H.; Lagrou, K.; Lass-Flörl, C.; Lewis, R.E.; Munoz, P.; Verweij, P.E.; et al. Diagnosis and Management of Aspergillus Diseases: Executive Summary of the 2017 ESCMID-ECMM-ERS Guideline. Clin. Microbiol. Infect. 2018, 24, e1–e38. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Aziz, M.H.; Alffenaar, J.-W.C.; Bassetti, M.; Bracht, H.; Dimopoulos, G.; Marriott, D.; Neely, M.N.; Paiva, J.-A.; Pea, F.; Sjovall, F.; et al. Antimicrobial Therapeutic Drug Monitoring in Critically Ill Adult Patients: A Position Paper. Intensive Care Med. 2020, 46, 1127–1153. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Sime, F.B.; Lipman, J.; Hernández-Mitre, M.P.; Baptista, J.P.; Brüggemann, R.J.; Darvall, J.; De Waele, J.J.; Dimopoulos, G.; Lefrant, J.-Y.; et al. Are Contemporary Antifungal Doses Sufficient for Critically Ill Patients? Outcomes from an International, Multicenter Pharmacokinetics Study for Screening Antifungal Exposure in Intensive Care Units—The SAFE-ICU Study. Intensive Care Med. 2025, 51, 302–317. [Google Scholar] [CrossRef] [PubMed]
- Hsu, A.J.; Tamma, P.D.; Fisher, B.T. Challenges in the Treatment of Invasive Aspergillosis in Immunocompromised Children. Antimicrob. Agents Chemother. 2022, 66, e02156-21. [Google Scholar] [CrossRef]
- Maertens, J.A.; Raad, I.I.; Marr, K.A.; Patterson, T.F.; Kontoyiannis, D.P.; Cornely, O.A.; Bow, E.J.; Rahav, G.; Neofytos, D.; Aoun, M.; et al. Isavuconazole versus Voriconazole for Primary Treatment of Invasive Mould Disease Caused by Aspergillus and Other Filamentous Fungi (SECURE): A Phase 3, Randomised-Controlled, Non-Inferiority Trial. Lancet 2016, 387, 760–769. [Google Scholar] [CrossRef]
- Marty, F.M.; Ostrosky-Zeichner, L.; Cornely, O.A.; Mullane, K.M.; Perfect, J.R.; Thompson, G.R.; Alangaden, G.J.; Brown, J.M.; Fredricks, D.N.; Heinz, W.J.; et al. Isavuconazole Treatment for Mucormycosis: A Single-Arm Open-Label Trial and Case-Control Analysis. Lancet Infect. Dis. 2016, 16, 828–837. [Google Scholar] [CrossRef]
- McCarthy, M.W.; Moriyama, B.; Petraitiene, R.; Walsh, T.J.; Petraitis, V. Clinical Pharmacokinetics and Pharmacodynamics of Isavuconazole. Clin. Pharmacokinet. 2018, 57, 1483–1491. [Google Scholar] [CrossRef]
- Desai, A.V.; Kovanda, L.L.; Hope, W.W.; Andes, D.; Mouton, J.W.; Kowalski, D.L.; Townsend, R.W.; Mujais, S.; Bonate, P.L. Exposure-Response Relationships for Isavuconazole in Patients with Invasive Aspergillosis and Other Filamentous Fungi. Antimicrob. Agents Chemother. 2017, 61, e01034-17. [Google Scholar] [CrossRef]
- Bertram, R.; Naumann, H.; Bartsch, V.; Hitzl, W.; Kinzig, M.; Haarmeyer, G.; Baumgärtel, M.; Geise, A.; Muschner, D.; Nentwich, J.; et al. Clinical and Demographic Factors Affecting Trough Levels of Isavuconazole in Critically Ill Patients with or without COVID-19. Mycoses 2023, 66, 1071–1078. [Google Scholar] [CrossRef]
- Höhl, R.; Bertram, R.; Kinzig, M.; Haarmeyer, G.; Baumgärtel, M.; Geise, A.; Muschner, D.; Prosch, D.; Reger, M.; Naumann, H.; et al. Isavuconazole Therapeutic Drug Monitoring in Critically Ill ICU Patients: A Monocentric Retrospective Analysis. Mycoses 2022, 65, 747–752. [Google Scholar] [CrossRef]
- Mikulska, M.; Melchio, M.; Signori, A.; Ullah, N.; Miletich, F.; Sepulcri, C.; Limongelli, A.; Giacobbe, D.R.; Balletto, E.; Russo, C.; et al. Lower Blood Levels of Isavuconazole in Critically Ill Patients Compared with Other Populations: Possible Need for Therapeutic Drug Monitoring. J. Antimicrob. Chemother. 2024, 79, 835–845. [Google Scholar] [CrossRef]
- DrugBank: Cytochrome P-450 CYP3A4 Inducers (Strong). Available online: https://go.drugbank.com/categories/DBCAT002649 (accessed on 17 May 2025).
- DrugBank: Cytochrome P-450 CYP3A4 Inhibitors (Strong). Available online: https://go.drugbank.com/categories/DBCAT002647 (accessed on 17 May 2025).
- Perez, L.; Corne, P.; Pasquier, G.; Konecki, C.; Sadek, M.; Le Bihan, C.; Klouche, K.; Mathieu, O.; Reynes, J.; Cazaubon, Y. Population Pharmacokinetics of Isavuconazole in Critical Care Patients with COVID-19-Associated Pulmonary Aspergillosis and Monte Carlo Simulations of High Off-Label Doses. J. Fungi 2023, 9, 211. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Xu, B.; Zheng, Y.; Huang, H.; Wei, Z.; Chen, S.; Huang, W.; Liu, M.; Zhang, Y.; Wu, X. Optimization of Oral Isavuconazole Dose for Population in Special Physiological or Pathological State: A Physiologically Based Pharmacokinetics Model-Informed Precision Dosing. J. Antimicrob. Chemother. 2024, 79, 2379–2389. [Google Scholar] [CrossRef] [PubMed]
- Furfaro, E.; Signori, A.; Di Grazia, C.; Dominietto, A.; Raiola, A.M.; Aquino, S.; Ghiggi, C.; Ghiso, A.; Ungaro, R.; Angelucci, E.; et al. Serial Monitoring of Isavuconazole Blood Levels during Prolonged Antifungal Therapy. J. Antimicrob. Chemother. 2019, 74, 2341–2346. [Google Scholar] [CrossRef]
- Cojutti, P.G.; Carnelutti, A.; Lazzarotto, D.; Sozio, E.; Candoni, A.; Fanin, R.; Tascini, C.; Pea, F. Population Pharmacokinetics and Pharmacodynamic Target Attainment of Isavuconazole against Aspergillus Fumigatus and Aspergillus Flavus in Adult Patients with Invasive Fungal Diseases: Should Therapeutic Drug Monitoring for Isavuconazole Be Considered as Mandatory as for the Other Mold-Active Azoles? Pharmaceutics 2021, 13, 2099. [Google Scholar] [CrossRef]
- Kosmidis, C.; Otu, A.; Moore, C.B.; Richardson, M.D.; Rautemaa-Richardson, R. Isavuconazole Therapeutic Drug Monitoring during Long-Term Treatment for Chronic Pulmonary Aspergillosis. Antimicrob. Agents Chemother. 2020, 65, e01511-20. [Google Scholar] [CrossRef] [PubMed]
- Borman, A.M.; Hughes, J.M.; Oliver, D.; Fraser, M.; Sunderland, J.; Noel, A.R.; Johnson, E.M. Lessons from Isavuconazole Therapeutic Drug Monitoring at a United Kingdom Reference Center. Med. Mycol. 2020, 58, 996–999. [Google Scholar] [CrossRef]
- Bury, D.; Tissing, W.J.E.; Muilwijk, E.W.; Wolfs, T.F.W.; Brüggemann, R.J. Clinical Pharmacokinetics of Triazoles in Pediatric Patients. Clin. Pharmacokinet. 2021, 60, 1103–1147. [Google Scholar] [CrossRef]
- Cojutti, P.G.; Rinaldi, M.; Giannella, M.; Viale, P.; Pea, F. Successful and Safe Real-Time TDM-Guided Treatment of Invasive Pulmonary and Cerebral Aspergillosis Using Low-Dose Isavuconazole in a Patient with Primary Biliary Cirrhosis: Grand Round/A Case Study. Ther. Drug Monit. 2023, 45, 140–142. [Google Scholar] [CrossRef]
- Couchepin, J.; Reinhold, I.; Kronig, I.; Guidi, M.; Buclin, T.; Schreiber, P.W.; Neofytos, D.; Lamoth, F.; for the Fungal Infection Network of Switzerland (FUNGINOS). Isavuconazole for the Treatment of Fungal Infections: A Real-Life Experience From the Fungal Infection Network of Switzerland (FUNGINOS). Open Forum Infect. Dis. 2024, 11, ofae223. [Google Scholar] [CrossRef] [PubMed]
- Monforte, A.; Los-Arcos, I.; Martín-Gómez, M.T.; Campany-Herrero, D.; Sacanell, J.; Berastegui, C.; Márquez-Algaba, E.; Sempere, A.; Nuvials, X.; Deu, M.; et al. Safety and Effectiveness of Isavuconazole Treatment for Fungal Infections in Solid Organ Transplant Recipients (ISASOT Study). Microbiol. Spectr. 2022, 10, e01784-21. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.; Gómez-Centurión, I.; Oarbeascoa, G.; Torres, M.; Martinez, A.P.; Suarez-Lledó, M.; Chinea, A.; Cascón, M.J.P.; Vazquez, L.; Espigado, I.; et al. Real-World Experience with Isavuconazole in Allogeneic Stem Cell Transplantation in Spain. Transplant. Cell. Ther. 2024, 30, 1033.e1–1033.e8. [Google Scholar] [CrossRef]
- Huang, E.; Wittenberg, R.; Dray, J.V.; Fine, J.; Robison, E.; Wilson, M.; Trigg, K.; Bays, D.J.; Chee, M.; Thompson, G.R. Isavuconazole Therapeutic Drug Monitoring and Association with Adverse Events. J. Antimicrob. Chemother. 2025, 80, 1702–1706. [Google Scholar] [CrossRef]
- Verweij, P.E.; Rijnders, B.J.A.; Brüggemann, R.J.M.; Azoulay, E.; Bassetti, M.; Blot, S.; Calandra, T.; Clancy, C.J.; Cornely, O.A.; Chiller, T.; et al. Review of Influenza-Associated Pulmonary Aspergillosis in ICU Patients and Proposal for a Case Definition: An Expert Opinion. Intensive Care Med. 2020, 46, 1524–1535. [Google Scholar] [CrossRef]
- Donnelly, J.P.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H.; et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020, 71, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Cornely, O.A.; Hoenigl, M.; Lass-Flörl, C.; Chen, S.C.-A.; Kontoyiannis, D.P.; Morrissey, C.O.; Thompson, G.R.; for the Mycoses Study Group Education and Research Consortium (MSG-ERC) and the European Confederation of Medical Mycology (ECMM). Defining Breakthrough Invasive Fungal Infection–Position Paper of the Mycoses Study Group Education and Research Consortium and the European Confederation of Medical Mycology. Mycoses 2019, 62, 716–729. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE); Version 5.0; US National Cancer Institute: Bethesda, MD, USA, 2017. [Google Scholar]
- Gatti, M.; Cojutti, P.G.; Bartoletti, M.; Tonetti, T.; Bianchini, A.; Ramirez, S.; Pizzilli, G.; Ambretti, S.; Giannella, M.; Mancini, R.; et al. Expert Clinical Pharmacological Advice May Make an Antimicrobial TDM Program for Emerging Candidates More Clinically Useful in Tailoring Therapy of Critically Ill Patients. Crit. Care 2022, 26, 178. [Google Scholar] [CrossRef] [PubMed]
- Chromsystems Instruments & Chemicals GmbH. MassTox TDM Series A. 2022. Available online: https://chromsystems.com/masstox-tdm-series-a (accessed on 17 May 2025).
| Variables | Overall Patients (n = 32) |
|---|---|
| Demographics | |
| Age (yrs; Median; [IQR]) | 62.0 (51.0–65.0) |
| Gender (male/female; n [%]) | 25/7 (78.1/21.9) |
| Weight (kg; Median; [IQR]) | 73.0 (60.8–85.8) |
| Body mass index (kg/m2; Median; [IQR]) | 25.5 (22.3–27.8) |
| Obesity (n; [%]) | 5 (15.6) |
| Underlying disease (n; [%]) | |
| Solid organ transplant recipient | 21 (65.6) |
| Hematological malignancies | 6 (18.8) |
| CAPA | 5 (15.6) |
| Pathophysiological conditions | |
| SOFA score (Median; [IQR]) | 8 (5–11) |
| Vasopressors (n; [%]) | 19 (59.4) |
| Mechanical ventilation (n; [%]) | 25 (78.1) |
| PaO2/FiO2 ratio (Median; [IQR]) | 166.0 (122.8–197.3) |
| PaO2/FiO2 ratio < 200 (n; [%]) | 24 (75.0) |
| Continuous renal replacement therapy (n; [%]) | 16 (50.0) |
| Cytosorb hemoadsorption (n; [%]) | 2 (6.3) |
| Extracorporeal membrane oxygenation (n; [%]) | 0 (0.0) |
| Serum albumin (g/dL; Median; [IQR]) | 2.8 (2.5–3.1) |
| Patients with moderate/severe hypoalbuminemia (n; [%]) | 22 (68.7) |
| Concomitant use of CYP3A4 strong inducers * (n; [%]) | 1 (3.1) |
| Concomitant use of CYP3A4 strong inhibitors * (n; [%]) | 0 (0.0) |
| IFI classification ** (n; [%]) | |
| Proven | 1 (3.1) |
| Probable | 25 (78.1) |
| Possible | 2 (6.3) |
| No IFI | 4 (12.5) |
| Documentation of IFI | |
| Patients with Aspergillus spp. isolated from BAL (n; [%]) | 6 (18.7) |
| Patients with positive galactomannan antigen on BAL (n; [%]) | 28 (87.5) |
| Patients with positive galactomannan antigen on serum (n; [%]) | 3 (9.4) |
| Galactomannan antigen index on BAL in positive patients (Median; [IQR]) | 2.6 (2.0–4.2) |
| Galactomannan antigen index on serum in positive patients (Median; [IQR]) | 2.9 (2.0–7.4) |
| Type of isavuconazole treatment (n; [%]) | |
| First-line therapy | 6 (18.7) |
| Second-line therapy | 22 (68.7) |
| First-line prophylaxis | 2 (6.3) |
| Second-line prophylaxis | 2 (6.3) |
| Isavuconazole treatment regimens and PK/PD target attainment | |
| Isavuconazole MD (mg/die; Median; [IQR]) | 200 (200–200) |
| Treatment duration (days; Median; [IQR]) | 35.0 (12.5–47.75) |
| Overall TDM-guided ECPA | 166 |
| TDM-guided ECPA per patient (Median; [IQR]) | 4 (2–7) |
| Average isavuconazole Cmin (mg/L; Median; [IQR]) | 3.5 (2.1–4.6) |
| (patients; n; [%]) | 29 (90.6) |
| Below desired range (patients; n; [%]) | 2 (6.3) |
| Above desired range (patients; n; [%]) | 1 (3.1) |
| Within desired range (ECPA; n; [%]) | 131 (78.9) |
| Below desired range (ECPA; n; [%]) | 7 (4.2) |
| Above desired range (ECPA; n; [%]) | 28 (16.9) |
| Outcome (n; [%]) | |
| HTD occurrence with no baseline alteration (n; [%]) | 5 (15.6) |
| Reduction ≥50% from baseline of galactomannan antigen on BAL within 14 days *** | 16/23 (69.6) |
| Breakthrough IFI | 0 (0.0) |
| 30-day mortality rate | 10 (31.3) |
| Variables | Patients with No Isavuconazole Cmin Below Desired Range (n = 30) | Patients with Isavuconazole Cmin Below Desired Range (n = 2) | p Value |
|---|---|---|---|
| Demographics | |||
| Age (yrs; Median; [IQR]) | 62.5 (51.0–65.0) | 53.0 (50.5–55.5) | 0.21 |
| Gender (male/female; n [%]) | 23/7 (76.7/23.3) | 2/0 (100.0/0.0) | 0.99 |
| Weight (kg; Median; [IQR]) | 72.5 (60.3–85.0) | 95.5 (83.3–107.8) | 0.24 |
| Body mass index (kg/m2; Median; [IQR]) | 25.5 (22.0–27.7) | 30.1 (26.6–33.6) | <0.001 |
| Obesity (n; [%]) | 4 (13.3) | 1 (50.0) | 0.29 |
| Underlying disease (n; [%]) | |||
| Solid organ transplant recipient | 21 (70.0) | 0 (0.0) | 0.11 |
| Hematological malignancies | 5 (16.7) | 1 (50.0) | 0.34 |
| CAPA | 4 (13.3) | 1 (50.0) | 0.29 |
| Pathophysiological conditions | |||
| SOFA score (Median; [IQR]) | 8.5 (5.25–11.0) | 6.5 (5.75–7.25) | 0.48 |
| Vasopressors (n; [%]) | 17 (56.7) | 2 (100.0) | 0.50 |
| Mechanical ventilation (n; [%]) | 23 (76.7) | 2 (100.0) | 0.99 |
| PaO2/FiO2 ratio (Median; [IQR]) | 166.0 (122.25–195.5) | 181.5 (171.75–191.25) | 0.56 |
| PaO2/FiO2 ratio < 200 (n; [%]) | 23 (76.7) | 1 (50.0) | 0.44 |
| Continuous renal replacement therapy (n; [%]) | 15 (50.0) | 1 (50.0) | 0.99 |
| Use of cytosorb hemoadsorption (n; [%]) | 1 (3.3) | 1 (50.0) | 0.12 |
| Extracorporeal membrane oxygenation (n; [%]) | 0 (0.0) | 0 (0.0) | 0.99 |
| Serum albumin (g/dL; Median; [IQR]) | 2.9 (2.6–3.1) | 2.4 (2.2–2.5) | 0.15 |
| Patients with moderate/severe hypoalbuminemia (n; [%]) | 20 (66.7) | 2 (100.0) | 0.99 |
| Concomitant use of CYP3A4 strong inducers * (n; [%]) | 0 (0.0) | 1 (50.0) | 0.06 |
| IFI classification ** (n; [%]) | |||
| Proven | 1 (3.3) | 0 (0.0) | 0.99 |
| Probable | 24 (80.0) | 1 (50.0) | 0.40 |
| Possible | 2 (6.7) | 0 (0.0) | 0.99 |
| No IFI | 3 (10.0) | 1 (50.0) | 0.24 |
| Type of isavuconazole treatment (n; [%]) | |||
| First-line therapy | 6 (20.0) | 0 (0.0) | 0.99 |
| Subsequent line of therapy | 21 (70.0) | 1 (50.0) | 0.53 |
| First-line prophylaxis | 1 (3.3) | 1 (50.0) | 0.12 |
| Second-line prophylaxis | 2 (6.7) | 0 (0.0) | 0.99 |
| Isavuconazole treatment regimens and outcome | |||
| Isavuconazole MD (mg/die; Median; [IQR]) | 200 (200–200) | 250 (200–300) | <0.001 |
| Treatment duration (days; Median; [IQR]) | 37.0 (13.25–49.25) | 19.0 (15.0–23.0) | 0.37 |
| HTD occurrence with no baseline alteration (n; [%]) | 5 (16.7) | 0 (0.0) | 0.99 |
| Reduction ≥ 50% from baseline of galactomannan antigen on BAL within 14 days *** (n; [%]) | 15/21 (71.4) | 1/2 (50.0) | 0.53 |
| Breakthrough IFI (n; [%]) | 0 (0.0) | 0 (0.0) | 0.99 |
| 30-day mortality rate (n; [%]) | 9 (30.0) | 1 (50.0) | 0.53 |
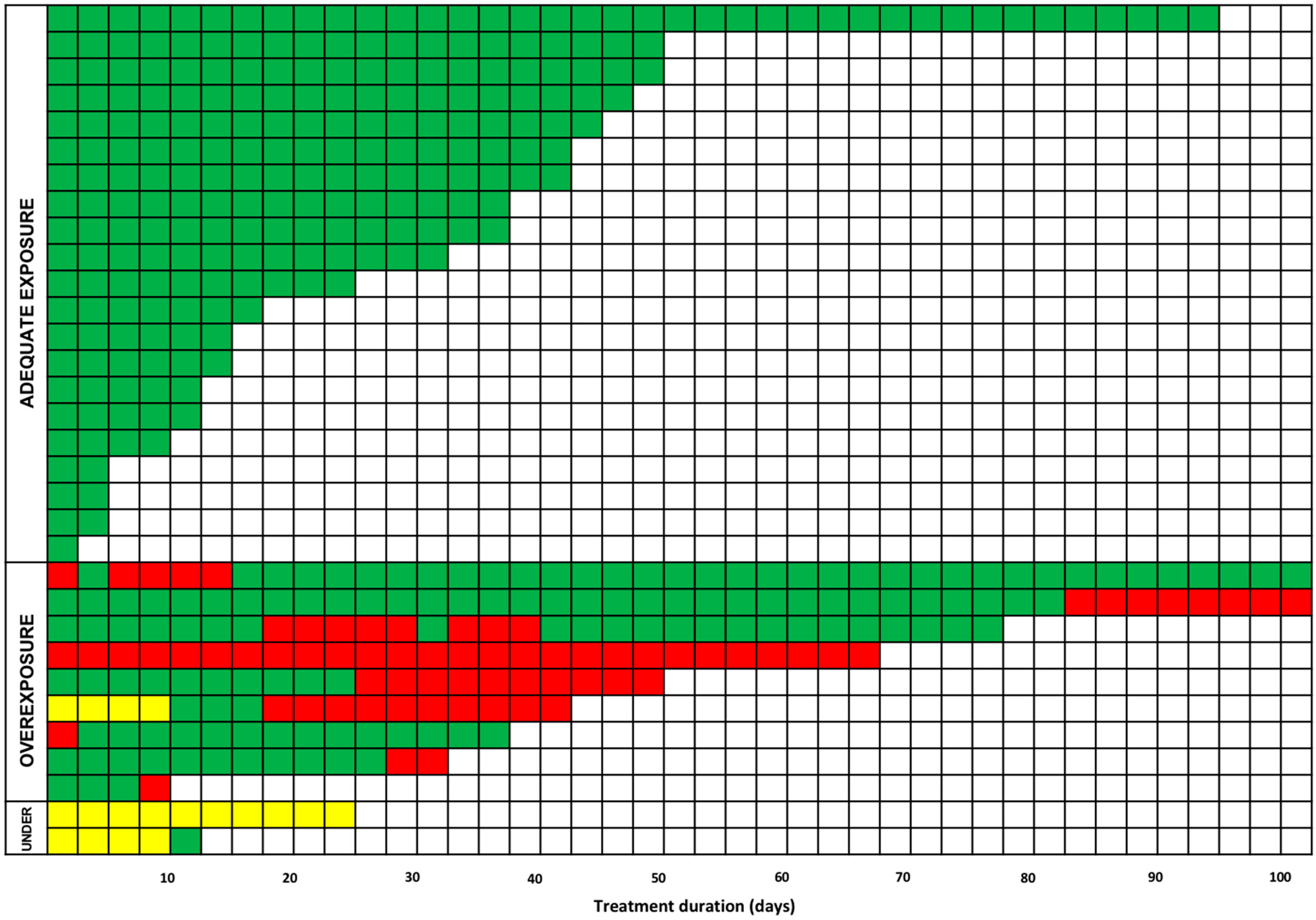
| Isavuconazole Cmin (mg/L) | Day < 7 | Day 7–28 | Day > 28 | p Value |
|---|---|---|---|---|
| <1.0 (n; [%]) | 4/38 (10.5%) | 3/70 (4.3%) | 0/58 (0.0%) | <0.001 |
| 1.0–5.1 (n; [%]) | 32/38 (84.2%) | 58/70 (82.9%) | 41/58 (70.7%) | |
| >5.1 (n; [%]) | 2/38 (5.3%) | 9/70 (12.8%) | 17/58 (29.3%) |
| Variables | SOT Patients (n = 21) | Non-SOT Patients (n = 11) | p Value |
|---|---|---|---|
| Demographics | |||
| Age (yrs; Median; [IQR]) | 62.0 (51.0–64.0) | 64.0 (54.5–65.0) | 0.55 |
| Gender (male/female; n [%]) | 17/4 (81.0/19.0) | 8/3 (72.7/27.3) | 0.67 |
| Weight (kg; Median; [IQR]) | 68.5 (60.0–80.0) | 84.0 (68.0–100.0) | 0.10 |
| Body mass index (kg/m2; Median; [IQR]) | 24.2 (21.1–26.6) | 27.4 (23.5–30.4) | 0.10 |
| Obesity (n; [%]) | 2 (9.5) | 3 (27.3) | 0.31 |
| Pathophysiological conditions | |||
| SOFA score (Median; [IQR]) | 8.0 (5.0–10.0) | 8.0 (6.5–11.0) | 0.56 |
| Vasopressors (n; [%]) | 12 (57.1) | 7 (63.6) | 0.99 |
| Mechanical ventilation (n; [%]) | 16 (76.2) | 9 (81.8) | 0.99 |
| PaO2/FiO2 ratio (Median; [IQR]) | 180.0 (135.0–209.0) | 136.0 (91.5–179.0) | 0.10 |
| PaO2/FiO2 ratio < 200 (n; [%]) | 15 (71.4) | 9 (81.8) | 0.68 |
| Continuous renal replacement therapy (n; [%]) | 12 (57.1) | 4 (36.4) | 0.46 |
| Use of cytosorb hemoadsorption (n; [%]) | 1 (4.8) | 1 (9.1) | 0.99 |
| Extracorporeal membrane oxygenation (n; [%]) | 0 (0.0) | 0 (0.0) | 0.99 |
| Serum albumin (g/dL; Median; [IQR]) | 2.88 (2.63–3.11) | 2.73 (2.41–3.22) | 0.76 |
| Patients with moderate/severe hypoalbuminemia (n; [%]) | 14 (66.7) | 8 (72.7) | 0.99 |
| Concomitant use of CYP3A4 strong inducers * (n; [%]) | 0 (0.0) | 1 (9.1) | 0.34 |
| IFI classification ** (n; [%]) | |||
| Proven | 1 (4.8) | 0 (0.0) | 0.99 |
| Probable | 18 (85.7) | 7 (63.6) | 0.20 |
| Possible | 2 (9.5) | 0 (0.0) | 0.53 |
| Prophylaxis | 0 (0.0) | 4 (36.4) | 0.009 |
| Type of isavuconazole treatment (n; [%]) | |||
| First-line therapy | 4 (19.0) | 2 (18.2) | 0.99 |
| Subsequent line of therapy | 17 (81.0) | 5 (45.4) | 0.06 |
| Prophylaxis | 0 (0.0) | 4 (36.4) | 0.009 |
| Isavuconazole treatment regimens and outcome | |||
| Isavuconazole MD (mg/die; Median; [IQR]) | 200 (200–200) | 200 (200–200) | 0.33 |
| Treatment duration (days; Median; [IQR]) | 42 (31–50) | 13 (8–26) | 0.02 |
| Average isavuconazole Cmin (mg/L; Median; [IQR]) | 3.44 (2.41–4.03) | 2.20 (1.36–2.70) | 0.006 |
| No. of TDM with isavuconazole underexposure *** (n; [%]) | 2/137 (1.5) | 5/29 (17.2) | 0.002 |
| No. of TDM with isavuconazole overexposure *** (n; [%]) | 27/137 (19.7) | 0/29 (0.0) | 0.005 |
| HTD occurrence in pts with normal baseline values (n; [%]) | 4 (19.0) | 1 (9.1) | 0.64 |
| Reduction ≥ 50% from baseline of galactomannan antigen on BAL within 14 days **** (n; [%]) | 10/16 (62.5) | 6/7 (85.7) | 0.37 |
| Breakthrough IFI (n; [%]) | 0 (0.0) | 0 (0.0) | 0.99 |
| 30-day mortality rate (n; [%]) | 5 (23.8) | 5 (45.5) | 0.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gatti, M.; Rinaldi, M.; De Paola, R.; Siniscalchi, A.; Tonetti, T.; Viale, P.; Pea, F. TDM-Based Approach for Properly Managing Intravenous Isavuconazole Treatment in a Complex Case Mix of Critically Ill Patients. Antibiotics 2025, 14, 777. https://doi.org/10.3390/antibiotics14080777
Gatti M, Rinaldi M, De Paola R, Siniscalchi A, Tonetti T, Viale P, Pea F. TDM-Based Approach for Properly Managing Intravenous Isavuconazole Treatment in a Complex Case Mix of Critically Ill Patients. Antibiotics. 2025; 14(8):777. https://doi.org/10.3390/antibiotics14080777
Chicago/Turabian StyleGatti, Milo, Matteo Rinaldi, Riccardo De Paola, Antonio Siniscalchi, Tommaso Tonetti, Pierluigi Viale, and Federico Pea. 2025. "TDM-Based Approach for Properly Managing Intravenous Isavuconazole Treatment in a Complex Case Mix of Critically Ill Patients" Antibiotics 14, no. 8: 777. https://doi.org/10.3390/antibiotics14080777
APA StyleGatti, M., Rinaldi, M., De Paola, R., Siniscalchi, A., Tonetti, T., Viale, P., & Pea, F. (2025). TDM-Based Approach for Properly Managing Intravenous Isavuconazole Treatment in a Complex Case Mix of Critically Ill Patients. Antibiotics, 14(8), 777. https://doi.org/10.3390/antibiotics14080777







